Abstract
The potential for immunogenicity is an ever-present concern during the development of biopharmaceuticals. Therapeutic antibodies occasionally elicit an antibody response in patients, which can result in loss of response or adverse effects. However, antibodies that bind a drug are sometimes found in pre-treatment serum samples, with the amount depending on drug, assay, and patient population. This review summarizes published data on pre-existing antibodies to therapeutic antibodies, including rheumatoid factors, anti-allotype antibodies, anti-hinge antibodies, and anti-glycan antibodies. Unlike anti-idiotype antibodies elicited by the drug, pre-formed antibodies in general appear to have little consequences during treatment. In the few cases where (potential) clinical consequences were encountered, antibodies were characterized and found to bind a distinct, unusual epitope of the therapeutic. Immunogenicity testing strategies should therefore always include a proper level of antibody characterization, especially when pre-formed antibodies are present. This minimizes false-positives, particularly due to rheumatoid factors, and helps to judge the potential threat in case a genuine pre-dose antibody reactivity is identified.
Keywords: pre-existing antibodies, anti-drug antibodies (ADA), glycan, idiotype, rheumatoid factor, allotype, immunogenicity
Abbreviations
- ADA
anti-drug antibody
- CDR
complementarity-determining region
- TNF
tumor necrosis factor
- VH
variable heavy
- VL
variable light
- RF
rheumatoid factor
- RA
rheumatoid arthritis
Introduction
The accurate prediction and assessment of (clinically relevant) immunogenicity remains a challenging endeavor. One issue that has gained considerable attention in recent years is the occasionally reported presence of pre-existing antibodies, which can bind to a drug already in treatment-naive individuals. These antibodies have been suggested to potentially induce adverse effects or diminish treatment efficacy, and are even believed by some to contribute to or be predictive of future loss of response due to immunogenicity. Therapeutic monoclonal antibodies in particular may be the target of such pre-existing antibodies, as will be elaborated in this review.
The first monoclonal antibody to be approved for therapeutic use, OKT3, was a murine antibody that was found to be highly immunogenic.1 This led to the development of methods to include human sequence in antibody therapeutics, and thus potentially reduce immunogenicity. Chimeric antibodies were developed by replacing the murine constant domains by human constant domains. Further humanization was accomplished by combining the murine complementarity-determining regions (CDRs) with a human variable domain framework region (Fig. 1), whereas more recent improvements led to the creation of so-called fully human antibodies. Although humanization greatly reduced the immunogenicity of therapeutic antibodies,2 even fully human antibodies are reported to elicit an immune response in some of the treated patients.3,4 Reported incidences of immunogenicity rates vary widely for a given drug (see e.g. ref. 5 for an overview of antibody formation to tumor necrosis factor (TNF) blockers). These large variations strongly reflect, among others, differences in the assays used to measure these anti-drug antibodies.4 However, factors such as dosage and the use of immunosuppressive co-medication such as methotrexate can also influence the immunogenic potential of a drug.6,7
Figure 1.
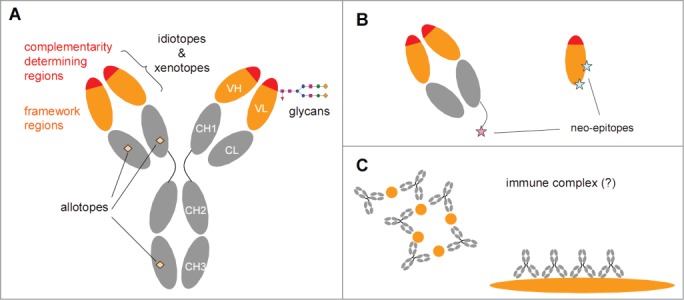
Potentially immunogenic determinants of antibodies. (A) An IgG antibody consists of variable domains (orange/red) and constant domains (gray). Chimeric, humanized and human therapeutic antibodies all contain human constant domains that may carry allotypic determinants. The variable domains can be subdivided in complementarity determining regions (CDRs) that are primarily involved in antigen binding and are highly variable, and framework regions (FRs) that are much less variable. The variation is partially germ-line encoded, partially the result of junctional variation and partially the result of somatic hypermutation. In chimeric antibodies the framework regions are of murine origin, whereas in humanized and human antibodies the framework regions will be largely human. Both CDRs and FRs can contain clone-specific determinants (idiotopes). Furthermore, in case of chimeric antibodies, the FRs may also contain murine-specific determinants (xenotopes). The variable domains may also express N-glycosylation sites that can contain non-human glycans depending on the expression system used to produce the therapeutic antibody. (B) Antibody fragments such as Fab (left) or single VH domains (right) will have regions exposed that are not exposed in an intact IgG antibody that can serve as neo-epitopes. In particular, anti-hinge antibodies can bind to a truncated hinge but will not bind intact IgG. (C) The antigenic trigger for rheumatoid factors is not known, but they might form in response to IgG-containing immune complexes.
Whether the antibody is human, humanized, or chimeric, determinants that are foreign to some or all individuals will still exist. In particular, the CDRs of any antibody clone (Fig. 1) contain unique stretches of amino acids, meaning that a certain degree of ‘foreign-ness’ will always be present in therapeutic antibodies.3,8,9 However, clone-specific determinants (idiotopes) are not necessarily the only parts of a therapeutic antibody that can be foreign to an individual. For instance, the variable regions of chimeric antibodies (as well as some of the early examples of humanized therapeutic antibodies) may also contain xenotopes, which are non-human determinants of murine origin that are germ-line encoded (Fig. 1). Xenotopes will be shared by multiple murine antibodies depending on their variable heavy (VH) and variable light (VL) gene usage. Other determinants that may be foreign to an individual include: i) polymorphic variation in the human constant domains, known as allotypes; ii) glycans attached to N-glycosylation sites in the variable domains; and iii) determinants that become exposed upon fragmentation of an IgG antibody (Fig. 1). These determinants will not be unique characteristics of a particular therapeutic antibody, but are potential targets of an antibody response.
Furthermore, these potential epitopes can also be present on (exogenous) IgG molecules other than the therapeutic antibody, or even on entirely different proteins in the case of glycans. Antibodies that bind to these epitopes may therefore also have formed on previous exposure to other materials. In addition, in a number of autoimmune diseases, patients are prone to produce rheumatoid factors, which are auto-antibodies specific for IgG and may therefore also cross-react with therapeutic antibodies.
Antibodies to unique, clone-specific determinants as well as to the various shared determinants described above have been demonstrated on numerous occasions. In the following sections, antibody reactivities to therapeutic antibodies will be discussed, focusing particularly on the existence, induction, and relevance of the different types of cross-reactive or pre-existing antibodies, and contrasting them with anti-idiotype antibodies. Furthermore, we will discuss their significance with respect to immunogenicity testing. Additionally, we will discuss how various types of non-specific associations between immunoglobulin molecules may render false-positive results in immunoassays that can be mistaken for pre-existing, as well as drug-induced, antibodies. Other important factors concerning immunogenicity assays, including drug interference, and the relationship between antibodies, drug levels, and clinical response, have been discussed elsewhere4,10-13 and will not be considered here.
Anti-Idiotype Antibodies
Antibodies that recognize a specific monoclonal antibody without cross-reacting to others are termed anti-idiotype antibodies. Anti-idiotype antibodies were originally described following immunizations of animals with myeloma antibody that led to the formation of specific antibodies that only recognized one particular myeloma antibody, but not others.14 This specific antigenic profile is named the idiotype, and may consist of one or more idiotopes, i.e., unique, immunogenic determinants on an antibody. It should be noted that the idiotype can be defined in various ways.15-18 Here, we adopt a practical rather than rigorous interpretation; see also the section ‘Anti-allotype antibodies’. A substantial part of the idiotype is made up by CDRs, which are the hypervariable loops of antibodies that determine its specificity and affinity. The actual antigen binding site of an antibody (the paratope) may involve the entire idiotype, but there may also exist idiotopes that are not part of the antigen binding site.
Antibodies that recognize idiotopes are expected to interfere with target binding (i.e., neutralize) to the extent that the idiotopes are part of the actual antigen binding site, or are in close proximity to the antigen binding site. Such antibodies can lead to loss of efficacy and ultimately treatment failure. Many studies on the immunogenicity of therapeutic antibodies indeed show an inverse correlation between anti-drug antibody levels that are developed in response to treatment, and the functional drug levels and clinical response, e.g., for natalizumab19 (anti-α4-integrin), infliximab20-22 and adalimumab,23,24 (both anti-TNF). This suggests that at least part of these anti-drug antibodies are neutralizing antibodies, and thus are likely anti-idiotype antibodies (see Textbox 1).
The specificities of the anti-drug antibodies have been characterized for different anti-TNF therapeutics.25-27 Notably, for both monoclonal and polyclonal anti-adalimumab antibodies, no cross-reactivity was observed to infliximab.26,27 A study on the actual binding sites of anti-adalimumab antibodies revealed that the antibody response against adalimumab is highly restricted. It was shown that more than 94% of the binding of anti-adalimumab antibodies could be blocked by the Fab fragment of a single monoclonal anti-adalimumab antibody.27 Furthermore, more than 97% of the anti-idiotype antibody response to adalimumab, golimumab, and certolizumab, all 3 of which are either human or humanized anti-TNF antibodies, could be inhibited by TNF.25 All-in-all, the vast majority of antibodies formed to these TNF blockers appear to be neutralizing, anti-idiotype antibodies. Strikingly, >90% of the antibody response to the chimeric infliximab could be inhibited by TNF.25 Several cases were identified with a small but definite fraction of non-neutralizing antibodies (i.e., <10%) that were not cross-reactive to polyclonal mouse IgG, suggesting that infliximab-specific idiotopes outside of the antigen binding site were recognized.
