Abstract
PRIMA-1Met has shown promising preclinical activity in various cancer types. However, its effect on Waldenström's Macroglobulinemia (WM) cells as well as its exact mechanism of action is still elusive. In this study, we evaluated the anti- tumor activity of PRIMA-1Met alone and in combination with dexamethasone or bortezomib in WM cell lines and primary samples. Treatment of WM cells with PRIMA-1Met resulted in induction of apoptosis, inhibition of migration and suppression of colony formation. Upon PRIMA-1Met treatment, p73 was upregulated and Bcl-xL was down-regulated while no significant change in expression of p53 was observed. Furthermore, siRNA knockdown of p53 in WM cell line did not influence the PRIMA-1Met-induced apoptotic response whereas silencing of p73 inhibited latter response in WM cells. Importantly, combined treatment of BCWM-1 cells with PRIMA-1Met and dexamethasone or bortezomib induced synergistic reduction in cell survival. Our study provides insights into the mechanisms of anti-WM activity of PRIMA-1Met and supports further clinical evaluation of PRIMA-1Met as a potential novel therapeutic intervention in WM.
Keywords: dexamethasone or bortezomib, PRIMA-1Met, apoptosis, p53, p73, Waldenström's Macroglobulinemia
Abbreviations
- WM
(Waldenström's Macroglobulinemia)
- MM
(Multiple Myeloma )
- PRIMA-1Met
methylated P53 Reactivation and Induction of Massive Apoptosis
- AML
acute myeloid Leukemia
- CLL
chronic lymphocytic leukemia
- PBMCs
peripheral blood mononuclear cells
- BMMCs
bone marrow mononuclear cells
Introduction
Waldenström Macroglobulinemia (WM) is a low grade lymphoplasmacytic lymphoma characterized by infiltration of bone marrow with malignant B cells and IgM monoclonal gammopathy.1 Reports show an increase in the incidence of WM over the past 20 y. In the United States, almost 1500 new cases of WM are reported annually.3 WM patients are quite heterogeneous with respect to clinical presentation, varying from asymptomatic to highly aggressive cases, and their responses to treatment. Given current therapies, WM remains incurable, and most patients eventually relapse.4
P53 is a well-known tumor suppressor protein responding to cellular stress through regulating cell cycle, activating DNA damage repair mechanism and inducing senescence and apoptosis.5,6 At steady state, p53 level is kept substantially low through a tight feedback regulation by negative regulators such as MDM2.7 Over the past decade, more than 150 trials exploiting p53 have been conducted taking advantage of its pro-apoptotic effects on tumor cells.7 PRIMA-1Met is a small molecule initially identified as an activator of mutant p53 in a cellular screening of a small molecular library.8 PRIMA-1Met has shown promising results in in-vitro and in xenograft models of several solid tumors such as breast, hepatic and colon cancer as well as haematological malignancies closely related to WM such as CLL.9-12 A recent phase I/II clinical trial of PRIMA-1Met in prostate cancer and AML also demonstrated promising results in terms of toxicity and general tolerance, making it a good candidate for further exploration in other neoplasias.13 Although initially thought to act through inducing apoptosis by restoring the wild type conformation to mutant p53,14 recent evidence points toward PRIMA-1Met's ability to induce apoptosis irrespective of p53 status or even in a p53-independent manner; therefore, the exact pathway affected by PRIMA-1Met is highly controversial and seems to be cell type specific.15-18
To date, the effects of PRIMA-1Met in WM have not been explored at either preclinical or clinical levels. The purpose of the current study is to examine the anti-tumor effects of PRIMA-1Met in WM cells and explore the underlying mechanism.
Results
PRIMA-1Met inhibits growth and induces apoptosis in-vitro in WM cells
PRIMA-1Met has shown cytotoxic effects on CLL and MM, 2 hematological cancers closely related to WM.15,18 To evaluate the effects of PRIMA-1Met on WM cells, we selected the only 2 existing WM cell lines, BCWM-1 (wild type p53) and MWCL-1 (Mutant p53). Both cell lines exhibited a gradual decline in cell viability in response to increasing doses of PRIMA-1Met with almost similar IC50 values of 21μM and 27.6μM respectively (Fig. 1).These values are in the range that was previously reported by our lab to be non-toxic to PBMCs and BMMCs.18 To confirm the anti-WM potential of PRIMA-1Met, primary cells derived from 2 previously untreated WM patients with more than 90% bone marrow involvement were treated with DMSO control or increasing doses of PRIMA-1Met for 48 h. Cells were then examined for viability by MTT assay. A significant decrease in the viability of WM primary cells was observed with similar or even lower IC50 values as were observed in the cell lines (Fig. 1).To explore whether this reduction in cell survival in WM cells was due to apoptosis, we performed Annexin V/PI staining to measure the percentage of apoptotic cells. PRIMA-1Met (25μM) induced more than 50% apoptosis in BCWM-1 cells which is in complete accordance with the results obtained from cell survival assay (Fig. 2).
Figure 1.
