Abstract
Although as an organ the root plays a pivotal role in nutrient and water uptake as well anchorage, individual cell types function distinctly. Cortex is regarded as the least differentiated cell type in the root, but little is known about its role in plant growth and physiology. In recent studies, we found that cortex proliferation can be induced by oxidative stress. Since all types of abiotic stress lead to oxidative stress, this finding suggests a role for cortex in coping with abiotic stress. This hypothesis was tested in this study using the spy mutant, which has an extra layer of cortex in the root. Interestingly, the spy mutant was shown to be hypersensitive to salt and oxidizing reagent applied to the leaves, but it was as tolerant as the wild type to these compounds in the soil. This result lends support to the notion that cortex has a protective role against abiotic stress arising from the soil.
Keywords: abiotic stress, Arabidopsis thaliana, cortex, reactive oxygen species, root, shoot
Plants are sessile; therefore, to survive in a precarious environment, they need to cope with various kinds of stress, biotic and abiotic. Although much has been learned about the genes and signaling pathways involved in stress responses,1 how plants respond to stress developmentally is poorly understood. Our recent studies suggest that root cortex proliferation is a protective mechanism against abiotic stress.2
The initial evidence suggesting this role for root cortex is derived from our studies of the spy mutant. In the root of one-week-old wild-type seedlings of Arabidopsis thaliana, there is a single layer of cortex; in contrast, in the spy mutant, an additional layer of cortex is produced from the endodermis within 3 days of germination.3 To determine how SPY affects root development, we conducted transcript profiling with roots from spy-3 mutant and wild type plants.2 This transcriptome analysis showed that genes involved in redox homeostasis are enriched among the genes whose expression is altered in the spy mutant. Consistent with this, we found that the spy mutant has an elevated level of reactive oxygen species (ROS). Based on these observations, we hypothesized that cortex proliferation might be caused by changes in cellular redox status. We confirmed this in further experiments showing that cortex proliferation was induced in the root of wild-type seedlings by hydrogen peroxide and that the extra cortex phenotype in the spy mutant root was suppressed by glutathione, a potent reducing agent.
Unexpectedly, we also found that the receptor kinase ERECTA is required for ROS-induced cortex proliferation.2 No cortex proliferation could be induced in plants with a mutation in the ERECTA gene (the Ler ecotype or the er105 mutant), regardless of the concentration of hydrogen peroxide for the treatment. Interestingly, further studies showed that STOMAGEN, but not other ERECTA ligands, is essential to this redox-mediated developmental response. Thus, cortex proliferation in response to oxidative stress is a regulated, not just a passive response.
Our finding that redox-mediated cortex proliferation is a regulated process raises a question about cortex proliferation's role in plant growth and physiology. Conceivably, the extra cortex cell layer could act as a water reservoir and thus help the plant survive in drought. Supporting this notion, extra cortex layers have been found in the roots of Arabidopsis plants that have experienced water deficiency (unpublished results).
The major sources of ROS in plants are the mitochondria and chloroplasts. Normally ROS are generated at low rates as by-products of the respiratory and photosynthetic reactions in these organelles, but they build up under stress and can reach levels lethal to plants. Cortex proliferation in response to oxidative stress is therefore likely to be a general mechanism protecting the plants from abiotic stress. Indeed, supporting this hypothesis, a recent study showed that the spy mutants are more tolerant of high salt in the soil.4
However, this seems to be at odds with our recent observation that the spy mutant develops a shorter root in normal growth medium than the wild type and its root growth is reduced to a greater extent in salt-containing medium.2 One plausible explanation for this paradox is that the extra cortex cell layer in the spy mutant root, along with its shorter length, causes reduction in salt uptake from the soil, thus maintaining a normal level of salt in the shoot. To test this hypothesis, we sprayed salt solution directly onto the leaves of the spy-3 mutant and wild type shoots. The leaves of the spy mutant became wilted when exposed to 50 mM NaCl, but the wild type showed no sign of stress under the same treatment (Fig. 1A). We also performed a similar experiment with methyl viologen, a ROS-inducing herbicide, and found that the spy-3 mutant was also more sensitive (Fig. 1). Similar results were obtained with the spy-8 and spy-12 mutants, which are in the Ler background (Fig. 1B). These results support the notion that cortex proliferation in the root plays a role in counteracting abiotic stresses derived from the soil only. The tolerance of the spy mutants to salt in the soil cannot be attributed solely to the extra layer of cortex, though. Because mutants with short roots only, such as the scr mutant, are more sensitive to high sugar and salt (Cui et al 2012; and unpublished results), we think cortex contributes significantly to counteracting soil-based abiotic stress.
Figure 1.