Anti-idiotype antibodies will, as a rule, not be detected in an individual that was never exposed to the therapeutic antibody. Thus, if any pre-existing antibodies are detected, these will often be anything but anti-idiotype antibodies. Therefore, in line with expectation, if pre-existing antibodies are detected, they are mostly classified as non-neutralizing, if further characterization was made.28-31
A note on neutralizing antibodies
Anti-drug antibodies can neutralize in different ways. Antibodies that interfere with target binding will in principle always be neutralizing, regardless of the mode of action of the therapeutic. For a therapeutic antibody that should merely block its target, this will be the only type of neutralizing antibody. Whether or not the drug will actually be (fully) neutralized ultimately depends on the amounts of both drug and anti-drug antibody, as well as the amounts of target molecules, and the relative affinities of these components. Nevertheless, non-neutralizing antibodies in this case may still contribute to enhanced clearance and thereby reduced efficacy. In case a therapeutic antibody needs to induce cell receptor cross-linking, or engage in Fc-mediated effector functions such as complement-dependent cytotoxicity (CDC) or antibody-dependent cell-mediated cytotoxicity (ADCC), antibodies that bind to the drug but do not interfere with target binding may still interfere with the functionality and neutralize the effects the drug is supposed to exert. In particular, antibodies that would block C1q or Fc receptor binding could result in diminished CDC, or ADCC, respectively.
Cross-Reactive and Pre-Existing Antibodies
Determinants that are not unique to the therapeutic antibody but potentially foreign to the patient, e.g., in case of allotypic mismatch, may elicit an antibody response. The available literature does not suggest that such responses to a therapeutic antibody are frequent, but this could be due to lack of assays set up to specifically address these types of antibodies, which may be of low affinity and of the IgM class (see below). However, since these reactivities are not specific to the therapeutic antibody, it is expected that they are occasionally encountered in individuals that have not been exposed to the therapeutic antibody. Different types of these pre-existing antibodies have been observed, and will be described below.
Rheumatoid factors
Rheumatoid factors (RF) are antibodies that bind the Fc part of human immunoglobulin G, and may also bind to IgG of several other species, including rabbit. Different epitopes on the IgG Fc may be recognized,32 and RFs are generally of low affinity.33-35 In healthy individuals RF has a prevalence of less than 5%, but in RA patients this increases to 70–90%.33 RF is usually of the IgM class, but can also be IgG or IgA. Due to its low affinity, IgM RF prefers binding to polymeric IgG (immune complexes) rather than IgG monomers because of the higher avidity upon multimeric interactions. By binding the Fc part of IgG, RF may form large antibody complexes that are able to activate the complement system and induce inflammation. The presence of RF therefore indicates a more destructive disease course.33 The exact mechanism by which these autoantibodies are formed remains elusive; immune complexes containing IgG have been implicated to serve as the antigen.
RFs will already be present before start of therapy in a large portion of RA patients and to various degrees in other autoimmune disorders, and therefore RF classifies as a pre-existing antibody. However, although clearance of IgG-containing immune complexes may be hampered or enhanced by the presence of RF,36,37 there are no indications that clearance of therapeutic antibodies is in general significantly affected by the presence of RF. This may be explained by the poor binding of RF to monomeric IgG. There are also no indications that individuals positive for RF have a higher chance of developing specific anti-drug antibodies.23,38 Furthermore, a number of studies report that RF levels actually declined in RA patients successfully treated with adalimumab or infliximab,39-41 although there are also a few case reports about raised RF titers after treatment with infliximab or tocilizumab (anti-interleukin-6 receptor).42,43
On the other hand, in an in vitro study the complement-dependent cytotoxicity of rituximab, an anti-CD20 antibody that depletes B cells, was found to be decreased in the presence of RF.44 This demonstrates that RF might in principle affect the cytolytic activity of a therapeutic antibody that binds a cellular target, which would lead to diminished efficacy in case of rituximab. However, the presence of RF actually predicts a better clinical response in RA patients treated with rituximab. The same holds true for tocilizumab and TNF blockers; evaluation of efficacy in RF+ and RF- patient groups might therefore be a useful part of a clinical trial, if applicable.45,46 Furthermore, although we are not aware of studies that explicitly evaluate or report RF-related safety issues of biologicals, therapeutic antibodies to both soluble and cell-bound targets are generally well-tolerated by RA patients.47,48 Therefore, RFs probably also do not significantly contribute to the occurrence of adverse effects. Taken together, with respect to the assessment of immunogenicity, it will normally not be of interest to detect RF activity to a therapeutic antibody.
In fact, RF can be a major confounding factor when assessing immunogenicity, severely limiting the sensitivity or specificity of anti-drug antibody assays.49-51 Direct ELISAs, in which serum is incubated on plates coated with the therapeutic antibody and bound antibodies revealed with, for example, an anti-lambda antibody, are especially prone to RF interference. Other assay formats, including the often-used bridging ELISA, which uses the therapeutic antibody both for capture and reveal, can also render positive signals from RF+ serum samples. A testimony to this is a recent overview of pre-dose positivity rates for a number of therapeutic antibodies and antibody-based constructs in healthy subjects as well as several disease populations, including RA.38 Whereas pre-dose positivity was 0.6% in healthy subjects, this was almost 15% in RA patients. Although detailed methodologies were not disclosed, it is likely that this elevated pre-dose positivity rate in RA patients is largely if not exclusively due to detection of RF, and may also explain the apparent bias of detecting pre-existing antibodies in autoimmune diseases.
Several approaches have been described to circumvent the detection of RF in immunogenicity assays.50-54 For instance, the use of F(ab')2 fragments of the therapeutic antibody instead of the intact IgG molecules for detection of ADA can effectively eliminate the detection of RF.50,53,54 However, one should take care not to detect anti-hinge antibodies when using this approach (see below).50 Similarly, pre-dose samples that tested positive for antibodies to tocilizumab were re-tested for antibodies to both Fab and Fc fragments to distinguish specific antibodies from RF.52,55 Furthermore, traces of aggregates in the drug conjugates used for detection were found to be a major contributor to signals from RF, and removal by size-exclusion chromatography was found to substantially reduce these signals.51 RF is sometimes removed from serum samples using IgG-coated latex beads.49 Alternatively, addition of polyclonal human IgG or rabbit anti-human IgM56 in the sample buffer may be used to block RF, although high concentrations of blocking reagent will be required.
Anti-allotype antibodies
Polymorphisms in the constant domains of the different IgG subclasses (as well as IgA) have resulted in slight differences in amino acid composition, known as allotypes.57,58 Taking IgG1 as an example, genetic variation includes the amino acid at position 214 in the CH1 domain, which can be a lysine or arginine, designated G1m17 and G1m3, respectively. Due to allotypic mismatch, antibodies with a certain allotype can in principle be immunogenic in certain individuals. A prevalence of anti-allotype antibodies of 1% is reported in the general adult population.59 These anti-allotype antibodies are described to be of the IgM class and have low affinity, similar to RF. Anti-allotype antibodies are probably formed in response to blood transfusion or parturition.58,60
Theoretically, anti-allotype antibodies may be elicited in response to treatment with therapeutic antibodies in case of allotypic mismatch. For both adalimumab and infliximab, immunogenicity due to allotypic mismatch has been examined. Adalimumab and infliximab are of the allotype G1m17,1 and thus patients homozygous for G1m3 are expected to develop anti-G1m17 or anti-G1m1 antibodies.58 Interestingly, one study reported increased in vitro CD4 T cell responsiveness to peptides derived from G1m1 antibodies in donors homozygous for the non-G1m1 allele versus those homozygous for the G1m1 allele.61 However, a cohort study comprising 250 adalimumab-treated RA patients examined the formation of anti-adalimumab antibodies and determined their binding to the G1m17,1 allotype, and anti-allotype antibodies were detected in none of the patients.62 Another cohort study examined anti-allotype antibody formation in 118 Crohn's disease patients treated with infliximab. As with adalimumab, no correlation was found between the patient's allotype and the formation of anti-infliximab antibodies.63 Apparently, in practice, allotopes of infliximab and adalimumab are only minor epitopes, especially when compared to the idiotype. Allotypic mismatch and anti-allotype antibody formation may therefore have no major impact on the efficacy of therapeutic antibodies.
In contrast to drug-induced anti-allotype antibodies, pre-formed anti-allotype antibodies reactive to a therapeutic antibody have been reported in a few cases.28 In order to detect these antibodies, assays were optimized for detection of low-affinity (IgM) antibodies, which contrasts with efforts that are usually taken to minimize RF interference in immunogenicity assays. Given the similar characteristics of RF and these (pre-formed) anti-allotype antibodies, it is unlikely that they will affect treatment efficacy, and it is advisable to avoid detection of these low-affinity IgM antibodies by using methods that are also used to minimize RF interference (see above).
