Abstract
The circadian clock modulates plant responses to environmental stimuli. In a recent study we showed that light and the circadian clock regulate daily changes in sensitivity to short treatments of high UV-B. Here we demonstrate that these time dependent changes in UV-B stress sensitivity are not mediated by the UV-B receptor UV RESISTANTCE LOCUS 8. We also discuss the potential mechanisms involved in this process and the role of the circadian clock in the acclimation to UV-B.
Keywords: UV-B, circadian, UVR8, stress, adaptation, transcription
Abbreviations
- COP1
CONSTITUTIVELY PHOTOMORPHOGENIC 1
- ELF3
EARLY FLOWERING 3
- ELF4
EARLY FLOWERING 4
- HY5
ELONGATED HYPOCOTYL 5
- HYH
HY5 HOMOLOGUE
- LUX
LUX ARRHYTHMO
- PHR1
PHOTOLYASE 1
- UVH1
ULTRAVIOLET HYPERSENSITIVE 1
- UVR3
UV RESISTANCE LOCUS 3
- UVR8
UV RESISTANCE LOCUS 8
The circadian clock modulates environmental signals and stress responses in plants. For example, the clock temporally regulates cold, heat and light quality mediated changes in gene expression1–5 as well as sensitivity to many biotic and abiotic stresses.2,6-9 We have recently shown that the circadian clock modulates both UV-B signaling as well as UV-B stress sensitivity in Arabidopsis. This work has raised some questions on the mechanisms of circadian control of UV-B acclimation and acute UV-B stress responses.
Plants grown under natural conditions are able to adapt to current levels of UV-B radiation and do not display signs of UV-B damage.10 However, plants grown in the absence of UV-B are more sensitive to subsequent UV-B applications indicating acclimation occurs in nature.11,12 Natural variation in UV-B sensitivity exists among plants and has been associated with differences in irradiation, such as the ones found at different altitudes.12–14 There are different fluence rate dependent responses to UV-B in plants. Continuous exposure to low levels of UV-B leads to morphogenic changes and protective acclimation to UV-B.15 These processes are regulated by the UV-B photoreceptor UV RESISTANCE LOCUS 8 (UVR8).16 In contrast, high UV-B irradiance leads to damage to plant cells and to stress responses that are thought to be regulated in a UVR8 independent manner.15
UVR8 is able to sense very low UV-B fluence and regulate gene expression.17 It forms a dimer in the absence of UV-B and quickly monomerizes upon UV-B perception.16,18,19 This monomerization allows UVR8 to interact with COP1 (CONSTITUTIVELY PHOTOMORPHOGENIC 1) and mediate the regulation of transcription in a manner that is still poorly understood.19 Principal targets of UVR8-COP1 include the transcription factors ELONGATED HYPOCOTYL 5 (HY5) and HYH (HY5 HOMOLOGUE) which control the expression of a large number of downstream genes in a UV-B dependent manner.20-23
The circadian clock modulates UVR8-COP1 UV-B-dependent regulation of gene expression. This activity is reflected in rhythmic changes in UV-B mediated transcription under either constant light or constant dark conditions.4,9 Most of the UV-B regulated genes are also circadian regulated under unstressed conditions and include components of the circadian clock.4,9 The misexpression of clock components strongly influences the effect of UV-B on transcription.4,9 Moreover, we have recently shown that clock transcriptional repressors are able to directly inhibit UV-B mediated gene expression of several UV-B regulated genes indicating that the circadian oscillator can regulate UV-B responses in a gene-by-gene manner.9 However, we have also observed that the loss of clock components ELF3 (EARLY FLOWERING 3), LUX (LUX ARRHYTHMO) or ELF4 (EARLY FLOWERING 4) leads to constitutive induction of all the genes tested so far, including genes not directly targeted by this transcriptional repressor complex.9 This result suggests that the circadian clock could also be acting further upstream in the UV-B signaling pathway.
In spite of the gated response in UV-B signaling under constant light, so far no changes in the time dependent UV-B stress sensitivity have been observed under these conditions.4,9 However, we have shown that wild type Arabidopsis plants are more resistant to UV-B stress during the night than during the day and this difference is reduced in elf3 and elf4 mutants.9 Under T-cycles of 6 h light and 6 h dark, the absence of visible light only increases UV-B stress sensitivity during the subjective night but not during the subjective day in wild type plants indicating that the circadian clock, in addition to light, affects UV-B stress sensitivity.9 In accordance, UV-B treatments of elf3 mutants in the dark always leads to an increase in stress sensitivity when compared to the treatments in the light, independent of the time of treatment, which is likely due to the absence of circadian control.
