Abstract
Mitogen-activated protein (MAP) kinase cascades play important roles in plant immunity. Upon pathogen associated molecular pattern (PAMP) treatment, MPK3, MPK6 and MPK4 are quickly activated by upstream MKKs through phosphorylation. Western blot analysis using α-phospho-p44/42-ERK antibody suggests that additional MPKs with similar size as MPK4 are also activated upon PAMP perception. To identify these MAP kinases, 7 candidate MPKs with similar sizes as MPK4 were selected for further analysis. Transgenic plants expressing these MPKs with a ZZ-3xFLAG double tag of 17 kD were generated and analyzed by western blot. MPK1, MPK11 and MPK13 were found to be phosphorylated upon treatment with flg22. Our study revealed additional MAPKs being activated during PAMP-triggered immunity.
Keywords: Arabidopsis, flg22, MAP kinases, MPK4, MPK1, MPK13, PAMP-triggered immunity
Mitogen-activated protein (MAP) kinases are serine/threonine-specific protein kinases. They are involved in signal transduction during many biological processes through responding to diverse arrays of stimuli. MAP kinase cascades play critical roles in plant defense against pathogens.1 During pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI), transmembrane receptors such as FLAGELLIN-SENSING 2 (FLS2) activate MAP kinase kinase kinases (MEKKs), which subsequently phosphorylate downstream MAP kinase kinases (MKKs) that in turn activate MAP kinases (MPKs).
In Arabidopsis, there are 20 MPKs, 10 MKKs and about 60 putative MEKKs.2 Two MAP kinase cascades have been shown to be activated downstream of PAMP receptors. One leads to activation of MKK4 and MKK5 and the downstream MPK3 and MPK6.3 The MEKK functioning in this cascade is still unknown. Downstream of the MAP kinases, ethylene response factor 6 (ERF6) was identified as a substrate of MPK3/MPK6 and ERF104 was identified as a substrate of MPK6.4,5 Activation of these ERF proteins is critical for defense against fungal pathogens.
Another cascade downstream of PAMP receptors leads to activation of MPK4 through MEKK1 and MKK1/MKK2.6-11 This cascade negatively regulates defense responses mediated by the NB-LRR resistance protein SUMM2.12 Inactivation of MPK4 by the bacterial effector protein HopAI1 leads to activation of SUMM2-mediated immune responses. The MEKK1-MKK1/MKK2-MPK4 kinase cascade was also found to positively regulate basal defense, as summ2 mekk1 and summ2 mkk1 mkk2 mutant plants exhibit enhanced susceptibility to pathogens.12
The α-phospho-p44/42-ERK antibody (Cell Signaling Technology, Inc., #4370s) recognizes a conserved phosphorylation motif of MAP kinases. It has been widely used to analyze MAP kinase phosphorylation in animals and plants. In Arabidopsis, 3 immunoreactive bands are usually detected in a protein gel blot analysis of samples treated with the elicitor flg22,13,14 a peptide derived from bacterial flagellin that is recognized by FLS2.15 In the mpk6 single mutant, the band of the highest molecular weight is absent, indicating that phosphorylated MPK6 is typically detected as the top band. In mpk3, the middle band is absent, indicating that phosphorylated MPK3 is detected as the central band. The intensity of the lower band with the smallest molecular weight is reduced in mpk4, but is not affected in the knockout mutant of its close homolog MPK11.13 In the mpk4 mpk11 double mutant, the lower band is still present, but its intensity is further reduced compared to that in mpk4,13 suggesting that MPK11 is also phosphorylated after flg22 treatment and flg22-treatment activates additional MAP kinases that co-migrates with MPK4 during sodium dodecyl sulfate polyacrylamide gel electrophoresis (PAGE).
In this study, we sought to identify MAPKs that have similar sizes as MPK4 and exhibit phosphorylation upon flg22 treatment. MAP kinase candidates were chosen based on their protein sizes. Prior to phosphorylation, MPK4 is 42.9 kD. Seven MAP kinases whose protein sizes are between 42.2 kD-43.2 kD and that are expressed in leaf tissue based on the microarray database at The Arabidopsis Information Resource, specifically MPK1, MPK2, MPK5, MPK7, MPK11, MPK12 and MPK13, were selected for further studies.
To test whether these candidate MAP kinases are phosphorylated upon treatment with flg22, we analyzed the knockout mutants of the predicted MAP kinases by western blot using the α-phospho-p44/42-ERK antibody, and did not observe any consistent difference between the wild type and the single mutants. Most likely there is redundancy between the MAP kinases that masks the phenotype in single mutants. We then took an alternative approach to detect single MAP kinase phosphorylation upon flg22 treatment using transgenic plants expressing epitope tagged candidate MAP kinases. The epitope used is a FLAG-ZZ double tag. The FLAG tag is approximately 1 kD in size, while the ZZ tag is approximately 16 kD, which was synthesized from the B domain of Protein A.16 By fusing the MAP kinase to the double tag, the protein size is expected to be increased by approximately 17 kD, allowing detection of the phosphorylated individual candidate MAP kinases apart from the endogenous proteins.