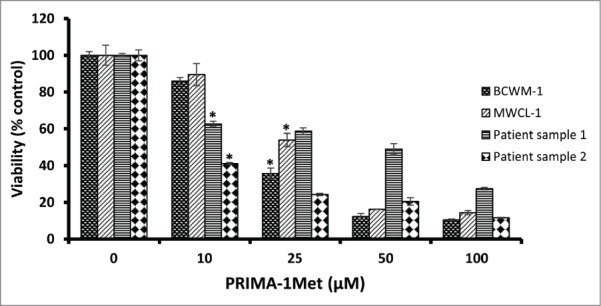
The effect of PRIMA-1Met on WM cell lines and patient samples. The growth suppressing effect of different concentrations of PRIMA-1Met in BCWM-1 (IC50 = 21µM), MWCL-1 (IC50 = 27.6), Patient sample 1 (IC50 = 10), Patient sample 2 (IC50 = 30) was studied using MTT assay after 48 h incubation; n = 3, error bars show SEM, * P = <0.05
Figure 2.
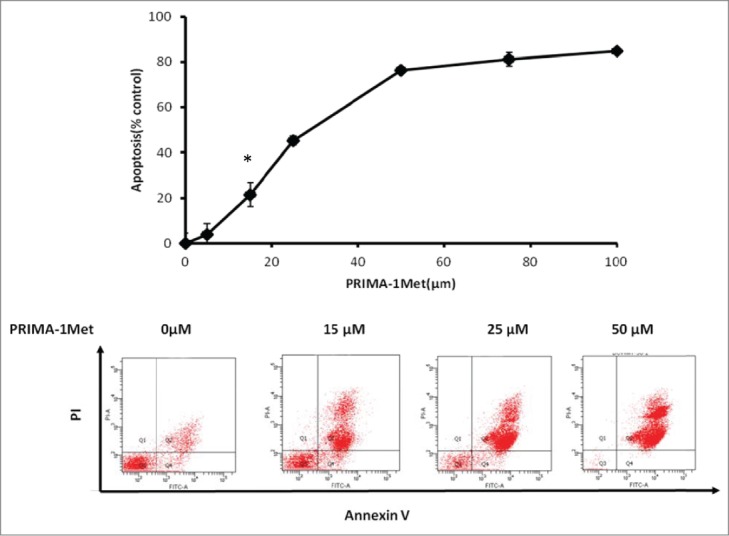
The apoptotic effect of PRIMA-1Met in BCWM-1 (wild type P53). The apoptotic effect of different concentrations of PRIMA-1Met in BCWM-1 was studied using Annexin-V/PI flow cytometry after 48 h incubation; n = 3, error bars show SEM, * P = <0.05
PRIMA-1Met inhibits colony formation and migration in BCWM-1 cells
Having shown the effect of PRIMA-1Met on viability and apoptosis, we next examined the effects of PRIMA-1Met on WM cells' migration and colony formation. PRIMA-1Met significantly inhibited colony formation in BCWM-1 cells in a dose-dependent manner (Fig. 3A, P < 0.005). The number of migrated BCWM-1 cells treated with increasing doses of PRIMA-1Met also significantly decreased compared with DMSO-treated control (Fig. 3B, P < 0.005). This difference was not due to cytotoxicity as the treated cells were found to be viable when stained with Trypan blue at the time of counting. These findings suggest that PRIMA-1Met suppressed the clonogenic and migratory potential of WM cells.
Figure 3.
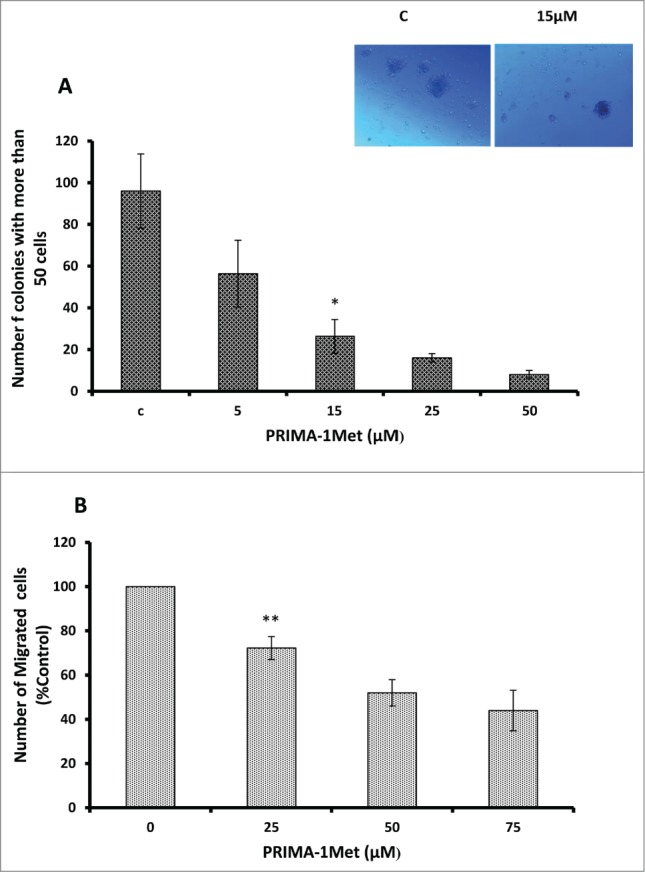
Anti-tumor activities of PRIMA-1Met in WM cells. (A) Dose dependent decrease in BCWM-1 colony formation abilities was measured by colony assay after 7 d. (B) Dose dependent decrease in BCWM-1 cell migratory abilities was measured by Boyden chamber assay after 8 h of incubation. Error bars = SEM, * P = <0.05,** P = <0.01.
