Abstract
After replication in the cytoplasm, viruses spread from the infected cell into the neighboring cells through plasmodesmata, membranous channels embedded by the cell wall. As obligate parasites, viruses have acquired the ability to utilize host factors that unwillingly cooperate for the viral infection process. For example, the viral movement proteins (MP) interacts with the host pectin methylesterase (PME) and both proteins cooperate to sustain the viral spread. However, how and where PMEs interact with MPs and how the PME/MP complexes favor the viral translocation is not well understood. Recently, we demonstrated that the overexpression of PME inhibitors (PMEIs) in tobacco and Arabidopsis plants limits the movement of Tobacco mosaic virus and Turnip vein clearing virus and reduces plant susceptibility to these viruses. Here we discuss how overexpression of PMEI may reduce tobamovirus spreading.
Keywords: cell wall, methanol, pectin methylesterase, pectin methylesterase inhibitors, pectin methylesterification, plasmodesmata, virus spreading
Abbreviations
- PME
pectin methylesterase
- PMEI
pectin methylesterase inhibitor
- MP
movement protein
- PD
plasmodesmata
- TMV
Tobacco mosaic virus
- CW
cell wall
- MeOH
methanol
- PM
Plasma membrane
- ER
Endoplasmic Reticulum
- CP
coat protein
After penetration through damaged cells, plant viruses utilize host proteins that assist their infection process. The viral cell-to-cell movement goes through the plasmodesmata (PD), dynamic and complex membranous channels surrounded by specialized cell wall regions.1,2 The movement is supported by virus-encoded movement proteins (MPs), which are able to increase the size exclusion limit of PD.3 MPs perform multiple interactions with host intracellular proteins, among which the cell wall-associated pectin methylesterases (PMEs).4,5 Specific interactions of MP of Tobacco mosaic virus (TMV) and Turnip vein clearing virus (TVCV) with PMEs from tomato, citrus and tobacco and, more recently, between MP of TVCV with PMEs from Arabidopsis have been characterized.4,5 Although both MP and PME have been found associated to PD structures the definition of the subcellular localization of the PME-MP complex is under debate.4,6,7 Plant PMEs contain a transmembrane (TM) domain preceding the mature enzymes that is considered a membrane-anchor domain required for targeting the enzyme to cell wall (CW).8 MP was found in cell wall where it is phosphorylated by wall associated kinases to regulate PD transport.9 MP of TMV has 2 putative transmembrane regions that enable the protein to expose its cytosolic and ER luminal domains.10 It can be hypothesized that these structural features enable MP to interact with membrane-associated PME at ER luminal face and/or in the apoplastic compartment. Consistently, the interaction between the MP of Chinese wheat mosaic virus and PME from Nicotiana benthamiana has been showed to occur at the plasma membrane-CW level of N. benthamiana epidermal cells.6
Several experimental evidences suggest that PMEs, by interacting with MP, play a functional role in tobamovirus local spreading.4,5,11 PME is also involved in TMV systemic movement mainly participating in the viral outcome from the vascular system.12 The activity of PME is modulated in the cell wall by pectin methylesterase inhibitors (PMEIs).13-18 PMEIs are targeted to the extracellular matrix and inhibit plant PMEs by forming a specific stoichiometric 1:1 complex.19 We have recently demonstrated that PMEIs affect plant susceptibility toward viruses by counteracting the action of plant PMEs. We overexpressed genes encoding 2 well-characterized PMEIs in tobacco and Arabidopsis plants and showed that overexpression of AcPMEI in tobacco and AtPMEI-2, in Arabidopsis, causes a significant reduction of PME activity, an increase of cell wall methylesterification and, as a consequence, the reduction of the local and systemic translocation of TMV and TVCV.5