Variable domain gene segments are also highly polymorphic, with some individuals lacking certain variable domain gene segments.64,65 In these individuals, a therapeutic antibody based on such a particular germ-line sequence might therefore elicit antibodies that recognize a germ-line encoded determinant. These antibodies would in principle qualify as anti-allotype. However, from a practical point of view, such determinants are likely to reside in the germ-line encoded parts of the CDRs rather than FRs. Therefore, antibodies binding such a determinant would be functionally indistinguishable from ‘true’ anti-idiotype antibodies. The distinction resembles that of ‘private’ vs ‘cross-reactive’ idiotopes, as discussed by Jefferis et al.17 It is unknown if immunogenicity rates are higher in individuals lacking a variable domain allele corresponding to a certain therapeutic antibody.
Heterophile and anti-isotype antibodies
This review focuses on antibodies that react with chimeric, humanized, or human therapeutic antibodies. Pre-existing antibodies that react with gammaglobulins of non-human species, so called heterophile antibodies (reviewed elsewhere66), will therefore in principle not be relevant. Nevertheless, rheumatoid factors may be cross-reactive to gammaglobulins of other species (see above), and heterophile antibodies are notorious for rendering false-positive results in immunoassays that use non-human antibodies for detection.67,68 Furthermore, antibodies that recognize xenotopes (non-human determinants of murine origin that are germ-line encoded) in the mouse variable domains of a chimeric antibody are at least a theoretical possibility, and may have formed on previous exposure to mice. However, there is no data available to support this notion, although the reverse, mouse antibodies binding to determinants specific to certain human VH domains is well-documented.69-71
Antibodies formed in response to treatment with murine therapeutic antibodies appear to be a combination of anti-idiotype antibodies and anti-isotype antibodies, the latter partly isotype-specific.1,72 This suggests that in addition to the idiotype, several immunodominant sites exist on the constant domains of the heavy chains of murine antibodies. Absence of these determinants in humanized antibodies explains in part their reduced immunogenicity. Interestingly, there are several reports suggesting that antibodies to murine therapeutic antibodies that target tumor cells may contribute to enhanced tumor cell removal.73–75 These reports did not further characterize the antibodies; therefore, the relative contributions of anti-idiotype and anti-isotype antibodies, or neutralizing and non-neutralizing antibodies, is unknown.
Anti-hinge antibodies and other antibodies to IgG fragments
The proteolytic cleavage of antibodies resulting in Fc and F(ab')2 or Fab fragments, can generate new epitopes that are potentially immunogenic.76 Antibodies to the hinge region of the F(ab')2 of IgG, as well as to the upper hinge region of Fab fragments, have indeed been demonstrated in numerous studies.50,77–80 However, the exact events that lead to the production of anti-hinge antibodies have not yet been elucidated. The incidence of anti-hinge antibodies is higher in RA patients than in healthy individuals, suggesting that chronic inflammation might result in their induction, possibly due to cleavage of IgG by endogenous proteases such as elastase or cathepsin G.50,81 Anti-hinge antibodies can be of high affinity,50,78 and are often specific for a particular C terminus, i.e., they will recognize Fab or F(ab')2 fragments generated by a certain protease, but not fragments obtained by a protease that cleaves at another site, even if just 1 or 2 amino acids apart.82 Furthermore, anti-hinge antibodies may have subclass-restricted specificity83 and do not bind to intact IgG molecules.50,78 The sometimes high specificity and affinity of these antibodies makes it likely that they are specifically elicited by Fab/F(ab')2 fragments. This in turn implies that therapeutic antibody fragments may also have the potential to elicit such antibody responses.
Anti-hinge antibodies are of no importance for most anti-TNF therapeutics because they are full-length, intact molecules. Certolizumab pegol, however, embodies an anti-TNF Fab' fragment that might elicit anti-hinge antibody formation. To our knowledge, the role of anti-hinge antibodies on the efficacy and clearance of certolizumab has not been investigated yet, and thus remains unclarified. In cases where the specificities of anti-certolizumab were investigated, the vast majority of antibodies was found to compete with TNF binding, thereby excluding a substantial anti-hinge response.25 Certolizumab has a polyethylene glycol tail attached to its C terminus, effectively shielding the exposed hinge epitope, which may lower the propensity to induce this particular type of antibody response. This also suggests that potentially immunogenic structures in the – branched – polyethylene glycol tail do not elicit a strong antibody response. In general, lack of assay standardization has thus far hampered assessment of the immunogenic potential of PEG and the prevalence of anti-PEG antibodies.84,85
The high prevalence of different kinds of anti-hinge antibodies implies that pre-existing antibodies may be found that cross-react with a particular therapeutic antibody Fab fragment in which the hinge region is exposed. An early study on one such therapeutic, the chimeric Fab fragment abciximab (anti-glycoprotein IIb/IIIa), indeed showed the presence of pre-existing antibodies directed to the C terminus of Fab fragments, but no adverse clinical events could be attributed to the presence of these antibodies.2 Further investigation of the immunogenicity of abciximab showed that the presence of antibodies to abciximab correlated with thrombocytopenia, an adverse effect of abciximab.86 A study of Lajus et al.87 demonstrated that these antibodies could not only be directed to abciximab, but also to a neo-epitope generated by the association of abciximab with its ligand, the platelet glycoprotein IIb/IIIa receptor. Despite this further characterization of the anti-abciximab antibody response, it remains unknown whether anti-hinge antibodies contribute to this response.
Another example of pre-existing antibodies to an antibody fragment comes from a study by Holland et al.29 About 50% of healthy individuals were found to have antibodies against a single VH domain antibody fragment that targets TNFR-1. In a dose-escalation study, physiological signs of cytokine release were observed in 2 individuals with anti-VH antibodies, and, in an in vitro experiment, complexes of anti-VH antibodies and the anti-TNFR-1 VH domain antibody were able to release cytokines from several human cell lines. The antibodies were found to bind to framework regions, but were not cross-reactive to the same VH domain as part of an intact antibody. The specific determinant was unfortunately not disclosed in the study, but may comprise an epitope that would be shielded if the VH domain is associated with a VL or CH1 domain.
Anti-hinge antibodies can also be a confounding factor in immunogenicity assays in case Fab or F(ab')2 fragments of the therapeutic antibody are used to capture the potential anti-drug antibodies, for instance, to circumvent the detection of RF.50 In particular, several studies aimed to characterize if anti-drug antibodies target the Fab or Fc part of the therapeutic antibody infliximab, but found substantial reactivities to Fab/F(ab')2 in pre-treatment samples or control samples, which were probably anti-hinge antibodies.88,89 In order to avoid the detection of anti-hinge antibodies, addition of Fab or F(ab')2 fragments of polyclonal human IgG can be used.50
Anti-glycan antibodies
All IgG antibodies possess a conserved N-glycosylation site in the Fc part. The attached bi-antennary glycan structure is usually heterogeneous in structure, both in case of in vivo produced antibodies as well as those produced in cell lines.90 Proper glycosylation depends on the cell line used to manufacture monoclonal antibodies. Some cell lines may introduce non-human glycan structures. One of these cell lines, SP2/0, used for the production of infliximab, is able to attach the non-human glycan structure galactose-α-1,3-galactose (α-gal) to antibodies.91 Alpha-gal is also a common antigen present on many animal proteins as well as bacteria, and it is not surprising that most individuals develop IgG antibodies against α-gal throughout life.92–94 However, no reports exist about anti-glycan antibodies cross-reacting with, for example, infliximab (see below).
In addition to Fc-linked glycans, the Fab portion of an antibody may also contain N-linked glycans in case a glycosylation site was introduced by somatic hypermutation. Most therapeutic antibodies do not contain Fab glycans, which is likely in part the result of lead selection against clones containing a Fab glycan, as well as by eliminating any such site if it does exist in an antibody with the potential of becoming a therapeutic. However, cetuximab, a therapeutic antibody targeting epidermal growth factor receptor, is produced in SP2/0 and contains an additional N-glycosylation site in the Fab portion.95 As a result, cetuximab has an exposed α-gal moiety on its Fab fragment.
No information exists on a potentially accelerated clearance of cetuximab due to (IgG) anti-α-gal antibodies. However, a high incidence of sometimes life-threatening hypersensitivity reactions at the first administration of cetuximab was observed in some areas of the United States.96 It was found that most patients who developed hypersensitivity reactions were positive for pre-existing IgE anti-α-gal antibodies. Subsequent studies indicate that the IgE antibodies were specifically developed after exposure to tick bites in these rural areas.97 (On the other hand, there are no indications that cetuximab-treated individuals without pre-existing IgE anti-α-gal develop these antibodies upon treatment.) Testing patients before start of treatment for the presence of IgE anti-α-gal may identify patients at risk for developing a severe hypersensitivity reaction.
Although infliximab and palivizumab also include α-gal, the α-gal epitopes of infliximab and palivizumab are exclusively located on Fc-linked glycans in relatively low abundance and were found not to be recognized by IgE anti-α-gal unless the Fc part was digested into fragments.91 Therefore, IgE anti-α-gal thus far appears to only be of significant importance to patients treated with cetuximab.
Non-Specific Associations Between Immunoglobulins
Antibody molecules will typically bind to a target via a specific interaction that is largely mediated by the CDRs. However, immunoglobulins can also associate in a variety of other ways.98 Depending on the type of immunoassay that is carried out, such non-specific associations may result in a positive signal, which will often reflect limitations of the assay format rather than a genuine interaction that may also take place in vivo. It is important to keep in mind that for some of the aforementioned examples of pre-existing antibodies, assays used to measure them can also suffer from these limitations, direct ELISAs in particular, as will be elaborated below.