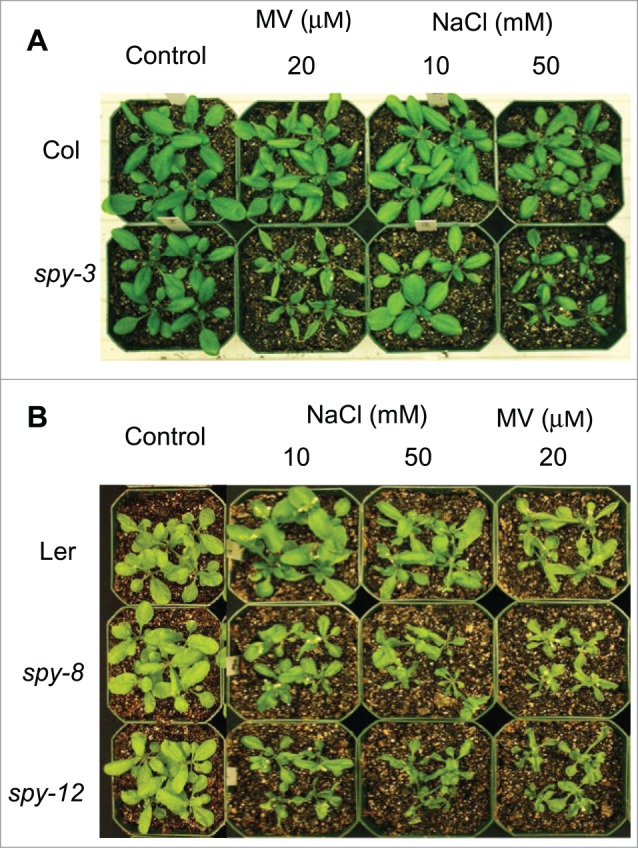
The spy mutant is hypersensitive to salt and methyl viologen. (A) Col and spy-3, (B) Ler (upper row), spy-8 (middle row), and spy-12 (lower row) plants, 9 h after spraying with water (control), 20 μM methyl viologen (MV), or 10 mM or 50 mM NaCl. Note that the shrunken leaves are due to wilting.
It is noteworthy that, in the wild-type root, cortex proliferation is induced only within a narrow range of hydrogen peroxide concentrations (0.5 – 2 mM).2 Above 2 mM, hydrogen peroxide causes cell damage, which explains the lack of cortex proliferation. This finding also means that cortex proliferation serves as a protective mechanism against mild but not acute stresses. Nevertheless, we believe that cortex proliferation is the predominant mechanism for plant crops to deal with abiotic stress, as mild, chronic stress occurs much more frequently than acute stress in agriculture. By forming an extra cell layer, plants would acquire permanent protection. Because there is no need to maintain the costly expression of proteins and antioxidants, plants can invest more resources in growth and development. Since cortex proliferation also takes resources, no extra cortex would be produced when the stress level is low. When stress is intense, the plants have to mount an array of responses to deal with it, but in this situation the plants’ priority is to survive.
Another intriguing observation from our studies is that only one extra layer of cortex is produced in the spy mutant or wild type plants treated with hydrogen peroxide. Two possible mechanisms can be conceived. First, this could result if the root has the energy to produce only a single extra cell layer, even though the cells can further divide. Alternatively, and more likely, if the endodermal cells that give rise to the extra cortex layer pause in a certain stage of the cell cycle, the ROS signal could reignite the cell cycle but be unable to reinitiate another cell cycle. Further studies are needed to distinguish between these possibilities.
As an organ, the root plays a pivotal role in nutrient and water uptake as well anchorage, but individual cell types function distinctly. For example, the endodermis acts as a gateway for selective nutrient uptake,5 whereas the columella cells are responsible for gravitropic response.6 In addition to long-distance transport of water and nutrients, the vascular tissue also has a role in nutrient storage and homeostasis, particularly for iron and sulfur.6 Among all root cell types, cortex is regarded as the least differentiated. Whether cortex has a role in plant growth and physiology is still unclear. This present study provides evidence that one function of cortex is protection against abiotic stress.
Materials and Methods
All plants used in this study are grown in soil at 20°C under a 16 h light/8 h dark regime in growth chambers. The spy mutant alleles used in this study were described previously (Jacobsen and Olszewski, 1993; Silverstone et al., 2007). For salt and methyl viologen treatment, 2-ml control (H2O + 0.1% Tween), NaCl (10 mM, 50 mM), or methyl viologen (20 μM, 50 μM, 100 μM) in control solution was sprayed onto the leaves of 4 3-week-old plants. The plants were kept under light during the treatment and photographed 9 h after the treatment.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by a set-up fund from Florida State University to H. Cui. The author is thankful to Jen Kennedy for help with editing.
References
- 1. Balderas-Hernandez VE, Alvarado-Rodriguez M, Fraire-Velazquez S Conserved versatile master regulators in signaling pathways in response to stress in plants. AoB Plants 2014; 5:plt055; PMID:24147216; http://dx.doi.org/25267734 10.1093/aobpla/plt033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cui H, Kong D, Wei P, Hao Y, Torii KU, Lee JS, Li J. SPINDLY, ERECTA, and Its Ligand STOMAGEN have a role in redox-mediated cortex proliferation in the Arabidopsis root. Mol Plant 2014; 7(12):1727-39; PMID:25267734; http://dx.doi.org/ 10.1093/mp/ssu106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cui H, Benfey PN. Interplay between SCARECROW, GA and LIKE HETEROCHROMATIN PROTEIN 1 in ground tissue patterning in the Arabidopsis root. Plant J 2009; 58:1016-27; PMID:19228333; http://dx.doi.org/ 10.1111/j.1365-313X.2009.03839.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Qin F, Kodaira KS, Maruyama K, Mizoi J, Tran LS, Fujita Y, Morimoto K, Shinozaki K, Yamaguchi-Shinozaki K. SPINDLY, a negative regulator of gibberellic acid signaling, is involved in the plant abiotic stress response. Plant physiol 2011; 157:1900-13; PMID:22013217; http://dx.doi.org/ 10.1104/pp.111.187302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dinneny JR. A gateway with a guard: how the endodermis regulates growth through hormone signaling. Plant Sci 2014; 214:14-9; PMID:24268159; http://dx.doi.org/ 10.1016/j.plantsci.2013.09.009 [DOI] [PubMed] [Google Scholar]
- 6. Iyer-Pascuzzi AS, Jackson T, Cui H, Petricka JJ, Busch W, Tsukagoshi H, Benfey PN. Cell identity regulators link development and stress responses in the Arabidopsis root. Dev Cell 2011; 21:770-82; PMID:22014526; http://dx.doi.org/ 10.1016/j.devcel.2011.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]