PMEs are a large class of cell wall-remodelling enzymes induced during growth and upon pathogen infection.8,20 Specific PME isoforms are up-regulated upon infection by different viruses.21-23 The accumulation of PME transcripts is induced by TMV in infected tobacco leaves.23 We have found that PME activity is strongly induced in tobacco and Arabidopsis leaves during TMV and TVCV infection and we demonstrated, that the overexpression of PMEIs in tobacco and Arabidopsis transgenic plants, not only affects the existing PME activity but also inhibits the PME activity induced during viral infection.5
PMEs catalyze the de-methylesterification of pectin and release both protons and methanol. PME activity is considered the main metabolic source of methanol in planta.24 It has been recently demonstrated that PME-dependent methanol emission triggers PD dilation and facilitates cell-to-cell communication and viral spreading.23 This effect has been related to expression of methanol-induced genes including β-1,3-glucanases cooperating to PD dilation by degrading callose, which is locally deposited at the cell wall embedded neck region of PD to restrict cell-to-cell movement of viruses.23,25 The overexpression of PMEI in transgenic plants limits cell-to-cell viral spreading by affecting the viral-induced PME activity and possibly by reducing the methanol-activated degradation of callose. PMEI expression has been shown to be induced by virus and after methanol treatment suggesting that the production of the inhibitor may be considered a defense strategy of the plant to hamper the activity of PME during viral infection.23,26,27
Immunoelectron microscopy studies indicate that PME is present in pectin-rich cell wall micro-domains around PD where acidic pectin and PME colocalize.1,4,27 Protons produced by PME activity, accumulate in the apoplast during pectin de-methylesterification and lead to acidification of the wall.28 A lower pH can promote the cell wall loosening by stimulating the activity of several cell wall-degrading enzymes (CWDEs), such as polygalacturonases, pectate lyases and expansins.29-31 In addition, a lower degree of methylesterification caused by PME may render the pectin more susceptible to the degradation by plant derived pectic enzymes.17,20,32 It can be postulated that the virus exploits the MP-PME interaction to recruit additional PMEs to perform a localized decrease of pH and pectin degree of esterification and to loosen the cell wall around PD to assist PD opening during infection. The overexpression of PMEI in transgenic plants may counteract this process and consequently limit viral spreading.
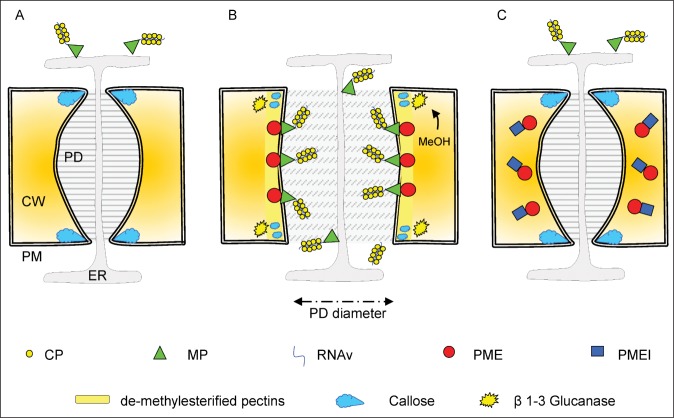
In conclusion a scenario is proposed that might explain the role of PME and PMEI in tobamovirus spreading. After viral penetration, plants respond to viral infection by depositing callose at the PD level to restrict the viral cell-to-cell diffusion (Fig. 1A). Viruses produce MPs and induce host PMEs and the interaction between the 2 proteins is exploited to localize additional PME activity and loosen the cell wall around PDs to promote the PD enlargement (Fig. 1B). The overexpression of PMEIs in transgenic plants may counteract the process by limiting PME/MP-mediated PD pore dilation and cell-to-cell viral spreading (Fig. 1C). Although this model proposes a novel vision on the impact of PME and PMEI on tobamovirus spreading, further experimental evidence is required to support this hypothesis and to clarify the mechanisms at the base of this process.
Figure 1.