Antibodies that are directly adsorbed onto a polystyrene microtiter plate will unfold to various degrees.99,100 As a result, parts of several immunoglobulin domains that are normally involved in interactions between light and heavy chains, and therefore shielded, may become exposed. These interaction surfaces exist between heavy and light chains (VH-VL; CH1-CL) and between heavy chains (CH3-CH3). Antibodies from a serum sample may subsequently associate to these partially unfolded antibodies in a non-specific fashion depending on, for example, their isotype as well as their structural integrity. A well-studied example is human IgG4, which can associate to immobilized/partially unfolded IgG exclusively via Fc-Fc interactions.101 The interaction involves (partial) dissociation and re-association of CH3 domains,102 which is most efficient for IgG3 and IgG4.103 Association of IgG4 is also observed to various degrees to immobilized IgG from other species,104 and is easily confused for e.g., rheumatoid factor activity.105 A similar association could be envisaged involving dissociation and re-association of light and heavy chains from certain antibody molecules, especially since interactions between VH and VL domains may greatly differ in association strength for different antibodies.106 This could explain the observation that in sera from healthy individuals, antibody reactivity to infliximab F(ab')2 fragments adsorbed to microtiter plates could be only partially depleted by repeated absorption using the same F(ab')2 fragments chemically attached to agarose.50
Another artificial interaction that may be relevant to immunogenicity testing is so-called ‘acquired polyreactivity’, a general increase in reactivity to many proteins, including immunoglobulins, upon treatment of antibodies with acid.107 Anti-drug antibody assays nowadays often include an acid-dissociation step to dissociate potential drug-antibody complexes. This sample treatment might result in an association of drug to serum antibodies that would not normally bind to the drug. It is therefore useful to test pre-treatment samples, which will not contain drug, both with and without an acid-dissociation step to ensure that no artificial reactivities are introduced following acidification. In general, one should be aware that assay steps that introduce structural modifications to a therapeutic antibody may lead to artificial antibody interactions.
Besides these non-physiological immunoglobulin interactions, another type of Fc interaction was recently found to contribute to IgG hexamer formation upon C1q binding.108,109 These low-affinity interactions will not result in association between monomeric IgG molecules, but may be responsible for the fact that IgG Fc fragments readily crystallize, and possibly facilitate PEG-induced immune complex precipitation reactions.98 Interference from these non-specific, low-affinity interactions in immunoassays may be minimized by ensuring that reagents, including drug molecules that are tagged (e.g., with biotin), are aggregate-free.
Immunogenicity Assays
There are several reasons to measure antibody responses to therapeutic antibodies, including purely scientific interest, clinical management, and evaluation of the immunogenic potential of a drug in development. In all cases, it is important to distinguish between an antibody response induced by the therapeutic antibody, which can be expected to be specific, targeting predominantly the idiotype and are clinically relevant, and pre-dose antibodies that can bind the therapeutic antibody, but are often irrelevant or the result of non-specific interactions. Nevertheless, several cases of pre-formed antibodies have been reported thus far that proved to be (potentially) clinically relevant (Table 1). The IgE anti-α-gal antibodies that cross-react with cetuximab and may induce hypersensitivity reactions is the most dramatic example. Perhaps the most important lesson from these examples is that understanding the potential risks of these pre-formed antibodies was possible in large part because of proper characterization of these antibody responses. Moreover, unusual features were demonstrable in the therapeutic antibody/antibody fragments, which were found to relate to the antibody reactivities that were observed.
Table 1.
Reports on consequences of pre-existing antibodies
| Drug | Effect | Reference |
|---|---|---|
| abciximab | presence of anti-hinge antibodies might be correlated with thrombocytopenia | 2, 86, 87 |
| cetuximab | IgE antibodies recognizing alpha-gal sugar moieties caused anaphylactic reactions in patients from certain regions in the United states | 96 |
| GSK1995057 | in vitro and in vivo cytokine release was associated with antibodies recognizing framework regions of the drug (a VH domain) but not the intact antibody | 29 |
| rituximab | decreased in vitro complement-dependent cytotoxicity of rituximab was observed in the presence of rheumatoid factor | 44 |
Currently, most therapeutic antibodies use ‘natural’ constant domains, but antibody formats are being developed with enhanced features compared to wild-type isotypes, such as improved or diminished binding to Fc receptors or enhanced capacity for complement activation.108,109 Another example is the use of single-chain VH domains, of which one example was discussed above.29 Whether or not these modifications will lead to substantial antibody formation remains to be seen. Furthermore, a variety of antibody conjugates are in development, such as molecules comprising toxins coupled to an antibody. Data on the potential immunogenicity as a result of specific determinants other than the idiotype are still limited, but the existing body of data suggests that, for example, allotypic mismatch may not result in a substantial anti-allotype response. However, predictions regarding the immunogenic potential of antibody modifications are troublesome. Addition of protein-based moieties to an antibody structure, such as an antibody-toxin conjugate, may have unexpected consequences with respect to immunogenicity. In any case, specific assessment of antibody reactivity to the modified parts is easily accomplished by cross-screening/inhibition experiments using the wild-type counterpart of such a therapeutic (see below).
More commonly observed pre-dose reactivities to ‘typical’ antibodies will in most cases largely consist of low-affinity antibodies such as RF. The current body of evidence suggests that there is no value in taking into account these antibodies for immunogenicity testing, but rather one should take measures to eliminate the detection of these antibodies. Importantly, pre-dose positivity does not appear to predict post-dose immunogenicity.38
Since many autoimmune diseases are characterized by elevated levels of such antibodies, it is necessary to base cut-points of immunogenicity assays not solely on samples from healthy individuals. Especially if one follows the advice of using a screening assay that by design identifies 1 out of 20 samples as positive,12 by itself a high false-positivity rate, the amount of positives in a patient group may easily reach double digits if the cut-point was based only on healthy individuals, while in fact none of the positive samples would represent a true positive. On the other hand, if no efforts are undertaken to eliminate the interference of irrelevant pre-existing reactivities, the resulting assay may be insensitive because of an artificially high cut-point. Furthermore, besides RF, other IgG-binding serum components such as C1q may render similar false-positive signals, especially in bridging formats. Specificity can be assessed, for instance, by checking for cross-reactivity with other antibodies with a different specificity;31 as well as inhibition experiments using, for example, (heat-aggregated) polyclonal human immunoglobulin to check for RF background, or using the same clone expressed as a different allotype to evaluate possible allo-reactivity.
In addition, as explained in the previous section, one should be aware of the fact that assay steps resulting in structural modifications of antibodies (e.g., by direct adsorption to a solid phase) may lead to non-specific associations of immunoglobulins and thereby false-positive signals. If in doubt, it is useful to (re-)design the assay in such a way that antibody-drug interactions take place in fluid phase. Assays should be set up in a manner that avoids structural modifications that can affect the interactions between the drug and serum antibodies.
To conclude, pre-formed antibodies in general appear to have little or no consequences during treatment, but a few exceptions exist where pre-formed antibodies could or did have clinical consequences. In these cases, specificities of the antibodies were analyzed and could be linked to distinct, unusual epitopes of the therapeutic. Therefore, immunogenicity testing strategies should always include a sufficient level of antibody characterization, especially in case of pre-formed antibodies (Table 2). On the one hand, this should ensure that false-positives, particularly due to rheumatoid factors, are largely eliminated. On the other hand, knowing which types of antibodies produce a genuine pre-dose signal to a certain drug will help to judge their potential threat.
Table 2.
Key points
| • Even fully human monoclonal antibodies are potentially immunogenic |
| • Antibodies formed in response to treatment are mostly neutralizing |
| • In many (but not all) cases, pre-existing antibodies have little consequences |
| • Pre-existing antibodies should be carefully distinguished from false-positive results |
| • Pre-existing antibodies should be evaluated on a case-by-case basis using assays that yield information on the specificity of the antibodies |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank Lucien Aarden for invaluable suggestions and discussions.
Funding
GJW has received a research grant from Pfizer and honoraria for lectures from Abbvie, Pfizer and UCB. TR has received honoraria for lecture from Pfizer and Abbvie.