Constructs expressing MPK1, MPK2, MPK5, MPK7, MPK11, MPK12 and MPK13 with a C-terminal FLAG-ZZ tag under their own promoters were generated and transformed in Col-0 wild type plants. Transgenic lines expressing the fusion proteins were identified by Western blot using an anti-FLAG antibody and used for subsequent phosphorylation analysis. As shown in Figure 1, treatment with flg22 results in strong increases in phosphorylated MPK1-FLAG-ZZ, MPK11-FLAG-ZZ and MPK13-FLAG-ZZ detected by the α-phospho-p44/42-ERK antibody, suggesting that MPK1, MPK11 and MPK13 are phosphorylated upon flg22 induction.
Figure 1.
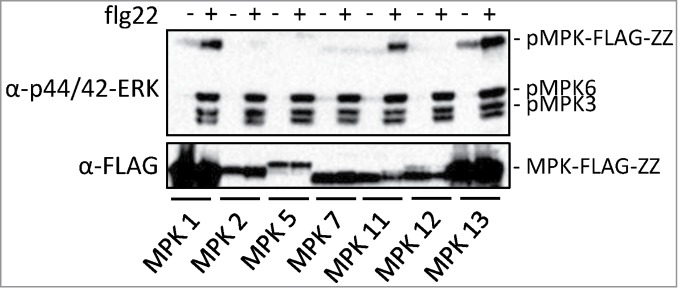
Activation of MPK-FLAG-ZZ fusion proteins in transgenic plants by flg22. 12-day-old seedlings grown on ½ MS medium were treated with or without 1 μM flg22. Samples were taken 10 min after treatment. The MPK-FLAG-ZZ fusion proteins were detected using an anti-FLAG antibody (Sigma). Phosphorylated MAP kinases were detected using the α-p44/42-ERK antibody.
To test whether the identified MAP kinases are important for PTI, the single mutants of mpk1, mpk11 and mpk13 were assayed for growth of the non-pathogenic bacteria Pseudomonas syringae pv. tomato DC3000 hrcC (P.s.t. DC3000 hrcC). As shown in Figure 2, the MAPK single mutants showed no enhanced susceptibility to P.s.t. DC3000 hrcC, suggesting that loss of individual MAP kinases does not affect PAMP-triggered immunity against P.s.t. DC3000 hrcC.
Figure 2.
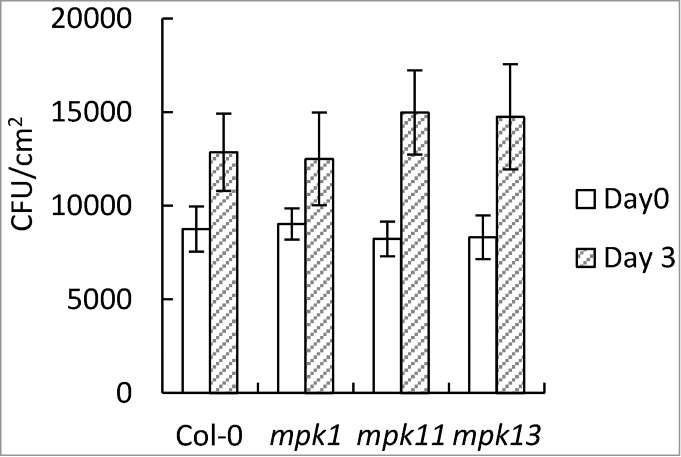
Growth of P. s. t. DC3000 hrcC in Col-0, mpk1, mpk11 and mpk13 plants. Leaves of five-week-old plants were inoculated with P. s.t. DC3000 hrcC (OD600 = 0.002). Leaf discs in the inoculated area were collected to measure bacterial titers at days 0 and 3. Plants were grown under short-day conditions (12-h day/12-h night cycles).
In summary, we have identified additional MAP kinases that are activated in response to flg22 treatment. Lack of obvious defects in PTI against P.s.t. DC3000 hrcC suggests potential functional redundancy among these MAPKs. Analysis of combined mutants of the MAPKs may be required to elucidate their roles in plant defense against pathogens. Future identification of the target proteins of these MAPKs and their upstream MEKKs and MKKs are also critical in understanding how they function in plant immunity.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Brian Ellis (University of British Columbia) for the mpk knockout mutants and Charles Copeland and Xin Li for discussion and editing of the manuscript.
Funding
This work is supported by Natural Sciences and Engineering Research Council (NSERC) of Canada and Canada Foundation for Innovation (CFI).
