Abstract
Gibberellin (GA) plays important roles through plant growth and development. However, where GA is synthesized inside a cell and how it regulates sex determination is obscure. We analyzed the classic dwarf1 (d1) mutant in maize and revealed that D1 encodes GA 3-oxidase converting inactive GA intermediates to bioactive GA. As such, the D1 protein marks the sites where GA is potentially synthesized. Interestingly, the D1 protein was found to localize in the cytosol and nucleus, a dual-localization coinciding with the GA receptor. The same result was found for GA 20-oxidase catalyzing the upstream reaction. These results suggest that GA can be synthesized in the cytosol and nucleus. The D1 protein was highly and specifically expressed in the stamen primordia in the ear florets, but low in the whole tassel. Hence it is possible that low level of GA in the tassel is insufficient to suppress stamen development. As jasmonic acid (JA) plays antagonistic role to GA in the tassel florets, here we propose a model to explain this antagonism effect on the regulation of the stamen and pistil organ development in the tassel florets in maize.
Keywords: gibberellin, GA 3-oxidase, maize, nucleus and cytosol, sex determination
Introduction
Gibberellin (GA) plays many important roles in plant growth and development such as promoting seed germination and stem elongation, regulating flower development. GA biosynthesis is believed to occur in 3 compartments within the cell.1 The first phase from geranylgeranyl diphosphate (GGDP) to ent-kaurene occurs in plastids; the second phase from ent-kaurene to GA12 in the plastid envelope and endoplasmic reticulum (ER); and the final phase from GA12 to bioactive GAs in the cytosol (Fig. 1).1 In contrary to this widely accepted conception, our recent study indicated that bioactive GA formation may be carried out in 2 compartments, the cytosol and the nucleus. Interestingly, this dual-localization coincides with the localization of GA receptor GID1.2,3
Figure 1.
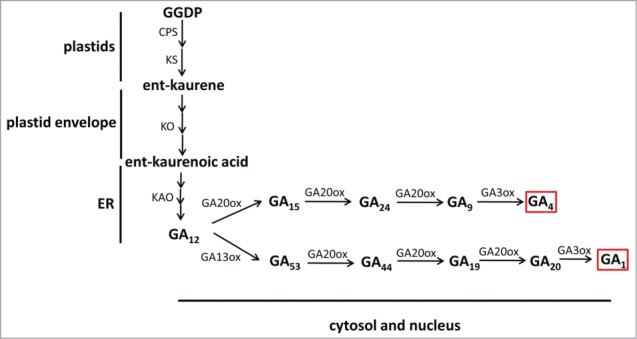
The GA biosynthetic pathway. Red rectangle indicates bioactive forms.
Maize carries unisexual flowers, male flowers (tassel) on top of the plant and female flowers (ear) in the axial of the leaf. In fact, maize develops bisexual flowers in both the tassel and the ear at early developmental stages.4 The unisexuality is achieved by selective suppression of pistil primordia in the tassel and the stamen primordia in the ear.4 The suppression of stamen primordia in the ear is attributed to GA, whereas the sexuality in the tassel florets is attributed to the antagonism between GA and jasmonic acid (JA). As the unisexual system is derived from bisexual system, unraveling the molecular mechanism of sex determination is important for understanding the evolution of flowers and plants.
The maize DWARF1 encodes a GA 3-oxidase
The maize dwarf1 (d1) mutant is a classic mutant known for decades.5,6 Genetic and biochemical analyses indicate that GA 3-oxidase (GA3ox) activity is absent in the d1 mutant,7 but the molecular basis for this mutation has not been revealed. Based on existing evidence, 2 possibilities were put forward: 1) D1 encoded a GA3ox that was directly involved in the GA biosynthesis; 2) D1 encoded a regulatory protein that was required for GA3ox expression or enzymatic function. Previous study mapped d1 on chromosome 3S;8 which was in the proximity of a putative GA3ox gene, hence strengthening the first possibility. By analyzing 4 d1 alleles, we revealed that each of the alleles contained mutation in ZmGA3ox2 gene. The mutation in d1–6016 allele was also independently found in GA3ox2 recently.9 Thus, these results confirmed that ZmGA3ox2 is the causative gene for d1 phenotype. Enzymatic analysis of D1 protein indicated that it can catalyze at least 4 reactions leading to bioactive GA formation: GA9 to GA4, GA20 to GA1, GA20 to GA3, and GA5 to GA3. These results confirm that D1 encodes a GA 3-oxidase converting GA intermediates to bioactive GAs. The maize genome contains another putative GA 3-oxidase ZmGA3ox1. However, expression of this gene was not detected, indicating that ZmGA3ox2 (D1) provides the predominant GA 3-oxidase activity in maize. This conclusion is also supported by the severe GA-deficient phenotype of d1.