These previously reported time dependent stress assays were performed by treating plants with high UV-B doses for short periods of time. We wanted to test whether the response under these conditions was dependent on the UV-B receptor UVR8. The loss of UVR8 leads to increased sensitivity to long treatments (≥1 day) at low UV-B fluence rates24,25 (Fig. 1A). We observed that the uvr8–1 mutants did not display a decrease in stress sensitivity to a 3 h treatment of high UV-B during the day when compared to the wild type (Fig. 1B). In addition, the single short UV-B pulses that are sufficient for UVR8-COP1 mediated regulation of gene expression4,9 did not affect changes in UV-B stress sensitivity (Fig. 1C). These results agree with UVR8 having a role of UVR8 in long-term acclimation to UV-B but not in short-term protection. These observations raise two questions about the interactions between UV-B and the circadian clock: What is the role of the circadian gated response to low UV-B in UV-B acclimation? And what causes the changes in sensitivity to short pulses of high UV-B radiation?
Figure 1.
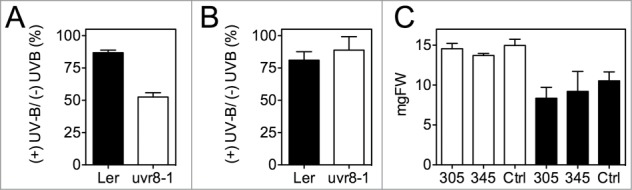
UVR8 is not involved in short-term responses to UV-B stress. (A) uvr8–1 plants were more sensitive to a long treatment of low UV-B radiation. Seedlings were grown under 12 h light/12 h dark for 7 d and transferred to constant light for another 5 d before treatment with 3 μmol m−2 s−1 of UV-B for 24 h in the presence of white light (70 μmol m−2 s−1). Seedlings were weighed 9 d after treatment. Values represent the averages and range of 2 independent experiments. (B) uvr8–1 seedlings displayed no differences in UV-B stress sensitivity under short UV-B treatments. Ten-day old seedlings grown under 12 h light/12 h dark conditions were treated with UV-B using a 305 nm long pass filter at ZT1 (3 μmol m−2 s−1). After 3 h, the seedlings were transferred to 7.7 μmol m−2 s−1 UV-B using a 305 nm longpass filter for 3 h in the presence of white light and before returning back to light/dark conditions. Seedlings were weighed 15 d after treatment. Values are the average and standard error of 4 independent experiments. For (A, B) values represent the ratio as a percentage of the weight between UV-B treated and control seedlings. Control seedlings were treated in the same manner but with a 345 nm long pass filter. (C) A short UV-B treatment did not affect the sensitivity to UV-B stress. Ten-day old wild type seedlings grown under 12 h light/12 h dark conditions were treated at ZT4 (white bars) or ZT16 (dark bars) with either UV-B under a 305 nm long pass filter (305) (3 μmol m−2 s−1), with UV-B but kept under a 345 nm long pass filter (345), or left untreated (Ctrl). After 3 h, the seedlings were transferred to 7.7 μmol m−2 s−1 UV-B using a 305 nm longpass filter for 3 h and before returning back to light/dark conditions. Seedlings were weighed 20 d after treatment. Values are the average and standard error of 3 independent experiments. Details about UV-B light conditions can be found in.9
Under natural conditions, plants are exposed to low UV-B fluence every morning and afternoon and to high fluence at midday. Together with the effect of the clock on UV-B signaling, it could be hypothesized that there might be times of day at which acclimation is more efficiently induced than at others. An analogous system is the acclimation of plants to cold temperatures. In Arabidopsis, the circadian clock is involved in the process of cold acclimation via the transcriptional regulation of key transcription factors.2,6,26 The misexpression of clock components leads to changes in sensitivity to freezing temperatures and some clock mutants are cold resistant even in the absence of acclimation.6 However, in both cold and UV-B acclimation processes it remains unknown whether the exposure to non-damaging treatments at certain times of day are more effective than others in protecting plants from subsequent stress.