References
- 1.Chatenoud L, Jonker M, Villemain F, Goldstein G, Bach JF. The human immune response to the OKT3 monoclonal antibody is oligoclonal. Science 1986; 232: 1406-1408; PMID:3086976; http://dx.doi.org/ 10.1126/science.3086976. [DOI] [PubMed] [Google Scholar]
- 2.Knight DM, Wagner C, Jordan R, McAleer MF, DeRita R, Fass DN, Coller BS, Weisman HF, Ghrayeb J. The immunogenicity of the 7E3 murine monoclonal Fab antibody fragment variable region is dramatically reduced in humans by substitution of human for murine constant regions. Mol Immunol 1995; 32: 1271-1281; PMID:8559151; http://dx.doi.org/ 10.1016/0161-5890(95)00085-2. [DOI] [PubMed] [Google Scholar]
- 3.Harding FA, Stickler MM, Razo J, DuBridge RB. The immunogenicity of humanized and fully human antibodies: residual immunogenicity resides in the CDR regions. MAbs 2010; 2: 256-265; PMID:20400861; http://dx.doi.org/ 10.4161/mabs.2.3.11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Schouwenburg PA, Rispens T, Wolbink GJ. Immunogenicity of anti-TNF biologic therapies for rheumatoid arthritis. Nat Rev Rheumatol 2013; 9: 164-172; PMID:23399692; http://dx.doi.org/ 10.1038/nrrheum.2013.4. [DOI] [PubMed] [Google Scholar]
- 5.Vincent FB, Morand EF, Murphy K, Mackay F, Mariette X, Marcelli C. Antidrug antibodies (ADAb) to tumour necrosis factor (TNF)-specific neutralising agents in chronic inflammatory diseases: a real issue, a clinical perspective. Ann Rheum Dis 2013; 72: 165-178; http://dx.doi.org/ 10.1136/annrheumdis-2012-202545. [DOI] [PubMed] [Google Scholar]
- 6.Somerfield J, Hill-Cawthorne GA, Lin A, Zandi MS, McCarthy C, Jones JL, Willcox M, Shaw D, Thompson SA, Compston AS, et al.. A novel strategy to reduce the immunogenicity of biological therapies. J Immunol 2010; 185: 763-768; http://dx.doi.org/ 10.4049/jimmunol.1000422. [DOI] [PubMed] [Google Scholar]
- 7.Krieckaert CL, Bartelds GM, Wolbink GJ. Therapy: Immunogenicity of biologic therapies-we need tolerance. Nat Rev Rheumatol 2010; 6: 558-559. [DOI] [PubMed] [Google Scholar]
- 8.Benjamin RJ, Cobbold SP, Clark MR, Waldmann H. Tolerance to rat monoclonal antibodies. Implications for serotherapy. J Exp Med 1986; 163: 1539-1552; http://dx.doi.org/ 10.1084/jem.163.6.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilliland LK, Walsh LA, Frewin MR, Wise MP, Tone M, Hale G, Kioussis D, Waldmann H. Elimination of the immunogenicity of therapeutic antibodies. J Immunol 1999; 162: 3663-3671. [PubMed] [Google Scholar]
- 10.Chamberlain P. Assessing immunogenicity of biosimilar therapeutic monoclonal antibodies: regulatory and bioanalytical considerations. Bioanalysis 2013; 5: 561-574; PMID:23425272; http://dx.doi.org/ 10.4155/bio.13.6. [DOI] [PubMed] [Google Scholar]
- 11.Krieckaert C, Rispens T, Wolbink G. Immunogenicity of biological therapeutics: from assay to patient. Curr Opin Rheumatol 2012; 24: 306-311; http://dx.doi.org/ 10.1097/BOR.0b013e3283521c4e. [DOI] [PubMed] [Google Scholar]
- 12.Shankar G, Devanarayan V, Amaravadi L, Barrett YC, Bowsher R, Finco-Kent D, Fiscella M, Gorovits B, Kirschner S, Moxness M, et al.. Recommendations for the validation of immunoassays used for detection of host antibodies against biotechnology products. J Pharm Biomed Anal 2008; 48: 1267-1281; http://dx.doi.org/ 10.1016/j.jpba.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 13.Shankar G, Arkin S, Cocea L, Devanarayan V, Kirshner S, Kromminga A, Quarmby V, Richards S, Schneider CK, Subramanyam M, et al.. Assessment and reporting of the clinical immunogenicity of therapeutic proteins and peptides-harmonized terminology and tactical recommendations. AAPS J 2014; 16: 658-673; PMID:24764037; http://dx.doi.org/ 10.1208/s12248-014-9599-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jerne NK. The generative grammar of the immune system. Science 1985; 229: 1057-1059; PMID:4035345; http://dx.doi.org/ 10.1126/science.4035345. [DOI] [PubMed] [Google Scholar]
- 15.Nisonoff A. On the nomenclature for V-region serological markers. Immunol Today 1995; 16: 191-193; PMID:7734047; http://dx.doi.org/ 10.1016/0167-5699(95)80120-0. [DOI] [PubMed] [Google Scholar]
- 16.Jefferis R. Nomenclature of V-region markers. Immunol Today 1995; 16: 207-208; PMID:7734050; http://dx.doi.org/ 10.1016/0167-5699(95)80123-5. [DOI] [PubMed] [Google Scholar]
- 17.Jefferis R. What is an idiotype? Immunol Today 1993; 14: 119-121; PMID:8466627; http://dx.doi.org/ 10.1016/0167-5699(93)90211-3. [DOI] [PubMed] [Google Scholar]
- 18.Kazatchkine MD, Coutinho A. Are lymphocytes concerned with our definition of idiotypes? Immunol Today 1993; 14: 513-515; PMID:8274191; http://dx.doi.org/ 10.1016/0167-5699(93)90268-P. [DOI] [PubMed] [Google Scholar]
- 19.Vennegoor A, Rispens T, Strijbis EM, Seewann A, Uitdehaag BM, Balk LJ, Barkhof F, Polman CH, Wolbink G, Killestein J. Clinical relevance of serum natalizumab concentration and anti-natalizumab antibodies in multiple sclerosis. Mult Scler 2013; 19: 593-600; http://dx.doi.org/ 10.1177/1352458512460604. [DOI] [PubMed] [Google Scholar]
- 20.Wolbink GJ, Vis M, Lems W, Voskuyl AE, de GE, Nurmohamed MT, Stapel S, Tak PP, Aarden L, Dijkmans B. Development of antiinfliximab antibodies and relationship to clinical response in patients with rheumatoid arthritis. Arthritis Rheum 2006; 54: 711-715; PMID:16508927; http://dx.doi.org/ 10.1002/art.21671. [DOI] [PubMed] [Google Scholar]
- 21.Radstake TR, Svenson M, Eijsbouts AM, van den Hoogen FH, Enevold C, van Riel PL, Bendtzen K. Formation of antibodies against infliximab and adalimumab strongly correlates with functional drug levels and clinical responses in rheumatoid arthritis. Ann Rheum Dis 2009; 68: 1739-1745; http://dx.doi.org/ 10.1136/ard.2008.092833. [DOI] [PubMed] [Google Scholar]
- 22.Svenson M, Geborek P, Saxne T, Bendtzen K. Monitoring patients treated with anti-TNF-α biopharmaceuticals: assessing serum infliximab and anti-infliximab antibodies. Rheumatology (Oxford) 2007; 46: 1828-1834; PMID:18032541; http://dx.doi.org/ 10.1093/rheumatology/kem261. [DOI] [PubMed] [Google Scholar]
- 23.Bartelds GM, Krieckaert CL, Nurmohamed MT, van Schouwenburg PA, Lems WF, Twisk JW, Dijkmans BA, Aarden L, Wolbink GJ. Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA 2011; 305: 1460-1468; PMID:21486979; http://dx.doi.org/ 10.1001/jama.2011.406. [DOI] [PubMed] [Google Scholar]
- 24.Lecluse LL, Driessen RJ, Spuls PI, De Jong EM, Stapel SO, van Doorn MB, Bos JD, Wolbink GJ. Extent and clinical consequences of antibody formation against adalimumab in patients with plaque psoriasis. Arch Dermatol 2010; 146: 127-132. [DOI] [PubMed] [Google Scholar]
- 25.van Schie KA, Hart MH, de Groot ER, Kruithof S, Aarden LA, Wolbink GJ, Rispens T. The antibody response against human and chimeric anti-TNF therapeutic antibodies primarily targets the TNF binding region. Ann Rheum Dis 2015; 74: 311-314; http://dx.doi.org/ 10.1136/annrheumdis-2014-206237. [DOI] [PubMed] [Google Scholar]
- 26.van Schouwenburg PA, Kruithof S, Votsmeier C, van Schie KA, Hart MH, de Jong RN, van Buren EE, van HM, Aarden L, Wolbink G, et al.. Functional Analysis of the Anti-adalimumab Response Using Patient-derived Monoclonal Antibodies. J Biol Chem 2014; 289: 34482-34488; http://dx.doi.org/ 10.1074/jbc.M114.615500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Schouwenburg PA, van de Stadt LA, de Jong RN, van Buren EE, Kruithof S, de GE, Hart M, van Ham SM, Rispens T, Aarden L, et al.. Adalimumab elicits a restricted anti-idiotypic antibody response in autoimmune patients resulting in functional neutralisation. Ann Rheum Dis 2013; 72: 104-109; http://dx.doi.org/ 10.1136/annrheumdis-2012-201445. [DOI] [PubMed] [Google Scholar]
- 28.Tatarewicz SM, Juan G, Swanson SJ, Moxness MS. Epitope characterization of pre-existing and developing antibodies to an aglycosylated monoclonal antibody therapeutic of G1m17,1 allotype. J Immunol Methods 2012; 382: 93-100; PMID:22609464; http://dx.doi.org/ 10.1016/j.jim.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Holland MC, Wurthner JU, Morley PJ, Birchler MA, Lambert J, Albayaty M, Serone AP, Wilson R, Chen Y, Forrest RM, Cordy JC, Lipson DA, Bayliffe AI. Autoantibodies to variable heavy (VH) chain Ig sequences in humans impact the safety and clinical pharmacology of a VH domain antibody antagonist of TNF-α receptor 1. J Clin Immunol 2013; 33: 1192-1203; http://dx.doi.org/ 10.1007/s10875-013-9915-0. [DOI] [PubMed] [Google Scholar]