GA can be synthesized in the nucleus and cytosol
The functions of GA20ox and GA3ox are required for the conversion from GA12 to bioactive GAs.1 Because of the lacking of apparent targeting sequences, these 2 proteins were believed to be localized in cytosol.1 However, by using 2 independent approaches, i.e. the GFP fusion and Western blot analysis on isolated organelles, we uncovered that both D1 and ZmGA20ox1 are dual-localized in the cytosol and the nucleus. This dual-localization is consistent with the dual-localization of GA receptor GID1.2,3 Because the D1 localization determines the potential sites of bioactive GA production, these results suggest that bioactive GA may be synthesized in both the cytosol and nucleus, although further experiments are required to fully confirm this conclusion. Nonetheless, our finding provides new insight to the understanding of the GA biosynthesis and perception in plants. However, several important questions invite attention:
One) Why do these enzymes co-exist in 2 compartments?
Two) How do cells regulate the expression of the enzymes in 2 compartments?
Three) Will GA be transported between cytosol and nucleus?
Four) Are there new components involved in the GA perception in these 2 compartments?
GA is antagonistic to JA in regulating stamen development in the tassel
We found that the D1 protein was highly expressed in the stamen cells of ear florets, but undetectable in the tassel with the same antibody. Although the mechanism is unknown, this result together with the genetic evidence invites the notion that high level of GA in specific cells suppresses cell growth and differentiation; whereas low level of GA promotes cell growth and differentiation. This notion is supported by the evidences: 1) the level of GA in the tassel is much lower than in the ear;10 2) application of GA converts male florets to female florets in the tassel.11 Thus, we postulate that high level of bioactive GA suppresses the stamen development in tassel florets. Interestingly, JA acts antagonistically to GA in regulating stamen cell development.4 This antagonism may be indirect or direct though affecting the synthesis and/or the signaling.
In Arabidopsis, GA and JA crosstalk through direct interaction between the core signaling repressors DELLA (GA) and JAZs (JA).12 Inspired by this finding, we proposed a model to explain the crosstalk of GA and JA in regulating stamen and pistil development in tassel florets. Protein A is a DELLA interacting protein and suppresses stamen development; protein B is a JAZs interacting protein and suppresses pistil development (Fig. 2). GA induces DELLA degradation to release protein A's function; while JA induces the degradation of JAZs to release protein B's function. When GA level is low, abundant DELLA will competitively bind to JAZs so that some protein Bs are released from JAZs to suppress pistil development. When JA is lacking, abundant JAZs will competitively bind to DELLA resulting in some free protein As to suppress stamen development. The identification of protein A and protein B will be a breakthrough for unraveling the regulation mechanism of maize sex determination in the future.
Figure 2.