There are several genes involved in the protection against short-term treatments of high UV-B. They include genes involved in DNA damage repair such as the photolyases (PHOTOLYASE 1/UV RESISTANCE 2) and UVR3, as well as UVH1 (ULTRAVIOLET HYPERSENSITIVE 1), which is likely to be involved in nucleotide excision repair.27–30 PHR1 and UVR3 RNA content oscillate under constant light conditions indicating that they are circadian regulated (Fig. 2). Furthermore, it has been shown that in Cucumis sativus extractable photorepair activity changes throughout the diurnal cycle with maximum activity occurring during the middle of the day.31 These changes in activity correlate with differences in stress sensitivity to high UV-B pulses in these plants.31 Therefore, it is possible that the circadian clock regulates changes in UV-B stress sensitivity by modulating DNA repair responses.
Figure 2.
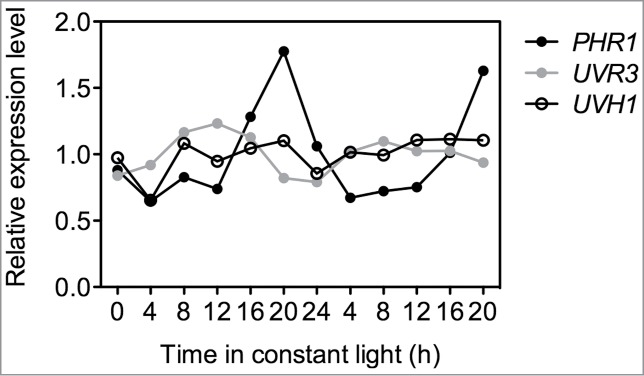
Expression level of DNA repair genes under constant light conditions. Data are from the Diurnal project.32 Expression values were normalized to the average expression across the experiment.
In summary, recent results have shown that the circadian clock in Arabidopsis regulates different aspects of UV-B signaling and stress responses. Further investigation is necessary to elucidate the mechanisms of circadian regulation and the clock's role in UV-B acclimation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Gareth Jenkins for the provision of the uvr8–1 lines and interesting discussions, and Tiffany Liu for critically reading the manuscript.
Funding
This work was supported by the National Science Foundation (IOS-1054243).
References
- 1. Thines B, Harmon FG. Ambient temperature response establishes ELF3 as a required component of the core Arabidopsis circadian clock. Proc Natl Acad Sci U S A 2010; 107:3257-62; PMID:20133619; http://dx.doi.org/ 10.1073/pnas.0911006107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dong MA, Farre EM, Thomashow MF. Circadian clock-associated 1 and late elongated hypocotyl regulate expression of the C-repeat binding factor (CBF) pathway in Arabidopsis. Proc Natl Acad Sci U S A 2011; 108:7241-6; PMID:21471455; http://dx.doi.org/ 10.1073/pnas.1103741108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McWatters HG, Bastow RM, Hall A, Millar AJ. The ELF3 zeitnehmer regulates light signalling to the circadian clock. Nature 2000; 408:716-20; PMID:11130072; http://dx.doi.org/ 10.1038/35047079 [DOI] [PubMed] [Google Scholar]
- 4. Feher B, Kozma-Bognar L, Kevei E, Hajdu A, Binkert M, Davis SJ, Schäfer E, Ulm R, Nagy F. Functional interaction of the circadian clock and UV RESISTANCE LOCUS 8-controlled UV-B signaling pathways in Arabidopsis thaliana. Plant J 2011; 67:37-48; PMID:21395889; http://dx.doi.org/ 10.1111/j.1365-313X.2011.04573.x [DOI] [PubMed] [Google Scholar]
- 5. Kolmos E, Chow BY, Pruneda-Paz JL, Kay SA. HsfB2b-mediated repression of PRR7 directs abiotic stress responses of the circadian clock. Proc Natl Acad Sci U S A 2014; 111:16172-7; PMID:25352668; http://dx.doi.org/ 10.1073/pnas.1418483111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nakamichi N, Kusano M, Fukushima A, Kita M, Ito S, Yamashino T, Saito K, Sakakibara H, Mizuno T. Transcript profiling of an Arabidopsis PSEUDO RESPONSE REGULATOR arrhythmic triple mutant reveals a role for the circadian clock in cold stress response. Plant Cell Physiol 2009; 50:447-62; PMID:19131357; http://dx.doi.org/ 10.1093/pcp/pcp004 [DOI] [PubMed] [Google Scholar]