PRIMA-1Met enhances the cytotoxicity of conventional and novel WM therapies
To examine the effect of PRIMA-1Met in combination with novel or conventional chemotherapeutic agents, we treated BCWM-1 cells with combinations of PRIMA-1Met with either dexamethasone or bortezomib. The cytotoxic effects of the drugs on the cells was analyzed by MTT assay. As shown in Figure 5, simultaneous treatment of BCWM-1 cell line with PRIMA-1Met and dexamethasone/bortezomib resulted in a significant decrease in cell survival as compared to the single agents (P < 0.005) after 72h treatment (Figs. 4A and B). When combined with low concentrations of these drugs, synergistic effects were observed (CI < 1.0) (Figs. 4A and B).
Figure 5.

The effect of PRIMA-1Met on BCWM-1 cells. Total levels of the indicated proteins were evaluated by Western blot analysis in BCWM-1 cells after treatment with 50 µM PRIMA-1Met at the displayed time points.
Figure 4.
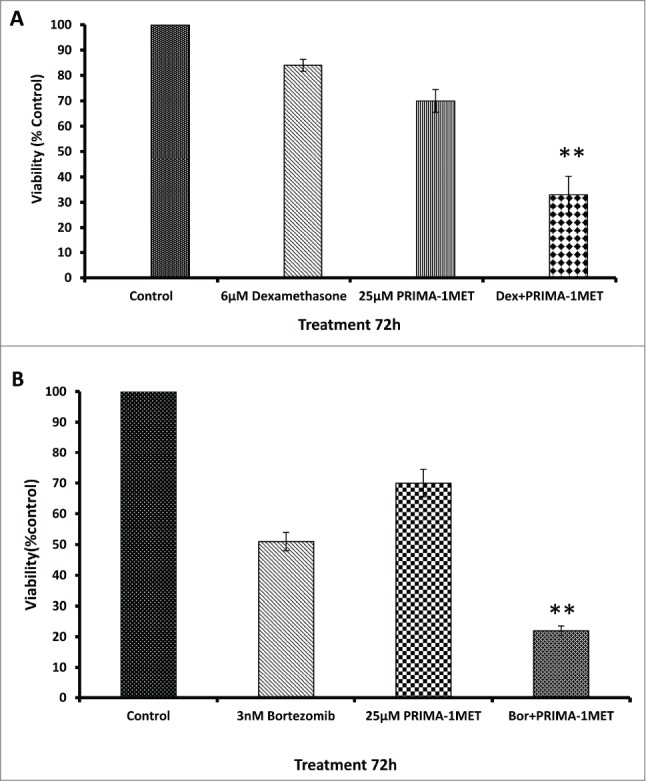
Effects of PRIMA-1Met in combination with current WM therapeutics. (A) Synergism was assessed by combination index (CI) analysis for dexamethasone and RIMA-1 after 72hrs, CI = 0.63. (B)RIMA-1met has synergistic effects with bortezomib velcade on BCWM-1 cells, 72hrs, CI = 0.85. Error bars = SEM, ** P = <0.01
PRIMA-1Met exerts its cytotoxic effect through a p73-dependent mechanism by modulation of Bcl2 family of proteins
Considering the fact that p53 signaling pathway was reported to mediate PRIMA-1Met-induced cytotoxic effects,9,14,19 we assessed the expression of p53 and its downstream targets through western blot analysis. Results showed activation of PARP in PRIMA-1Met-treated BCWM-1cells. However, expression of p53 and its transcriptionally regulated downstream targets such as MDM2 was not significantly affected (Fig. 5). To further examine the involvement of p53 in PRIMA-1Met cytotoxicity in WM cells, we suppressed p53 expression by siRNA and confirmed the knockdown through both Western Blot (Fig. 6A) and q-PCR analysis (Data not shown). In p53- silenced WM cells, PRIMA-1Met was still able to reduce the cell survival judged by an MTT assay (Fig. 6B). These results suggest that p53 does not have a direct role in PRIMA-1Met-induced apoptosis of WM cells. Due to the functional and structural similarity between members of p53 super family,20 we next investigated the status of p73 by protein gel blot analysis. BCWM-1 cells treated with PRIMA-1Met exhibited time-dependent activation of p73 (Fig 5). Furthermore, PRIMA-1Met decreased the expression of anti-apoptotic protein Bcl-xL and increased the level of cleaved caspase-9 (Fig 5). These results indicate that PRIMA-1Met-induced apoptosis in WM cells is associated with mitochondrial/intrinsic pathway of apoptosis. Finally, to confirm the role of p73 in PRIMA-1Met-induced cell death, we silenced p73 gene using siRNA. Knockdown was confirmed through q-PCR analysis using p73-specific primer (Data not shown), followed by Western Blot analysis and quantification of the knockdown (Fig. 7A). P73 knocked-down cells were unable to undergo cell death in response to PRIMA-1Met treatment as much as the scrambled RNA-treated cells did (Fig. 7B), suggesting at least partial dependency of PRIMA-1Met on p73 to exert its cytotoxic effects.