- 30.Weeraratne D, Chen A, Pennucci JJ, Wu CY, Zhang K, Wright J, Perez-Ruixo JJ, Yang BB, Kaliyaperumal A, Gupta S, et al.. Immunogenicity of panitumumab in combination chemotherapy clinical trials. BMC Clin Pharmacol 2011; 11: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szolar OH, Stranner S, Zinoecker I, Mudde GC, Himmler G, Waxenecker G, Nechansky A. Qualification and application of a surface plasmon resonance-based assay for monitoring potential HAHA responses induced after passive administration of a humanized anti Lewis-Y antibody. J Pharm Biomed Anal 2006; 41: 1347-1353; http://dx.doi.org/ 10.1016/j.jpba.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 32.Sasso EH, Barber CV, Nardella FA, Yount WJ, Mannik M. Antigenic specificities of human monoclonal and polyclonal IgM rheumatoid factors. The C gamma 2-C gamma 3 interface region contains the major determinants. J Immunol 1988; 140: 3098-3107; PMID:2452199. [PubMed] [Google Scholar]
- 33.Dorner T, Egerer K, Feist E, Burmester GR. Rheumatoid factor revisited. Curr Opin Rheumatol 2004; 16: 246-253; http://dx.doi.org/ 10.1097/00002281-200405000-00013. [DOI] [PubMed] [Google Scholar]
- 34.Mierau R, Genth E. Diagnosis and prognosis of early rheumatoid arthritis, with special emphasis on laboratory analysis. Clin Chem Lab Med 2006; 44: 138-143; PMID:16475897; http://dx.doi.org/ 10.1515/CCLM.2006.026. [DOI] [PubMed] [Google Scholar]
- 35.Westwood OM, Nelson PN, Hay FC. Rheumatoid factors: what's new? Rheumatology (Oxford) 2006; 45: 379-385; PMID:16418203; http://dx.doi.org/ 10.1093/rheumatology/kei228. [DOI] [PubMed] [Google Scholar]
- 36.Van Snick JL, Van RE, Markowetz B, Cambiaso CL, Masson PL. Enhancement by IgM rheumatoid factor of in vitro ingestion by macrophages and in vivo clearance of aggregated IgG or antigen-antibody complexes. Eur J Immunol 1978; 8: 279-285; PMID:668802; http://dx.doi.org/ 10.1002/eji.1830080412. [DOI] [PubMed] [Google Scholar]
- 37.Ng YC, Peters DK, Walport MJ. Monoclonal rheumatoid factor-IgG immune complexes. Poor fixation of opsonic C4 and C3 despite efficient complement activation. Arthritis Rheum 1988; 31: 99-107; PMID:3345233; http://dx.doi.org/ 10.1002/art.1780310114. [DOI] [PubMed] [Google Scholar]
- 38.Xue L, Rup B. Evaluation of pre-existing antibody presence as a risk factor for posttreatment anti-drug antibody induction: analysis of human clinical study data for multiple biotherapeutics. AAPS J 2013; 15: 893-896; PMID:23761225; http://dx.doi.org/ 10.1208/s12248-013-9497-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alessandri C, Bombardieri M, Papa N, Cinquini M, Magrini L, Tincani A, Valesini G. Decrease of anti-cyclic citrullinated peptide antibodies and rheumatoid factor following anti-TNFalpha therapy (infliximab) in rheumatoid arthritis is associated with clinical improvement. Ann Rheum Dis 2004; 63: 1218-1221; PMID:15361374; http://dx.doi.org/ 10.1136/ard.2003.014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bos WH, Bartelds GM, Wolbink GJ, de Koning MH, van de Stadt RJ, van SD, Dijkmans BA, Nurmohamed MT. Differential response of the rheumatoid factor and anticitrullinated protein antibodies during adalimumab treatment in patients with rheumatoid arthritis. J Rheumatol 2008; 35: 1972-1977; PMID:18785316. [PubMed] [Google Scholar]
- 41.De RL, Verhelst X, Kruithof E, Van den Bosch F, Hoffman IE, Veys EM, De KF. Rheumatoid factor, but not anti-cyclic citrullinated peptide antibodies, is modulated by infliximab treatment in rheumatoid arthritis. Ann Rheum Dis 2005; 64: 299-302; PMID:15166003; http://dx.doi.org/ 10.1136/ard.2004.023523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mendonca JA, Marques-Neto JF, Samara AM, Appenzeller S. Increased levels of rheumatoid factors after TNF inhibitor in rheumatoid arthritis. Rheumatol Int 2012; 32: 815-818; PMID:21327431; http://dx.doi.org/ 10.1007/s00296-011-1812-3. [DOI] [PubMed] [Google Scholar]
- 43.Faillace C, de Carvalho JF. Rheumatoid factor appearance after tocilizumab treatment seems to predict bad therapeutical response in rheumatoid arthritis. Rheumatol Int 2013; 33: 1909-1910; PMID:22441966; http://dx.doi.org/ 10.1007/s00296-012-2409-1. [DOI] [PubMed] [Google Scholar]
- 44.Jones JD, Shyu I, Newkirk MM, Rigby WF. A rheumatoid factor paradox: inhibition of rituximab effector function. Arthritis Res Ther 2013; 15: R20; PMID:23351360; http://dx.doi.org/ 10.1186/ar4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chatzidionysiou K, Lie E, Nasonov E, Lukina G, Hetland ML, Tarp U, Gabay C, van Riel PL, Nordstrom DC, Gomez-Reino J, et al.. Highest clinical effectiveness of rituximab in autoantibody-positive patients with rheumatoid arthritis and in those for whom no more than one previous TNF antagonist has failed: pooled data from 10 European registries. Ann Rheum Dis 2011; 70: 1575-1580; PMID:21571731; http://dx.doi.org/ 10.1136/ard.2010.148759. [DOI] [PubMed] [Google Scholar]
- 46.Maneiro RJ, Salgado E, Carmona L, Gomez-Reino JJ. Rheumatoid factor as predictor of response to abatacept, rituximab and tocilizumab in rheumatoid arthritis: Systematic review and meta-analysis. Semin Arthritis Rheum 2013; 43: 9-17; PMID:23290690; http://dx.doi.org/ 10.1016/j.semarthrit.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 47.Burmester GR, Panaccione R, Gordon KB, McIlraith MJ, Lacerda AP. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn's disease. Ann Rheum Dis 2013; 72: 517-524; PMID:22562972; http://dx.doi.org/ 10.1136/annrheumdis-2011-201244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song SN, Yoshizaki K. Tocilizumab for treating rheumatoid arthritis: an evaluation of pharmacokinetics/pharmacodynamics and clinical efficacy. Expert Opin Drug Metab Toxicol 2015; 11: 307-316; PMID:25491492; http://dx.doi.org/ 10.1517/17425255.2015.992779. [DOI] [PubMed] [Google Scholar]
- 49.Aarden L, Ruuls SR, Wolbink G. Immunogenicity of anti-tumor necrosis factor antibodies-toward improved methods of anti-antibody measurement. Curr Opin Immunol 2008; 20: 431-435; PMID:18619538; http://dx.doi.org/ 10.1016/j.coi.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 50.Rispens T, de VH, de GE, Wouters D, Stapel S, Wolbink GJ, Aarden LA. Antibodies to constant domains of therapeutic monoclonal antibodies: anti-hinge antibodies in immunogenicity testing. J Immunol Methods 2012; 375: 93-99; PMID:21986105; http://dx.doi.org/ 10.1016/j.jim.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 51.Tatarewicz S, Miller JM, Swanson SJ, Moxness MS. Rheumatoid factor interference in immunogenicity assays for human monoclonal antibody therapeutics. J Immunol Methods 2010; 357: 10-16; PMID:20347831; http://dx.doi.org/ 10.1016/j.jim.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 52.Stubenrauch K, Wessels U, Vogel R, Schleypen J. Evaluation of a biosensor immunoassay for simultaneous characterization of isotype and binding region of human anti-tocilizumab antibodies with control by surrogate standards. Anal Biochem 2009; 390: 189-196; PMID:19379704; http://dx.doi.org/ 10.1016/j.ab.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 53.Schmidt E, Hennig K, Mengede C, Zillikens D, Kromminga A. Immunogenicity of rituximab in patients with severe pemphigus. Clin Immunol 2009; 132: 334-341; PMID:19502112; http://dx.doi.org/ 10.1016/j.clim.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 54.Vaisanen V, Peltola MT, Lilja H, Nurmi M, Pettersson K. Intact free prostate-specific antigen and free and total human glandular kallikrein 2. Elimination of assay interference by enzymatic digestion of antibodies to F(ab')2 fragments. Anal Chem 2006; 78: 7809-7815; PMID:17105175; http://dx.doi.org/ 10.1021/ac061201+. [DOI] [PubMed] [Google Scholar]