- 7. Wang W, Barnaby JY, Tada Y, Li H, Tor M, Caldelari D, Lee DU, Fu XD, Dong X. Timing of plant immune responses by a central circadian regulator. Nature 2011; 470:110-4; PMID:21293378; http://dx.doi.org/ 10.1038/nature09766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lai AG, Doherty CJ, Mueller-Roeber B, Kay SA, Schippers JH, Dijkwel PP. CIRCADIAN CLOCK-ASSOCIATED 1 regulates ROS homeostasis and oxidative stress responses. Proc Natl Acad Sci U S A 2012; 109:17129-34; PMID:23027948; http://dx.doi.org/ 10.1073/pnas.1209148109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takeuchi T, Newton L, Burkhardt A, Mason S, Farre EM. Light and the circadian clock mediate time-specific changes in sensitivity to UV-B stress under light/dark cycles. J Exp Bot 2014; 65:6003-12; PMID:25147271; http://dx.doi.org/ 10.1093/jxb/eru339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ballare CL, Caldwell MM, Flint SD, Robinson SA, Bornman JF. Effects of solar ultraviolet radiation on terrestrial ecosystems. Patterns, mechanisms, and interactions with climate change. Photochem Photobiol Sci 2011; 10:226-41; PMID:21253661; http://dx.doi.org/ 10.1039/c0pp90035d [DOI] [PubMed] [Google Scholar]
- 11. Casati P, Walbot V. Gene expression profiling in response to ultraviolet radiation in maize genotypes with varying flavonoid content. Plant Physiol 2003; 132:1739-54; PMID:12913132; http://dx.doi.org/ 10.1104/pp.103.022871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jansen MA, Martret BL, Koornneef M. Variations in constitutive and inducible UV-B tolerance; dissecting photosystem II protection in Arabidopsis thaliana accessions. Physiol Plant 2010; 138:22-34; PMID:19843242; http://dx.doi.org/ 10.1111/j.1399-3054.2009.01293.x [DOI] [PubMed] [Google Scholar]
- 13. Yao Y, Xuan Z, He Y, Lutts S, Korpelainen H, Li C. Principal component analysis of intraspecific responses of tartary buckwheat to UV-B radiation under field conditions. Environ Exp Bot 2007; 61:237-45; http://dx.doi.org/ 10.1016/j.envexpbot.2007.06.003 [DOI] [Google Scholar]
- 14. Sullivan JH, Teramura AH, Ziska LH. Variation in UV-B Sensitivity in Plants from a 3,000-m Elevational Gradient in Hawaii. Am J Bot 1992; 79:737-43; http://dx.doi.org/ 10.2307/2444938 [DOI] [Google Scholar]
- 15. Jenkins GI. Signal transduction in responses to UV-B radiation. Annu Rev Plant Biol 2009; 60:407-31; PMID:19400728; http://dx.doi.org/ 10.1146/annurev.arplant.59.032607.092953 [DOI] [PubMed] [Google Scholar]
- 16. Jenkins GI. The UV-B photoreceptor UVR8: from structure to physiology. Plant Cell 2014; 26:21-37; PMID:24481075; http://dx.doi.org/ 10.1105/tpc.113.119446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brown BA, Jenkins GI. UV-B signaling pathways with different fluence-rate response profiles are distinguished in mature Arabidopsis leaf tissue by requirement for UVR8, HY5, and HYH. Plant Physiol 2008; 146:576-88; PMID:18055587; http://dx.doi.org/ 10.1104/pp.107.108456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rizzini L, Favory JJ, Cloix C, Faggionato D, O'Hara A, Kaiserli E, Baumeister R, Schäfer E, Nagy F, Jenkins GI, et al. Perception of UV-B by the Arabidopsis UVR8 protein. Science 2011; 332:103-6; PMID:21454788; http://dx.doi.org/ 10.1126/science.1200660 [DOI] [PubMed] [Google Scholar]