Figure 6.
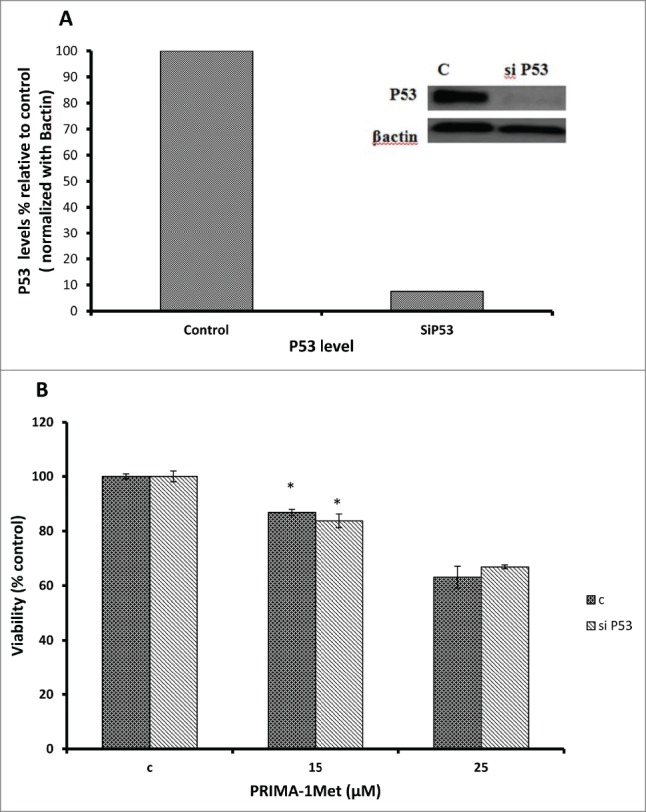
PRIMA-1Met cytotoxicity is p53 independent. (A) The efficiency of p53 knockdown by siRNA in BCWM.1 cells was confirmed by protein gel blot using β-actin as a loading control. (B) PRIMA-1Met reduces the cell survival measured by MTT assay in P53-silenced cells to the same extend as the scrambled si-RNA knocked-down control. Error bars = SEM, * P = <0.05
Figure 7.
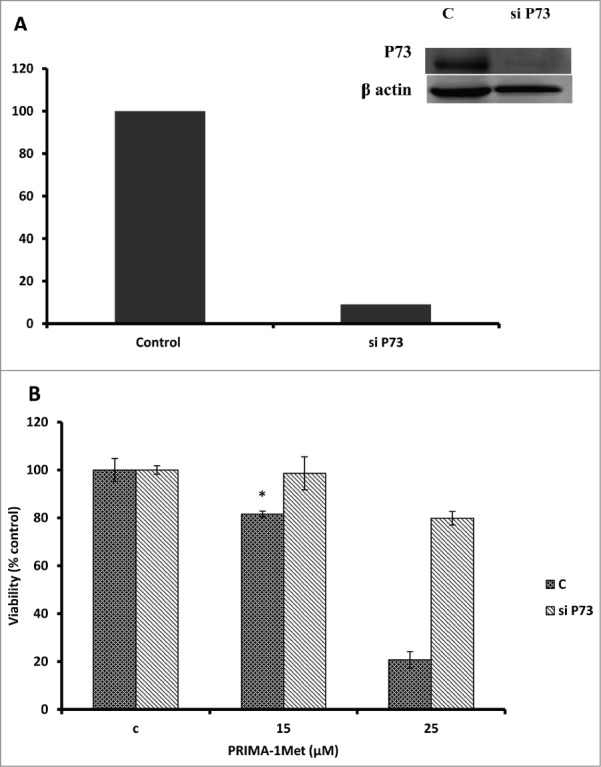
PRIMA-1Met cytotoxicity is P73 dependent. (A) The efficiency of p73 knockdown by siRNA in BCWM.1 cells was confirmed by western blot using β-actin as a loading control. (B)- PRIMA-1Met was unable to reduce the cell survival measured by MTT assay in p73-silenced cells as much as scrambled control. Error bars = SEM, * P = <0.05.