- 55.Stubenrauch K, Wessels U, Birnboeck H, Ramirez F, Jahreis A, Schleypen J. Subset analysis of patients experiencing clinical events of a potentially immunogenic nature in the pivotal clinical trials of tocilizumab for rheumatoid arthritis: Evaluation of an antidrug antibody ELISA using clinical adverse event-driven immunogenicity testing. Clin Ther 2010; 32: 1597-1609; PMID:20974317; http://dx.doi.org/ 10.1016/j.clinthera.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 56.Araujo J, Zocher M, Wallace K, Peng K, Fischer SK. Increased rheumatoid factor interference observed during immunogenicity assessment of an Fc-engineered therapeutic antibody. J Pharm Biomed Anal 2011; 55: 1041-1049; PMID:21466939; http://dx.doi.org/ 10.1016/j.jpba.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 57.de Lange GG. Polymorphisms of human immunoglobulins: Gm, Am, Em and Km allotypes. Exp Clin Immunogenet 1989; 6: 7-17; PMID:2698222. [PubMed] [Google Scholar]
- 58.Jefferis R, Lefranc MP. Human immunoglobulin allotypes: possible implications for immunogenicity. MAbs 2009; 1: 332-338; PMID:20073133; http://dx.doi.org/ 10.4161/mabs.1.4.9122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jerne D. Antibody suppression and antiallotype antibodies. II. Interference of circulating antiallotype antibodies with prophylactic treatment against Rh sensitization. Scand J Immunol 1976; 5: 1085-1088; PMID:827012; http://dx.doi.org/ 10.1111/j.1365-3083.1976.tb03061.x. [DOI] [PubMed] [Google Scholar]
- 60.Fudenberg HH, Fudenberg BR. Antibody to hereditary human gamma-globulin (gm) factor resulting from maternal-fetal incompatibility. Science 1964; 145: 170-171; PMID:14171557; http://dx.doi.org/ 10.1126/science.145.3628.170. [DOI] [PubMed] [Google Scholar]
- 61.Stickler MM, Reddy A, Xiong JM, Hinton PR, DuBridge R, Harding FA. The human G1m1 allotype associates with CD4+ T-cell responsiveness to a highly conserved IgG1 constant region peptide and confers an asparaginyl endopeptidase cleavage site. Genes Immun 2011; 12: 213-221; PMID:21326320; http://dx.doi.org/ 10.1038/gene.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bartelds GM, de GE, Nurmohamed MT, Hart MH, van Eede PH, Wijbrandts CA, Crusius JB, Dijkmans BA, Tak PP, Aarden L, et al.. Surprising negative association between IgG1 allotype disparity and anti-adalimumab formation: a cohort study. Arthritis Res Ther 2010; 12: R221; PMID:21187010; http://dx.doi.org/ 10.1186/ar3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Magdelaine-Beuzelin C, Vermeire S, Goodall M, Baert F, Noman M, Assche GV, Ohresser M, Degenne D, Dugoujon JM, Jefferis R, Rutgeerts P, Lefranc MP, Watier H. IgG1 heavy chain-coding gene polymorphism (G1m allotypes) and development of antibodies-to-infliximab. Pharmacogenet Genomics 2009; 19: 383-387; PMID:19319024; http://dx.doi.org/ 10.1097/FPC.0b013e32832a06bf. [DOI] [PubMed] [Google Scholar]
- 64.Milner EC, Hufnagle WO, Glas AM, Suzuki I, Alexander C. Polymorphism and utilization of human VH Genes. Ann N Y Acad Sci 1995; 764: 50-61; PMID:7486575; http://dx.doi.org/ 10.1111/j.1749-6632.1995.tb55806.x. [DOI] [PubMed] [Google Scholar]
- 65.Watson CT, Breden F. The immunoglobulin heavy chain locus: genetic variation, missing data, and implications for human disease. Genes Immun 2012; 13: 363-373; PMID:22551722; http://dx.doi.org/ 10.1038/gene.2012.12. [DOI] [PubMed] [Google Scholar]
- 66.Klee GG. Human anti-mouse antibodies. Arch Pathol Lab Med 2000; 124: 921-923; PMID:10835540. [DOI] [PubMed] [Google Scholar]
- 67.Koshida S, Asanuma K, Kuribayashi K, Goto M, Tsuji N, Kobayashi D, Tanaka M, Watanabe N. Prevalence of human anti-mouse antibodies (HAMAs) in routine examinations. Clin Chim Acta 2010; 411: 391-394; PMID:20006593; http://dx.doi.org/ 10.1016/j.cca.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 68.Kaplan IV, Levinson SS. When is a heterophile antibody not a heterophile antibody? When it is an antibody against a specific immunogen. Clin Chem 1999; 45: 616-618; PMID:10222346. [PubMed] [Google Scholar]
- 69.Potter KN, Li Y, Mageed RA, Jefferis R, Capra JD. Molecular characterization of the VH1-specific variable region determinants recognized by anti-idiotypic monoclonal antibodies G6 and G8. Scand J Immunol 1999; 50: 14-20; PMID:10404046; http://dx.doi.org/ 10.1046/j.1365-3083.1999.00524.x. [DOI] [PubMed] [Google Scholar]
- 70.Mageed RA, Dearlove M, Goodall DM, Jefferis R. Immunogenic and antigenic epitopes of immunoglobulins. XVII–Monoclonal antibodies reactive with common and restricted idiotopes to the heavy chain of human rheumatoid factors. Rheumatol Int 1986; 6: 179-183; PMID:2431452; http://dx.doi.org/ 10.1007/BF00541285. [DOI] [PubMed] [Google Scholar]
- 71.Potter KN, Li Y, Mageed RA, Jefferis R, Capra JD. Anti-idiotypic antibody D12 and superantigen SPA both interact with human VH3-encoded antibodies on the external face of the heavy chain involving FR1, CDR2 and FR3. Mol Immunol 1998; 35: 1179-1187; PMID:10199392; http://dx.doi.org/ 10.1016/S0161-5890(98)00103-5. [DOI] [PubMed] [Google Scholar]
- 72.Legendre C, Kreis H, Bach JF, Chatenoud L. Prediction of successful allograft rejection retreatment with OKT3. Transplantation 1992; 53: 87-90; PMID:1733090; http://dx.doi.org/ 10.1097/00007890-199201000-00016. [DOI] [PubMed] [Google Scholar]
- 73.Ott MG, Marme F, Moldenhauer G, Lindhofer H, Hennig M, Spannagl R, Essing MM, Linke R, Seimetz D. Humoral response to catumaxomab correlates with clinical outcome: results of the pivotal phase II/III study in patients with malignant ascites. Int J Cancer 2012; 130: 2195-2203; PMID:21702044; http://dx.doi.org/ 10.1002/ijc.26258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mirick GR, Bradt BM, DeNardo SJ, DeNardo GL. A review of human anti-globulin antibody (HAGA, HAMA, HACA, HAHA) responses to monoclonal antibodies. Not four letter words. Q J Nucl Med Mol Imaging 2004; 48: 251-257; PMID:15640788. [PubMed] [Google Scholar]
- 75.DeNardo GL, Bradt BM, Mirick GR, DeNardo S. Human antiglobulin response to foreign antibodies: therapeutic benefit? Cancer Immunol Immunother 2003; 52: 309-316; PMID:12700946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brezski RJ, Knight DM, Jordan RE. The origins, specificity, and potential biological relevance of human anti-IgG hinge autoantibodies. Sci World J 2011; 11: 1153-1167; PMID:21623461; http://dx.doi.org/ 10.1100/tsw.2011.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brezski RJ, Luongo JL, Petrone D, Ryan MH, Zhong D, Tam SH, Schmidt AP, Kruszynski M, Whitaker BP, Knight DM, Jordan RE. Human anti-IgG1 hinge autoantibodies reconstitute the effector functions of proteolytically inactivated IgGs. J Immunol 2008; 181: 3183-3192; PMID:18713989; http://dx.doi.org/ 10.4049/jimmunol.181.5.3183. [DOI] [PubMed] [Google Scholar]
- 78.Osterland CK, Harboe M, Kunkel HG. Anti-gamma-globulin factors in human sera revealed by enzymatic splitting of anti-Rh antibodies. Vox Sang 1963; 8: 133-152; PMID:13940587; http://dx.doi.org/ 10.1111/j.1423-0410.1963.tb03290.x. [DOI] [PubMed] [Google Scholar]
- 79.Mellbye OJ, Natvig JB. Evidence for immune complexes containing antibody to the pepsin site of IgG in rheumatoid synovial fluids. Clin Exp Immunol 1971; 8: 889-899; PMID:4933318. [PMC free article] [PubMed] [Google Scholar]
- 80.Terness P, Kohl I, Hubener G, Battistutta R, Moroder L, Welschof M, Dufter C, Finger M, Hain C, Jung M. The natural human IgG anti-F(ab')2 antibody recognizes a conformational IgG1 hinge epitope. J Immunol 1995; 154: 6446-6452; PMID:7539020. [PubMed] [Google Scholar]
- 81.Brezski RJ, Jordan RE. Cleavage of IgGs by proteases associated with invasive diseases: an evasion tactic against host immunity?. MAbs 2010; 2: 212-220; PMID:20400859; http://dx.doi.org/ 10.4161/mabs.2.3.11780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brezski RJ, Kinder M, Grugan KD, Soring KL, Carton J, Greenplate AR, Petley T, Capaldi D, Brosnan K, Emmell E, et al.. A monoclonal antibody against hinge-cleaved IgG restores effector function to proteolytically-inactivated IgGs in vitro and in vivo. MAbs 2014; 6: 1265-1273; PMID:25517311; http://dx.doi.org/ 10.4161/mabs.29825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van de Stadt LA, de VH, Derksen NI, Brouwer M, Wouters D, van SD, Wolbink G, Rispens T. Antibodies to IgG4 hinge can be found in rheumatoid arthritis patients during all stages of disease and may exacerbate chronic antibody-mediated inflammation. Arthritis Rheumatol 2014; 66: 1133-1140; PMID:24782178; http://dx.doi.org/ 10.1002/art.38335. [DOI] [PubMed] [Google Scholar]