Dynamics of pectin methylesterase (PME) and pectin methylesterase inhibitors (PMEIs) in the viral cell-to-cell movement through the plasmodesmata (PD). (A) After viral penetration plants reduce size exclusion limit of PD by locally depositing callose at the neck regions of PD. (B) Virus infection alters the PD gating capacity by inducing PME that in cooperation with MP enlarges the pore diameter of PD by affecting the cell wall microdomains embedding PD. PME activity, localized by MP at the level of the cell wall embedding PD, can decrease pH and pectin degree of methylsterification which, in turn, favor the cell wall degradation by CWDEs. In addition viruses degrade the callose ring by inducing a methanol-mediated expression of β 1–3 glucanases. (C) The overexpression of PMEIs in transgenic plants counteracts these processes by limiting PME/MP-mediated PD pore dilatation and cell-to-cell viral spreading. PM, plasma membrane; ER, endoplasmic reticulum; CW, cell wall; PD, plasmodesmata; CP, coat protein; MP, movement protein; RNAv, viral RNA; MeOH, methanol.
Funding Statement
This research was supported by the Institute Pasteur-Fondazione Cenci Bolognetti and the Ministero dell’Università e della Ricerca (PRIN 2010T7247Z)
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Orfila C, Knox JP. Spatial regulation of pectic polysaccharides in relation to pit fields in cell walls of tomato fruit pericarp. Plant Physiol 2000; 122:775-81; PMID:10712541; http://dx.doi.org/ 10.1104/pp.122.3.775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu C, Nelson RS. The cell biology of Tobacco mosaic virus replication and movement. Front Plant Sci 2013; 4: 12; PMID:23403525; http://dx.doi.org/ 10.3389/fpls.2013.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benitez-Alfonso Y, Faulkner C, Ritzenthaler C, Maule AJ. Plasmodesmata: gateways to local and systemic virus infection. Mol Plant-Microbe Interact 2010; 23:1403-12; PMID:20687788; http://dx.doi.org/ 10.1094/MPMI-05-10-0116 [DOI] [PubMed] [Google Scholar]
- 4. Chen MH, Sheng J, Hind G, Handa AK, Citovsky V. Interaction between the tobacco mosaic virus movement protein and host cell pectin methylesterases is required for viral cell-to-cell movement. EMBO J 2000; 19:913-20; PMID:10698933; http://dx.doi.org/ 10.1093/emboj/19.5.913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lionetti V, Raiola A, Cervone F, Bellincampi D. Transgenic expression of pectin methylesterase inhibitors limits tobamovirus spread in tobacco and Arabidopsis. Mol Plant Pathol 2014; 15:265-74; PMID:24127644; http://dx.doi.org/ 10.1111/mpp.12090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andika IB, Zheng SL, Tan ZL, Sun LY, Kondo H, Zhou XP, Chen JP. Endoplasmic reticulum export and vesicle formation of the movement protein of Chinese wheat mosaic virus are regulated by two transmembrane domains and depend on the secretory pathway. Virology 2013; 435:493-503; PMID:23137810; http://dx.doi.org/ 10.1016/j.virol.2012.10.024 [DOI] [PubMed] [Google Scholar]
- 7. Morvan O, Quentin M, Jauneau A, Mareck A, Morvan C. Immunogold localization of pectin methylesterases in the cortical tissues of flax hypocotyl. Protoplasma 1998; 202:175-84; http://dx.doi.org/ 10.1007/BF01282545 [DOI] [Google Scholar]
- 8. Pelloux J, Rusterucci C, Mellerowicz EJ. New insights into pectin methylesterase structure and function. Trends Plant Sci 2007; 12:267-77; PMID:17499007; http://dx.doi.org/ 10.1016/j.tplants.2007.04.001 [DOI] [PubMed] [Google Scholar]