Discussion
In this report for the first time we demonstrated the anti-tumor activity of PRIMA-1Met in WM cell lines and patient samples, and treatment of BCWM-1 cells with PRIMA-1Met resulted in significant inhibition of viability associated with apoptosis induction. PRIMA-1Met also inhibited colony formation and migration of WM cells in a dose-dependent manner. These observations pinpoint the potential antiproliferative and apoptotic effects of PRIMA-1Met on WM cells. It also prompts us to speculate that it may antagonize WM cells viability and migration in the context of bone marrow microenvironment which is known to play an important role in WM pathogenesis.21 Importantly, similar effects of PRIMA-1Met have also been observed in other tumor cell types.10,22,23
We found that PRIMA-1Met-induced apoptosis in BCWM-1 cells was associated with downregulation of Bcl-xL and cleavage of caspase 9 but not caspase 8 (Data not shown), implying the activation of intrinsic/mitochondrial pathway of apoptosis. These findings are in accordance with previous reports in breast cancer and melanoma cells treated with PRIMA-1Met.10,11,16 Although PRIMA-1Met was initially discovered as a p53 reactivating agent,14 further studies especially in hematological malignancies could not confirm the role of p53 in PRIMA-1Met-induced apoptosis.15-18 Our initial western blot analysis did not show any significant change in p53 level after PRIMA-1Met treatment. Furthermore, selective knockdown of p53 did not influence PRIMA-1Met-induced apoptosis of WM cells implying a probable p53-independent mechanism. Additionally, the same p53-independent effects of PRIMA-1Met was reported by our group in MM and by others in AML, CLL and prostate cancer cell lines.18,15,25 Interestingly, PRIMA-1Met treatment of WM cells resulted in activation of p73, another member of p53 super family which shares structural and functional similarities with p53.20 p73 is a well-known tumor suppressor which due to its non-mutated state in most cancers has attracted much attention as a potential drug target. Our knock-down study also demonstrated that p73-silenced cells did not undergo apoptosis in response to PRIMA-1Met treatment supporting the possible role of p73 in PRIMA-1Met-induced apoptosis. The latter results are consistent with the findings in our previous study in multiple myeloma.18 It should be noted that other possible mediators of PRIMA-1Met effects in WM couldn't be ruled out, especially in light of recent findings highlighting the significance of ROS production in PRIMA-1Met-induced cell death;26 thus it would be interesting to analyze the oxidative stress pathways in PRIMA-1Met-treated WM cells in future studies.
Moreover, we found down-regulation of anti-apoptotic marker Bcl-xL in WM cells following PRIMA-1Met treatment. This finding together with above-mentioned cleavage of caspase 9 imply that mitochondrial/intrinsic pathway of apoptosis may be involved in PRIMA-1Met-induced apoptosis in WM cells. In fact, involvement of latter pathway in PRIMA-1Met-induced cell death has been indicated in lung cancer and MM cells.9,18 Nonetheless, further investigation is required to decipher the mechanism of PRIMA-1Met-induced apoptosis in WM cells.
Finally, we showed that PRIMA-1Met-induced cell death could be synergistically enhanced in combination with dexamethasone or bortezomib. It is interesting to note that both agents are known to inhibit NF-kB which in turn inhibits p53 super family,27,28 denoting a possible mechanism underlying the synergistic effects of PRIMA-1Met in combination with dexamethasone or bortezomib.
Taken all together, our findings indicate that treatment of WM cells with PRIMA-1Met leads to induction of p73-mediated, p53-independent apoptosis by downregulation of Bcl-xL and possibly through the intrinsic pathway of apoptosis. Our study provides the rationale for further investigation of PRIMA-1Met as a novel therapeutic agent to improve the outcome of patients with WM.
Materials and Methods
Patient samples and cell lines
Bone marrow samples were collected from WM patients during routine diagnostic procedures. This study received written approval from the University Health Network Research Ethics Board, Toronto, in accordance with the Declaration of Helsinki. WM cell line, BCWM-129, was kindly provided by Dr. Treon's lab. MWCL−1 cells were kindly provided by Dr. Ansell's lab.30 Both cell lines were maintained in standard culture medium RPMI 1640 medium containing 10% fetal bovine serum, 2 mM L-glutamine, 50U/ml penicillin, and 50 µg/mL streptomycin at 37°C in a 5% CO2 incubator. Freshly isolated primary WM cells separated by Ficoll Hypaque (Sigma Aldrich, St. Louis, MO, USA) were re-suspended in above culture medium and incubated at 37°C in a 5% CO2 incubator.
Drug treatment
PRIMA-1Met was purchased from Cayman Chemical and dissolved in dimethyl sulfoxide (DMSO) to make a 10 mM stock solution and stored at −20°C. In each experiment, the final DMSO concentration was kept constant and did not exceed 0.05% (v/v). In some experiments, cells were simultaneously exposed to PRIMA-1Met and dexamethasone (Cayman Chemical, Ann Arbor, MI, USA) or bortezomib (Orthobiotech, Horsham, PA, USA). After drug treatment, cells were harvested and subjected to further analysis as described below.
Cell viability, apoptosis, colony formation and migration assays
Cell viability was assessed by MTT ((3-[4,5-dimethilthiazol-2yl]-2,5-diphenyl tetrazolium bromide)). Briefly, cells were cultured in 96-well micro-titer plates with different concentrations of the drugs for 48 h. To assess the effect of PRIMA-1Met on cell viability and proliferation of primary samples, 2 × 104 cells/100 µl were cultured in 96-well plates and then treated with the drugs for 48 h. After incubation, MTT (0.5 mg/ml) was added and the cells were further incubated for an additional 4 h. This was followed by the addition of acidified isopropanol to the wells and overnight incubation at 37°C to solubilize the dye crystals. Following incubation, the optical density of the wells was read with a microplate reader set at a test wavelength of 570 nm and a reference wavelength of 630 nm.
To examine apoptotic cell death, WM cells were treated with various concentrations of PRIMA-1Met and stained with Annexin V-FITC (Abcam, MA, USA) and propidium iodide (Sigma-Aldrich, St. Louis, MO, USA) for flow cytometric analysis. Data were analyzed using FlowJo software. The extent of apoptosis was quantified as percentage of Annexin-V positive cells. All the readouts were from triplicate measurements of at least 2 experiments.