References
- 1. Meng X, Zhang S. MAPK cascades in plant disease resistance signaling. Annu Rev Phytopathol 2013; 51:245-66; PMID:23663002; http://dx.doi.org/ 10.1146/annurev-phyto-082712-102314 [DOI] [PubMed] [Google Scholar]
- 2. MAPK-Group . Mitogen-activated protein kinase cascades in plants: a new nomenclature. Trends Plant Sci 2002; 7:301-8; PMID:12119167; http://dx.doi.org/ 10.1016/S1360-1385(02)02302-6 [DOI] [PubMed] [Google Scholar]
- 3. Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 2002; 415:977-83; PMID:11875555; http://dx.doi.org/ 10.1038/415977a [DOI] [PubMed] [Google Scholar]
- 4. Meng X, Xu J, He Y, Yang KY, Mordorski B, Liu Y, Zhang S. Phosphorylation of an ERF transcription factor by Arabidopsis MPK3MPK6 regulates plant defense gene induction and fungal resistance. Plant Cell 2013; 25:1126-42; PMID:23524660; http://dx.doi.org/ 10.1105/tpc.112.109074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bethke G, Unthan T, Uhrig JF, Poschl Y, Gust AA, Scheel D, Lee J. Flg22 regulates the release of an ethylene response factor substrate from MAP kinase 6 in Arabidopsis thaliana via ethylene signaling. Proc Natl Acad Sci U S A 2009; 106:8067-72; PMID:19416906; http://dx.doi.org/ 10.1073/pnas.0810206106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gao M, Liu J, Bi D, Zhang Z, Cheng F, Chen S, Zhang Y. MEKK1, MKK1MKK2 and MPK4 function together in a mitogen-activated protein kinase cascade to regulate innate immunity in plants. Cell Res 2008; 18:1190-8; PMID:18982020; http://dx.doi.org/ 10.1038/cr.2008.300 [DOI] [PubMed] [Google Scholar]
- 7. Qiu JL, Zhou L, Yun BW, Nielsen HB, Fiil BK, Petersen K, Mackinlay J, Loake GJ, Mundy J, Morris PC. Arabidopsis mitogen-activated protein kinase kinases MKK1 and MKK2 have overlapping functions in defense signaling mediated by MEKK1, MPK4, and MKS1. Plant Physiol 2008; 148:212-22; PMID:18599650; http://dx.doi.org/ 10.1104/pp.108.120006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ichimura K, Casais C, Peck SC, Shinozaki K, Shirasu K. MEKK1 is required for MPK4 activation and regulates tissue-specific and temperature-dependent cell death in Arabidopsis. J Biol Chem 2006; 281:36969-76; PMID:17023433; http://dx.doi.org/ 10.1074/jbc.M605319200 [DOI] [PubMed] [Google Scholar]
- 9. Nakagami H, Soukupova H, Schikora A, Zarsky V, Hirt H. A Mitogen-activated protein kinase kinase kinase mediates reactive oxygen species homeostasis in Arabidopsis. J Biol Chem 2006; 281:38697-704; PMID:17043356; http://dx.doi.org/ 10.1074/jbc.M605293200 [DOI] [PubMed] [Google Scholar]
- 10. Pitzschke A, Djamei A, Bitton F, Hirt H. A major role of the MEKK1-MKK12-MPK4 pathway in ROS signalling. Mol Plant 2009; 2:120-37; PMID:19529823; http://dx.doi.org/ 10.1093/mp/ssn079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Suarez-Rodriguez MC, Adams-Phillips L, Liu Y, Wang H, Su SH, Jester PJ, Zhang S, Bent AF, Krysan PJ. MEKK1 is required for flg22-induced MPK4 activation in Arabidopsis plants. Plant Physiol 2007; 143:661-9; PMID:17142480; http://dx.doi.org/ 10.1104/pp.106.091389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang Z, Wu Y, Gao M, Zhang J, Kong Q, Liu Y, Ba H, Zhou J, Zhang Y. Disruption of PAMP-induced MAP kinase cascade by a Pseudomonas syringae effector activates plant immunity mediated by the NB-LRR protein SUMM2. Cell Host Microbe 2012; 11:253-63; PMID:22423965; http://dx.doi.org/ 10.1016/j.chom.2012.01.015 [DOI] [PubMed] [Google Scholar]
- 13. Bethke G, Pecher P, Eschen-Lippold L, Tsuda K, Katagiri F, Glazebrook J, Glazebrook J, Scheel D, Lee J. Activation of the Arabidopsis thaliana mitogen-activated protein kinase MPK11 by the flagellin-derived elicitor peptide, flg22. Mol Plant Microbe Interact 2012; 25:471-80; PMID:22204645; http://dx.doi.org/ 10.1094/MPMI-11-11-0281 [DOI] [PubMed] [Google Scholar]
- 14. Liu J, Ding P, Sun T, Nitta Y, Dong O, Huang X, Yang W, Li X, Botella JR, Zhang Y. Heterotrimeric G proteins serve as a converging point in plant defense signaling activated by multiple receptor-like kinases. Plant Physiol 2013; 161:2146-58; PMID:23424249; http://dx.doi.org/ 10.1104/pp.112.212431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gomez-Gomez L, Boller T. FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell 2000; 5:1003-11; PMID:10911994; http://dx.doi.org/ 10.1016/S1097-2765(00)80265-8 [DOI] [PubMed] [Google Scholar]
- 16. Forler D, Kocher T, Rode M, Gentzel M, Izaurralde E, Wilm M. An efficient protein complex purification method for functional proteomics in higher eukaryotes. Nat Biotechnol 2003; 21:89-92; PMID:12483225; http://dx.doi.org/ 10.1038/nbt773 [DOI] [PubMed] [Google Scholar]