- 9. Waigmann E, Chen MH, Bachmaier R, Ghoshroy S, Citovsky V. Regulation of plasmodesmal transport by phosphorylation of tobacco mosaic virus cell-to-cell movement protein. EMBO J 2000; 19:4875-84; PMID:10990451; http://dx.doi.org/ 10.1093/emboj/19.18.4875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brill LM, Nunn RS, Kahn TW, Yeager M, Beachy RN. Recombinant tobacco mosaic virus movement protein is an RNA-binding, alpha-helical membrane protein. Proc Natl Acad Sci USA 2000; 97:7112-7; PMID:10840061; http://dx.doi.org/ 10.1073/pnas.130187897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dorokhov YL, Makinen K, Frolova OY, Merits A, Saarinen J, Kalkkinen N, Atabekov JG, Saarma M. A novel function for a ubiquitous plant enzyme pectin methylesterase: the host-cell receptor for the tobacco mosaic virus movement protein. FEBS Lett 1999; 461:223-8; PMID:10567701; http://dx.doi.org/ 10.1016/S0014-5793(99)01447-7 [DOI] [PubMed] [Google Scholar]
- 12. Chen M-H, Citovsky V. Systemic movement of a tobamovirus requires host cell pectin methylesterase. Plant J 2003; 35:386-92; PMID:12887589; http://dx.doi.org/ 10.1046/j.1365-313X.2003.01818.x [DOI] [PubMed] [Google Scholar]
- 13. Bellincampi D, Camardella L, Delcour JA, Desseaux V, D'Ovidio R, Durand A, Elliot G, Gebruers K, Giovane A, Juge N, et al. Potential physiological role of plant glycosidase inhibitors. Biochim Biophys Acta 2004; 1696:265-74; PMID:14871667; http://dx.doi.org/ 10.1016/j.bbapap.2003.10.011 [DOI] [PubMed] [Google Scholar]
- 14. Reca IB, Lionetti V, Camardella L, D'Avino R, Giardina T, Cervone F, Bellincampi D. A functional pectin methylesterase inhibitor protein (SolyPMEI) is expressed during tomato fruit ripening and interacts with PME-1. Plant Mol Biol 2012; 79:429-42; PMID:22610346; http://dx.doi.org/ 10.1007/s11103-012-9921-2 [DOI] [PubMed] [Google Scholar]
- 15. Raiola A, Camardella L, Giovane A, Mattei B, De Lorenzo G, Cervone F, Bellincampi D. Two Arabidopsis thaliana genes encode functional pectin methylesterase inhibitors. FEBS Lett 2004; 557:199-203; PMID:14741367; http://dx.doi.org/ 10.1016/S0014-5793(03)01491-1 [DOI] [PubMed] [Google Scholar]
- 16. Lionetti V, Raiola A, Camardella L, Giovane A, Obel N, Pauly M, Favaron F, Cervone F, Bellincampi D. Overexpression of pectin methylesterase inhibitors in Arabidopsis restricts fungal infection by Botrytis cinerea. Plant Physiol 2007; 143:1871-80; PMID:17277091; http://dx.doi.org/ 10.1104/pp.106.090803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Volpi C, Janni M, Lionetti V, Bellincampi D, Favaron F, D'Ovidio R. The ectopic expression of a pectin methyl esterase inhibitor increases pectin methyl esterification and limits fungal diseases in wheat. Mol Plant-Microbe Interact 2011; 24:1012-9; PMID:21585271; http://dx.doi.org/ 10.1094/MPMI-01-11-0021 [DOI] [PubMed] [Google Scholar]
- 18. Raiola A, Lionetti V, Elmaghraby I, Immerzeel P, Mellerowicz EJ, Salvi G, Cervone F, Bellincampi D. Pectin methylesterase is induced in Arabidopsis upon infection and is necessary for a successful colonization by necrotrophic pathogens. Mol Plant-Microbe Interact 2011; 24:432-40; PMID:21171891; http://dx.doi.org/ 10.1094/MPMI-07-10-0157 [DOI] [PubMed] [Google Scholar]
- 19. Di Matteo A, Giovane A, Raiola A, Camardella L, Bonivento D, De Lorenzo G, Cervone F, Bellincampi D, Tsernoglou D. Structural basis for the Interaction between pectin methylesterase and a specific inhibitor protein. Plant Cell 2005; 17:849-858; PMID:15722470; http://dx.doi.org/ 10.1105/tpc.104.028886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lionetti V, Cervone F, Bellincampi D. Methyl esterification of pectin plays a role during plant-pathogen interactions and affects plant resistance to diseases. J Plant Physiol 2012; 169:1623-30; PMID:22717136; http://dx.doi.org/ 10.1016/j.jplph.2012.05.006 [DOI] [PubMed] [Google Scholar]