For colony formation assays, WM cells (5 × 104 cells/mL) were plated into 6-well plates in 1 mL RPMI medium (20% FBS) containing 1% methylcellulose and maintained with DMSO control or the indicated concentration of PRIMA-1Met. Seven days after plating, the total number of colonies was calculated and enumerated by morphologic assessment, as previously described.31
Migration assays were conducted in triplicate with 24-well Transwell insert chambers (8 µm insert; Costar, Corning Inc., Corning, NY, USA) according to the manufacturer's instruction. In brief, WM cells (5 × 104 cells/mL) in RPMI medium without FBS were added to the upper chamber in the presence or absence of PRIMA-1Met at the indicated concentrations and allowed to migrate for 8 h to the lower chamber containing RPMI medium with 20% FBS at 37°C. The migration of control DMSO-treated cells on the Transwell was normalized to 100%.
Immunoblotting
Western blot analysis was performed to evaluate several protein targets in whole cell lysates obtained from the cells treated with PRIMA-1Met in the absence or presence of siRNAs. Whole cell lysates were prepared by lysing the cell pellets for 10 min on ice in a buffer composed of 150 mM NaCl, 50 mM Tris-HCl (pH 8.0), 5 mM EDTA, 1% (v/v) Nonidet P-40, 1 mM phenylmethylsulfonyl fluoride (PMSF), 20 µg/ml aprotinin and 25 µg/ml leupeptin. Protein concentrations were measured by using a Nano Drop 1000 spectrophotometer (ThermoFisher Scientific Inc.., San Diego, CA, USA). Equal amounts of protein were resolved using 12% SDS-polyacrylamide gel electrophoresis and transferred to a polyvinylidene diflouride (PVDF) membrane (Perkin Elmer Inc., Waltham, MA, USA). After blocking for 1 h at room temperature with PBS containing 3% bovine serum albumin (BSA), the membrane was incubated with specific antibodies for at least 1 h at room temperature or overnight at 4°C. After washing, the membrane was incubated with a horseradish peroxidase (HRP)-labeled secondary antibody for 1 h at room temperature and the blots were developed using a chemiluminescent detection system (ECL, Perkin Elmer). Primary antibodies were from the following manufacturers: Santa Cruz Biotechnology (Santa Cruz, CA,USA): MDM2, P73 (H-79) and β-actin; Biolegend (San Diego, CA, USA): p53 (DO-7); Roche (Manheim, Germany): PARP. Goat anti-mouse and anti-rabbit secondary antibodies conjugated to horseradish peroxidase were purchased from Cell Signaling Technology (Beverly, MA, USA).
Knockdown of selective target genes
BCWM-1 cells were transfected with target specific siRNAs for p53 (Invitrogen, Carslbad, CA, USA) or p73 (Invitrogen) or control scrambled siRNA (Invitrogen) using the Cell Line Solution Kit V (Amaxa, GmbH, Cologne, Germany) according to the manufacturer's instruction with the Amaxa Nucleofector II device (Amaxa) Program T-030. Following transfection, cells were treated with PRIMA-1Met and analyzed for its effect on cell viability by MTT assay.
Statistical analysis
The synergistic effect [combination index (CI) <1.0] of the combination of PRIMA-1Met and Dexmethasone or bortezomib was analyzed using the Chou-Talalay method. The dose-effect curve for each drug alone was determined based on the experimental observations using the median-effect principle; the combination index (CI) for each experimental combination was then calculated according to the following equation: CI = (D)1/(Dx)1 + (D)2/(Dx)2 + (D)1(D)2/(Dx)1(Dx)2, where (D) are the doses of drug 1 and drug 2 that have × effect when used in combination and (D)1 and (D) are the doses of drug 1 and drug 2 that have the same × effect when used alone. The combination is additive when CI = 1, synergistic when CI <1.0, and antagonistic when CI > 1.0.Statistical significance levels were determined by 2-tailed t-test analysis. p values of < 0.05 were considered significant.32
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported in part by research grants from Leukemia & Lymphoma Society of Canada (LLSC), Cancer Research Society (CRS). MS is the recipient of the Canada Graduate Scholarships Master's Awards, Frederick Banting and Charles Best Canada Graduate Scholarships.