- 19. Jenkins GI. Structure and function of the UV-B photoreceptor UVR8. Curr Opin Struct Biol 2014; 29:52-7; PMID:25300065; http://dx.doi.org/ 10.1016/j.sbi.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 20. Brown BA, Cloix C, Jiang GH, Kaiserli E, Herzyk P, Kliebenstein DJ, Jenkins GI. A UV-B-specific signaling component orchestrates plant UV protection. Proc Natl Acad Sci U S A 2005; 102:18225-30; PMID:16330762; http://dx.doi.org/ 10.1073/pnas.0507187102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ulm R, Baumann A, Oravecz A, Mate Z, Adam E, Oakeley EJ, Schäfer E, Nagy F. Genome-wide analysis of gene expression reveals function of the bZIP transcription factor HY5 in the UV-B response of Arabidopsis. Proc Natl Acad Sci U S A 2004; 101:1397-402; PMID:14739338; http://dx.doi.org/ 10.1073/pnas.0308044100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oravecz A, Baumann A, Mate Z, Brzezinska A, Molinier J, Oakeley EJ, Adám E, Schäfer E, Nagy F, Ulm R. CONSTITUTIVELY PHOTOMORPHOGENIC1 is required for the UV-B response in Arabidopsis. Plant Cell 2006; 18:1975-90; PMID:16829591; http://dx.doi.org/ 10.1105/tpc.105.040097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Binkert M, Kozma-Bognar L, Terecskei K, De Veylder L, Nagy F, Ulm R. UV-B-Responsive Association of the Arabidopsis bZIP Transcription Factor ELONGATED HYPOCOTYL5 with Target Genes, Including Its Own Promoter. Plant Cell 2014; 26:4200-13; PMID:25351492; http://dx.doi.org/ 10.1105/tpc.114.130716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kliebenstein DJ, Lim JE, Landry LG, Last RL. Arabidopsis UVR8 regulates ultraviolet-B signal transduction and tolerance and contains sequence similarity to human regulator of chromatin condensation 1. Plant Physiol 2002; 130:234-43; PMID:12226503; http://dx.doi.org/ 10.1104/pp.005041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Favory JJ, Stec A, Gruber H, Rizzini L, Oravecz A, Funk M, Albert A, Cloix C, Jenkins GI, Oakeley EJ, et al. Interaction of COP1 and UVR8 regulates UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. EMBO J 2009; 28:591-601; PMID:19165148; http://dx.doi.org/ 10.1038/emboj.2009.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu T, Carlsson J, Takeuchi T, Newton L, Farre EM. Direct regulation of abiotic responses by the Arabidopsis circadian clock component PRR7. Plant J 2013; 76(1):101-14; Epub; PMID:23808423 [DOI] [PubMed] [Google Scholar]
- 27. Landry LG, Stapleton AE, Lim J, Hoffman P, Hays JB, Walbot V, Last RL. An Arabidopsis photolyase mutant is hypersensitive to ultraviolet-B radiation. Proc Natl Acad Sci U S A 1997; 94:328-32; PMID:8990208; http://dx.doi.org/ 10.1073/pnas.94.1.328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nakajima S, Sugiyama M, Iwai S, Hitomi K, Otoshi E, Kim ST, Jiang CZ, Todo T, Britt AB, Yamamoto K. Cloning and characterization of a gene (UVR3) required for photorepair of 6-4 photoproducts in Arabidopsis thaliana. Nucleic Acids Res 1998; 26:638-44; PMID:9421527; http://dx.doi.org/ 10.1093/nar/26.2.638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jiang CZ, Yee J, Mitchell DL, Britt AB. Photorepair mutants of Arabidopsis. Proc Natl Acad Sci U S A 1997; 94:7441-5; PMID:9750104; http://dx.doi.org/ 10.1073/pnas.94.14.7441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fidantsef AL, Mitchell DL, Britt AB. The Arabidopsis UVH1 gene is a homolog of the yeast repair endonuclease RAD1. Plant Physiol 2000; 124:579-86; PMID:11027708; http://dx.doi.org/ 10.1104/pp.124.2.579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takahashi S, Nakajima N, Saji H, Kondo N. Diurnal change of cucumber CPD photolyase gene (CsPHR) expression and its physiological role in growth under UV-B irradiation. Plant Cell Physiol 2002; 43:342-9; PMID:11917089; http://dx.doi.org/ 10.1093/pcp/pcf038 [DOI] [PubMed] [Google Scholar]
- 32. Mockler TC, Michael TP, Priest HD, Shen R, Sullivan CM, Givan SA, McEntee C, Kay SA, Chory J. The DIURNAL project: DIURNAL and circadian expression profiling, model-based pattern matching, and promoter analysis. Cold Spring Harb Symp Quant Biol 2007; 72:353-63; PMID:18419293; http://dx.doi.org/ 10.1101/sqb.2007.72.006 [DOI] [PubMed] [Google Scholar]