- 21. Yang CL, Guo R, Jie F, Nettleton D, Peng JQ, Carr T, Yeakley JM, Fan JB, Whitham SA. Spatial analysis of Arabidopsis thaliana gene expression in response to Turnip mosaic virus infection. Mol Plant-Microbe Interact 2007; 20:358-70; PMID:17427806; http://dx.doi.org/ 10.1094/MPMI-20-4-0358 [DOI] [PubMed] [Google Scholar]
- 22. Ascencio-Ibanez JT, Sozzani R, Lee TJ, Chu TM, Wolfinger RD, Cella R, Hanley-Bowdoin L. Global analysis of Arabidopsis gene expression uncovers a complex array of changes impacting pathogen response and cell cycle during geminivirus infection. Plant Physiol 2008; 148:436-54; PMID:18650403; http://dx.doi.org/ 10.1104/pp.108.121038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dorokhov YL, Komarova TV, Petrunia IV, Frolova OY, Pozdyshev DV, Gleba YY. Airborne signals from a wounded leaf facilitate viral spreading and induce antibacterial resistance in neighboring plants. Plos Pathogens 2012; 8: e1002640; PMID:22496658; http://dx.doi.org/ 10.1371/journal.ppat.1002640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fall R, Benson AA. Leaf methanol–the simplest natural product from plants. Trends Plant Sci 1996; 1:296-301; http://dx.doi.org/ 10.1016/S1360-1385(96)88175-0 [DOI] [Google Scholar]
- 25. Zavaliev R, Levy A, Gera A, Epel BL. Subcellular dynamics and role of Arabidopsis b-1,3-glucanases in cell-to-cell movement of tobamoviruses. Mol Plant-Microbe Interact 2013; 26: 1016-30; PMID:23656331; http://dx.doi.org/ 10.1094/MPMI-03-13-0062-R [DOI] [PubMed] [Google Scholar]
- 26. Kogovsek P, Pompe-Novak M, Baebler S, Rotter A, Gow L, Gruden K, Foster GD, Boonham N, Ravnikar M. Aggressive and mild Potato virus Y isolates trigger different specific responses in susceptible potato plants. Plant Pathol 2010; 59:1121-32; http://dx.doi.org/ 10.1111/j.1365-3059.2010.02340.x [DOI] [Google Scholar]
- 27. Yadav RK, Chattopadhyay D. Differential soybean gene expression during early phase of infection with Mungbean yellow mosaic India virus. Mol Biol Rep 2014; 41:5123-34; PMID:24752408; http://dx.doi.org/ 10.1007/s11033-014-3378-0 [DOI] [PubMed] [Google Scholar]
- 28. Gaffe J, Tieman DM, Handa AK. Pectin methylesterase isoforms in tomato (Lycopersicon esculentum) tissues. Effects of expression of a pectin methylesterase antisense gene. Plant Physiol 1994; 105:199-203; PMID:12232199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wen FS, Zhu YM, Hawes MC. Effect of pectin methylesterase gene expression on pea root development. Plant Cell 1999; 11:1129-40; PMID:10368183; http://dx.doi.org/ 10.1105/tpc.11.6.1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ren C, Kermode AR. An increase in pectin methyl esterase activity accompanies dormancy breakage and germination of yellow cedar seeds. Plant Physiol 2000; 124:231-42; PMID:10982438; http://dx.doi.org/ 10.1104/pp.124.1.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bellincampi D, Cervone F, Lionetti V. Plant cell wall dynamics and wall-related susceptibility in plant-pathogen interactions. Front Plant Sci 2014; 5:228; PMID:24904623; http://dx.doi.org/ 10.3389/fpls.2014.00228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pogorelko G, Lionetti V, Bellincampi D, Zabotina O. Cell wall integrity: targeted post-synthetic modifications to reveal its role in plant growth and defense against pathogens. Plant Signal Behav 2013; 8: e25435; PMID:23857352; http://dx.doi.org/ 10.4161/psb.25435 [DOI] [PMC free article] [PubMed] [Google Scholar]