Figure 1.
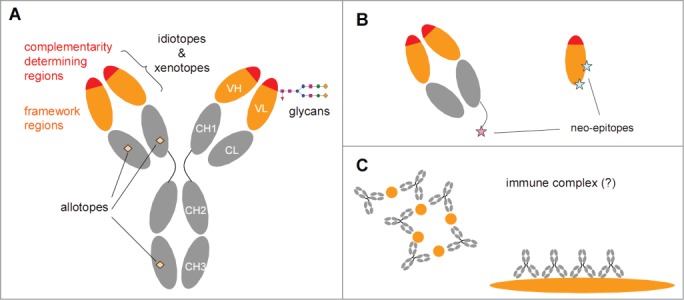
Potentially immunogenic determinants of antibodies. (A) An IgG antibody consists of variable domains (orange/red) and constant domains (gray). Chimeric, humanized and human therapeutic antibodies all contain human constant domains that may carry allotypic determinants. The variable domains can be subdivided in complementarity determining regions (CDRs) that are primarily involved in antigen binding and are highly variable, and framework regions (FRs) that are much less variable. The variation is partially germ-line encoded, partially the result of junctional variation and partially the result of somatic hypermutation. In chimeric antibodies the framework regions are of murine origin, whereas in humanized and human antibodies the framework regions will be largely human. Both CDRs and FRs can contain clone-specific determinants (idiotopes). Furthermore, in case of chimeric antibodies, the FRs may also contain murine-specific determinants (xenotopes). The variable domains may also express N-glycosylation sites that can contain non-human glycans depending on the expression system used to produce the therapeutic antibody. (B) Antibody fragments such as Fab (left) or single VH domains (right) will have regions exposed that are not exposed in an intact IgG antibody that can serve as neo-epitopes. In particular, anti-hinge antibodies can bind to a truncated hinge but will not bind intact IgG. (C) The antigenic trigger for rheumatoid factors is not known, but they might form in response to IgG-containing immune complexes.