References
- 1.Ansell SM, Kyle RA, Reeder CB, Fonseca R, Mikhael JR, Morice WG, Bergsagel PL, Buadi FK, Colgan JP, Dingli D, et al.. Diagnosis and Management of Waldenstr?m Macroglobulinemia: Mayo Stratification of Macroglobulinemia and Risk-Adapted Therapy (mSMART) Guidelines. Mayo Clin Proc 2010; 85:824-833; PMID:20702770; http://dx.doi.org/ 10.4065/mcp.2010.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gertz MA. Waldenström macroglobulinemia: 2012 update on diagnosis, risk stratification, and management. Am J Hematol 2012; 87:503-510; PMID:22508368; http://dx.doi.org/ 10.1002/ajh.23192. [DOI] [PubMed] [Google Scholar]
- 3.Shaheen SP, Talwalkar SS, Lin P, Medeiros LJ. Waldenström macroglobulinemia: a review of the entity and its differential diagnosis. Adv Anat Pathol 2012; 19:11-27; PMID:22156831; http://dx.doi.org/ 10.1097/PAP.0b013e31824019d0. [DOI] [PubMed] [Google Scholar]
- 4.Gertz M. Waldenström macroglobulinemia: my way. Leuk. Lymphoma 2013; 54:464-471; http://dx.doi.org/ 10.3109/10428194.2012.717173. [DOI] [PubMed] [Google Scholar]
- 5.Vousden KH, Prives C. Blinded by the Light: The Growing Complexity of p53. Cell 2009; 137:413-431; PMID:19410540; http://dx.doi.org/ 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 6.Green DR, Kroemer G. Cytoplasmic functions of the tumour suppressor p53. Nature 2009; 458:1127-1130; PMID:19407794; http://dx.doi.org/ 10.1038/nature07986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheok CF, Verma CS, Baselga J, Lane DP. Translating p53 into the clinic. Nat Rev Clin Oncol 2011; 8:25-37; PMID:20975744; http://dx.doi.org/ 10.1038/nrclinonc.2010.174. [DOI] [PubMed] [Google Scholar]
- 8.Bykov VJN, Issaeva N, Shilov A, Hultcrantz M, Pugacheva E, Chumakov P, Bergman J, Wiman KG, Selivanova G. Restoration of the tumor suppressor function to mutant p53 by a low-molecular-weight compound. Nat Med 2002; 8:282-288; PMID:11875500; http://dx.doi.org/ 10.1038/nm0302-282. [DOI] [PubMed] [Google Scholar]
- 9.Zandi R, Selivanova G, Christensen CL, Gerds TA, Willumsen BM, Poulsen HS. PRIMA-1Met/APR-246 induces apoptosis and tumor growth delay in small cell lung cancer expressing mutant p53. Clin Cancer Res 2011; 17:2830-2841; PMID:21415220; http://dx.doi.org/ 10.1158/1078-0432.CCR-10-3168. [DOI] [PubMed] [Google Scholar]
- 10.Bao W, Chen M, Zhao X, Kumar R, Spinnler C, Thullberg M, Issaeva N, Selivanova G, Strömblad S. PRIMA-1Met/APR-246 induces wild-type p53-dependent suppression of malignant melanoma tumor growth in 3D culture and in vivo. Cell Cycle 2011; 10:301-307; PMID:21239882; http://dx.doi.org/ 10.4161/cc.10.2.14538. [DOI] [PubMed] [Google Scholar]
- 11.Liang Y, Besch-Williford C, Hyder SM. PRIMA-1 inhibits growth of breast cancer cells by re-activating mutant p53 protein. Int J Oncol 2009; 35:1015-1023; PMID:19787255. [DOI] [PubMed] [Google Scholar]
- 12.Nahi H, Selivanova G, Lehmann S, Möllgård L, Bengtzen S, Concha H, Svensson A, Wiman KG, Merup M, Paul C. Mutated and non-mutated TP53 as targets in the treatment of leukaemia. Br J Haematol 2008; 141:445-453; PMID:18341636; http://dx.doi.org/ 10.1111/j.1365-2141.2008.07046.x. [DOI] [PubMed] [Google Scholar]
- 13.Lehmann S, Bykov VJN, Ali D, Andrén O, Cherif H, Tidefelt U, Uggla B, Yachnin J, Juliusson G, Moshfegh A, et al.. Targeting p53 in vivo: a first-in-human study with p53-targeting compound APR-246 in refractory hematologic malignancies and prostate cancer. J Clin Oncol 2012; 30:3633-3639; PMID:22965953; http://dx.doi.org/ 10.1200/JCO.2011.40.7783. [DOI] [PubMed] [Google Scholar]
- 14.Lambert JMR, Gorzov P, Veprintsev DB, Söderqvist M, Segerbäck D, Bergman J, Fersht AR, Hainaut P, Wiman KG, Bykov VJN. PRIMA-1 reactivates mutant p53 by covalent binding to the core domain. Cancer Cell 2009; 15:376-388; PMID:19411067; http://dx.doi.org/ 10.1016/j.ccr.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Nahi H, Lehmann S, Mollgard L, Bengtzen S, Selivanova G, Wiman KG, Paul C, Merup M. Effects of PRIMA-1 on chronic lymphocytic leukaemia cells with and without hemizygous p53 deletion. Br J Haematol 2004; 127:285-291; PMID:15491287; http://dx.doi.org/ 10.1111/j.1365-2141.2004.05210.x. [DOI] [PubMed] [Google Scholar]