The model of GA and JA crosstalk through direct interaction in regulating tassel floret development. (A) When GA level is low (e.g. WT and d1 tassel florets), abundant DELLA proteins restrict protein A to suppress stamen development and interact with JAZs to release some protein Bs to suppress pistil development. (B) When GA level is high (e.g., exogenous GA in WT tassel florets), DELLA proteins are degraded and protein As are released to suppress stamen development; while JAZs restrict protein B to suppress pistil development. (C) When JA level is low (e.g. ts1 or ts2 mutant), abundant JAZs restrict protein B to suppress pistil development and interact with DELLA to release some protein As from DELLA to suppress stamen development. (D) When both GA and JA levels are low (e.g., ts2 d1 double mutant), DELLA and JAZs restrict protein A and protein B, respectively. The development of stamen and pistil are not suppressed.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Yamaguchi S. Gibberellin metabolism and its regulation. Annu Rev Plant Biol 2008; 59:225-51; PMID:18173378; http://dx.doi.org/ 10.1146/annurev.arplant.59.032607.092804 [DOI] [PubMed] [Google Scholar]
- 2. Ueguchi-Tanaka M, Ashikari M, Nakajima M, Itoh H, Katoh E, Kobayashi M, Chow TY, Hsing YI, Kitano H, Yamaguchi I, et al. GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature 2005; 437:693-8; PMID:16193045; http://dx.doi.org/ 10.1038/nature04028 [DOI] [PubMed] [Google Scholar]
- 3. Willige BC, Ghosh S, Nill C, Zourelidou M, Dohmann EM, Maier A, Schwechheimer C. The DELLA domain of GA INSENSITIVE mediates the interaction with the GA INSENSITIVE DWARF1A gibberellin receptor of Arabidopsis. Plant Cell 2007; 19:1209-20; PMID:17416730; http://dx.doi.org/ 10.1105/tpc.107.051441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dellaporta SL, Calderon-Urrea A. The sex determination process in maize. Science 1994; 266:1501-5; PMID:7985019; http://dx.doi.org/ 10.1126/science.7985019 [DOI] [PubMed] [Google Scholar]
- 5. Fujioka S, Yamane H, Spray CR, Gaskin P, MacMillan J, Phinney BO, Takahashi N. Qualitative and Quantitative Analyses of Gibberellins in Vegetative Shoots of Normal, dwarf-1, dwarf-2, dwarf-3, and dwarf-5 Seedlings of Zea mays L. Plant Physiol 1988; 88:1367-72; PMID:16666468; http://dx.doi.org/ 10.1104/pp.88.4.1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Phinney BO. GROWTH RESPONSE OF SINGLE-GENE DWARF MUTANTS IN MAIZE TO GIBBERELLIC ACID. Proc Natl Acad Sci USA 1956; 42:185-189; PMID:16589846; http://dx.doi.org/ 10.1073/pnas.42.4.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spray CR, Kobayashi M, Suzuki Y, Phinney BO, Gaskin P, MacMillan J. The dwarf-1 (dt) Mutant of Zea mays blocks three steps in the gibberellin-biosynthetic pathway. Proc Natl Acad Sci USA 1996; 93:10515-8; PMID:11607708; http://dx.doi.org/ 10.1073/pnas.93.19.10515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Neuffer MG, England D. Induced mutations with confirmed locations. Maize Genet Coop Newsl 1995. 69:43-6 [Google Scholar]
- 9. Teng F, Zhai L, Liu R, Bai W, Wang L, Huo D, Tao Y, Zheng Y, Zhang Z. ZmGA3ox2, a candidate gene for a major QTL, qPH3.1, for plant height in maize. Plant J 2013; 73:405-16; PMID:23020630; http://dx.doi.org/ 10.1111/tpj.12038 [DOI] [PubMed] [Google Scholar]
- 10. Rood SB, Pharis RP. Changes of endogenous gibberellin-like substances with sex reversal of the apical inflorescence of corn. Plant Physiol 1980; 66:793-6; PMID:16661527; http://dx.doi.org/ 10.1104/pp.66.5.793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nickerson NH. Sustained treatment with gibberellic acid of five different kinds of maize. Ann Mo Bot Gard 1959; 46:19-37.6; http://dx.doi.org/ 10.2307/2394566 [DOI] [Google Scholar]
- 12. Hou X, Lee LY, Xia K, Yan Y, Yu H. DELLAs modulate jasmonate signaling via competitive binding to JAZs. Dev Cell 2010; 19:884-94; PMID:21145503; http://dx.doi.org/ 10.1016/j.devcel.2010.10.024 [DOI] [PubMed] [Google Scholar]