- 84.Schellekens H, Hennink WE, Brinks V. The immunogenicity of polyethylene glycol: facts and fiction. Pharm Res 2013; 30: 1729-1734; PMID:23673554; http://dx.doi.org/ 10.1007/s11095-013-1067-7. [DOI] [PubMed] [Google Scholar]
- 85.Verhoef JJ, Carpenter JF, Anchordoquy TJ, Schellekens H. Potential induction of anti-PEG antibodies and complement activation toward PEGylated therapeutics. Drug Discov Today 2014; 19: 1945-1952; PMID:25205349; http://dx.doi.org/ 10.1016/j.drudis.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 86.Dery JP, Braden GA, Lincoff AM, Kereiakes DJ, Browne K, Little T, George BS, Sane DC, Cines DB, Effron MB, Mascelli MA, Langrall MA, Damaraju L, Barnathan ES, Tcheng JE. Final results of the ReoPro readministration registry. Am J Cardiol 2004; 93: 979-984; PMID:15081439; http://dx.doi.org/ 10.1016/j.amjcard.2003.12.051. [DOI] [PubMed] [Google Scholar]
- 87.Lajus S, Clofent-Sanchez G, Jais C, Coste P, Nurden P, Nurden A. Thrombocytopenia after abciximab use results from different mechanisms. Thromb Haemost 2010; 103: 651-661; PMID:20076853; http://dx.doi.org/ 10.1160/TH09-08-0603. [DOI] [PubMed] [Google Scholar]
- 88.Ben-Horin S, Yavzori M, Katz L, Kopylov U, Picard O, Fudim E, Coscas D, Bar-Meir S, Goldstein I, Chowers Y. The immunogenic part of infliximab is the F(ab')2, but measuring antibodies to the intact infliximab molecule is more clinically useful. Gut 2011; 60: 41-48; PMID:20519742; http://dx.doi.org/ 10.1136/gut.2009.201533. [DOI] [PubMed] [Google Scholar]
- 89.Steenholdt C, Palarasah Y, Bendtzen K, Teisner A, Brynskov J, Teisner B, Nielsen CH. Pre-existing IgG antibodies cross-reacting with the Fab region of infliximab predict efficacy and safety of infliximab therapy in inflammatory bowel disease. Aliment Pharmacol Ther 2013; 37: 1172-1183; PMID:23650912; http://dx.doi.org/ 10.1111/apt.12330. [DOI] [PubMed] [Google Scholar]
- 90.Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol 2014; 5: 520; PMID:25368619; http://dx.doi.org/ 10.3389/fimmu.2014.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lammerts van Bueren JJ, Rispens T, Verploegen S, Palen-Merkus T, Stapel S, Workman LJ, James H, van Berkel PH, van de Winkel JG, Platts-Mills TA, Parren PW. Anti-galactose-α-1,3-galactose IgE from allergic patients does not bind α-galactosylated glycans on intact therapeutic antibody Fc domains. Nat Biotechnol 2011; 29: 574-576; PMID:21747378; http://dx.doi.org/ 10.1038/nbt.1912. [DOI] [PubMed] [Google Scholar]
- 92.Galili U. The α-gal epitope and the anti-Gal antibody in xenotransplantation and in cancer immunotherapy. Immunol Cell Biol 2005; 83: 674-686; PMID:16266320; http://dx.doi.org/ 10.1111/j.1440-1711.2005.01366.x. [DOI] [PubMed] [Google Scholar]
- 93.Galili U, Rachmilewitz EA, Peleg A, Flechner I. A unique natural human IgG antibody with anti-α-galactosyl specificity. J Exp Med 1984; 160: 1519-1531; PMID:6491603; http://dx.doi.org/ 10.1084/jem.160.5.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rispens T, Derksen NI, Commins SP, Platts-Mills TA, Aalberse RC. IgE production to α-gal is accompanied by elevated levels of specific IgG1 antibodies and low amounts of IgE to blood group B. PLoS ONE 2013; 8: e55566; PMID:23390540; http://dx.doi.org/ 10.1371/journal.pone.0055566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qian J, Liu T, Yang L, Daus A, Crowley R, Zhou Q. Structural characterization of N-linked oligosaccharides on monoclonal antibody cetuximab by the combination of orthogonal matrix-assisted laser desorption/ionization hybrid quadrupole-quadrupole time-of-flight tandem mass spectrometry and sequential enzymatic digestion. Anal Biochem 2007; 364: 8-18; PMID:17362871; http://dx.doi.org/ 10.1016/j.ab.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 96.Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, Murphy BA, Satinover SM, Hosen J, Mauro D, et al.. Cetuximab-induced anaphylaxis and IgE specific for galactose-α-1,3-galactose. N Engl J Med 2008; 358: 1109-1117; PMID:18337601; http://dx.doi.org/ 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Commins SP, James HR, Kelly LA, Pochan SL, Workman LJ, Perzanowski MS, Kocan KM, Fahy JV, Nganga LW, Ronmark E, et al.. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-α-1,3-galactose. J Allergy Clin Immunol 2011; 127: 1286-1293; PMID:21453959; http://dx.doi.org/ 10.1016/j.jaci.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nezlin R. Interactions between immunoglobulin G molecules. Immunol lett 2010; 132(1-2):1-5; PMID:20600325. [DOI] [PubMed] [Google Scholar]
- 99.Butler JE, Ni L, Brown WR, Joshi KS, Chang J, Rosenberg B, Voss EW Jr. The immunochemistry of sandwich ELISAs–VI. Greater than 90% of monoclonal and 75% of polyclonal anti-fluorescyl capture antibodies (CAbs) are denatured by passive adsorption. Mol Immunol 1993; 30: 1165-1175; PMID:8413321; http://dx.doi.org/ 10.1016/0161-5890(93)90135-X. [DOI] [PubMed] [Google Scholar]
- 100.Qian W, Yao D, Yu F, Xu B, Zhou R, Bao X, Lu Z. Immobilization of antibodies on ultraflat polystyrene surfaces. Clin Chem 2000; 46: 1456-1463; PMID:10973890. [PubMed] [Google Scholar]
- 101.Rispens T, Ooievaar-De HP, Vermeulen E, Schuurman J, van der Neut KM, Aalberse RC. Human IgG4 binds to IgG4 and conformationally altered IgG1 via Fc-Fc interactions. J Immunol 2009; 182: 4275-4281; PMID:19299726; http://dx.doi.org/ 10.4049/jimmunol.0804338. [DOI] [PubMed] [Google Scholar]
- 102.Rispens T, Meesters J, den Bleker TH, Ooijevaar-de HP, Schuurman J, Parren PW, Labrijn A, Aalberse RC. Fc-Fc interactions of human IgG4 require dissociation of heavy chains and are formed predominantly by the intra-chain hinge isomer. Mol Immunol 2013; 53: 35-42. [DOI] [PubMed] [Google Scholar]
- 103.Rispens T, Davies AM, Ooijevaar-de HP, Absalah S, Bende O, Sutton BJ, Vidarsson G, Aalberse RC. Dynamics of inter-heavy chain interactions in human immunoglobulin G (IgG) subclasses studied by kinetic Fab arm exchange. J Biol Chem 2014; 289: 6098-6109; PMID:24425871; http://dx.doi.org/ 10.1074/jbc.M113.541813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ito T, Kitahara K, Umemura T, Ota M, Shimozuru Y, Kawa S, Bahram S. A novel heterophilic antibody interaction involves IgG4. Scand J Immunol 2010; 71: 109-114; PMID:20384862; http://dx.doi.org/ 10.1111/j.1365-3083.2009.02353.x. [DOI] [PubMed] [Google Scholar]
- 105.Zack DJ, Stempniak M, Wong AL, Weisbart RH. Localization of an Fc-binding reactivity to the constant region of human IgG4. Implications for the pathogenesis of rheumatoid arthritis. J Immunol 1995; 155: 5057-5063; PMID:7594514. [PubMed] [Google Scholar]
- 106.Ewert S, Huber T, Honegger A, Pluckthun A. Biophysical properties of human antibody variable domains. J Mol Biol 2003; 325: 531-553; PMID:12498801; http://dx.doi.org/ 10.1016/S0022-2836(02)01237-8. [DOI] [PubMed] [Google Scholar]
- 107.McMahon MJ, O'Kennedy R. Polyreactivity as an acquired artefact, rather than a physiologic property, of antibodies: evidence that monoreactive antibodies may gain the ability to bind to multiple antigens after exposure to low pH. J Immunol Methods 2000; 241: 1-10; PMID:10915844; http://dx.doi.org/ 10.1016/S0022-1759(00)00196-4. [DOI] [PubMed] [Google Scholar]
- 108.Diebolder CA, Beurskens FJ, de Jong RN, Koning RI, Strumane K, Lindorfer MA, Voorhorst M, Ugurlar D, Rosati S, Heck AJ, et al.. Complement is activated by IgG hexamers assembled at the cell surface. Science 2014; 343: 1260-1263; PMID:24626930; http://dx.doi.org/ 10.1126/science.1248943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.An Z, Forrest G, Moore R, Cukan M, Haytko P, Huang L, Vitelli S, Zhao JZ, Lu P, Hua J, et al.. IgG2m4, an engineered antibody isotype with reduced Fc function. MAbs 2009; 1: 572-579; PMID:20073128; http://dx.doi.org/ 10.4161/mabs.1.6.10185. [DOI] [PMC free article] [PubMed] [Google Scholar]