- 16.Supiot S, Zhao H, Wiman K, Hill RP, Bristow RG. PRIMA-1(met) radiosensitizes prostate cancer cells independent of their MTp53-status. Radiother Oncol 2008; 86:407-411; PMID:18237796; http://dx.doi.org/ 10.1016/j.radonc.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Ali D, Jönsson-Videsäter K, Deneberg S, Bengtzén S, Nahi H, Paul C, Lehmann S. APR-246 exhibits anti-leukemic activity and synergism with conventional chemotherapeutic drugs in acute myeloid leukemia cells. Eur J Haematol 2011; 86:206-215; PMID:21114538; http://dx.doi.org/ 10.1111/j.1600-0609.2010.01557.x. [DOI] [PubMed] [Google Scholar]
- 18.Saha MN, Jiang H, Yang Y, Reece D, Chang H. PRIMA-1Met/APR-246 displays high antitumor activity in multiple myeloma by induction of p73 and Noxa. Mol Cancer Ther 2013; 12:2331-2341; PMID:24030633; http://dx.doi.org/ 10.1158/1535-7163.MCT-12-1166. [DOI] [PubMed] [Google Scholar]
- 19.Lambert JMR, Moshfegh A, Hainaut P, Wiman KG, Bykov VJN. Mutant p53 reactivation by PRIMA-1MET induces multiple signaling pathways converging on apoptosis. Oncogene 2010; 29:1329ne (20; http://dx.doi.org/ 10.1038/onc.2009.425. [DOI] [PubMed] [Google Scholar]
- 20.Levrero M, De Laurenzi V, Costanzo A, Gong J, Wang JY, Melino G. The p53/p63/p73 family of transcription factors: overlapping and distinct functions. J Cell Sci 2000; 113 (Pt 10):16610) 10). [DOI] [PubMed] [Google Scholar]
- 21.Agarwal A, Ghobrial IM. The bone marrow microenvironment in Waldenström macroglobulinemia. Clin Lymphoma Myeloma Leuk 2013; 13, 218-221; PMID:23490994; http://dx.doi.org/ 10.1016/j.clml.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Messina RL, Sanfilippo M, Vella V, Pandini G, Vigneri P, Nicolosi ML, Gianì F, Vigneri R, Frasca F. Reactivation of p53 mutants by prima-1 [corrected] in thyroid cancer cells. Int J Cancer 2012; 130:2259-2270; PMID:21647879; http://dx.doi.org/ 10.1002/ijc.26228. [DOI] [PubMed] [Google Scholar]
- 23.Aryee DNT, Niedan S, Ban J, Schwentner R, Muehlbacher K, Kauer M, Kofler R, Kovar H. Variability in functional p53 reactivation by PRIMA-1(Met)/APR-246 in Ewing sarcoma. Br J Cancer 2013; 109:2696-2704; PMID:24129240; http://dx.doi.org/ 10.1038/bjc.2013.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braggio E, Philipsborn C, Novak A, Hodge L, Ansell S, Fonseca R. Molecular pathogenesis of Waldenström's macroglobulinemia. Haematologica 2012; 97:1281-1290; PMID:22773606; http://dx.doi.org/ 10.3324/haematol.2012.068478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nahi H, Merup M, Lehmann S, Bengtzen S, Möllgård L, Selivanova G, Wiman KG, Paul C. PRIMA-1 induces apoptosis in acute myeloid leukaemia cells with p53 gene deletion. Br J Haematol 2006; 132:230-236; PMID:16398657. [DOI] [PubMed] [Google Scholar]
- 26.Tessoulin B, Descamps G, Moreau P, MaG S, Lod L, Godon C, Marionneau-Lambot S, Oullier T, Le Gouill S, Amiot M, et al.. PRIMA-1Met induces myeloma cell death independent of p53 by impairing the GSH/ROS balance. Blood 2014; 124:1626 et al; http://dx.doi.org/ 10.1182/blood-2014-01-548800. [DOI] [PubMed] [Google Scholar]
- 27.Mujtaba T, Dou QP. Advances in the understanding of mechanisms and therapeutic use of bortezomib. Discov Med 2011; 12:471-480; PMID:22204764. [PMC free article] [PubMed] [Google Scholar]
- 28.Distelhorst CW. Recent insights into the mechanism of glucocorticosteroid-induced apoptosis. Cell Death Differ 2002; 9:6-19; PMID:11803370; http://dx.doi.org/ 10.1038/sj.cdd.4400969. [DOI] [PubMed] [Google Scholar]
- 29.Ditzel Santos D, Ho AW, Tournilhac O, Hatjiharissi E, Leleu X, Xu L, Tassone P, Neri P, Hunter ZR, Chemaly MAZ, et al.. Establishment of BCWM.1 cell line for Waldenström's macroglobulinemia with productive in vivo engraftment in SCID-hu mice. Exp Hematol 2007; 35:1366-1375; PMID:17761288. [DOI] [PubMed] [Google Scholar]
- 30.Hodge LS, Novak AJ, Grote DM, Braggio E, Ketterling RP, Manske MK, Price Troska TL, Ziesmer SC, Fonseca R, Witzig TE, et al.. Establishment and characterization of a novel Waldenstrom macroglobulinemia cell line, MWCL−1. Blood 2011; 117:e190-197; PMID:21415268; http://dx.doi.org/ 10.1182/blood-2010-12-326868. [DOI] [PubMed] [Google Scholar]
- 31.Trudel S, Stewart AK, Li Z, Shu Y, Liang S-B, Trieu Y, Reece D, Paterson J, Wang D, Wen X-Y. The Bcl−2 family protein inhibitor, ABT-737, has substantial antimyeloma activity and shows synergistic effect with dexamethasone and melphalan. Clin Cancer Res 2007; 13:621-629; PMID:17255285. [DOI] [PubMed] [Google Scholar]
- 32.Chou T-C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev 2006; 58:621-681; PMID:16968952; http://dx.doi.org/ 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]


