Abstract
In response to herbivore attack, plants perceive herbivore associated elicitors (HAE) and rapidly accumulate jasmonic acid (JA) and other phytohormones, which interact in complex ways, such as the crosstalk between JA and salicylic acid (SA). Although recent studies have shown that HAE-induced individual phytohormones can be highly specific among closely related species, it remains unclear how conserved and specific the relationships among HAE-induced phytohormones are. Here we analyzed the correlations among 4 different phytohormones, JA, JA-isoleucine (JA-Ile), SA, and abscisic acid (ABA) in 6 closely related Nicotiana species that were induced by 3 different HAEs. Our results showed that while no clear association between ABA and other phytohormones were found, the positive association between JA and JA-Ile is mostly conserved among closely related Nicotiana species. Interestingly, the association between JA and SA are highly variable and can be regulated by different HAEs.
Keywords: ABA, herbivore associated elicitors, JA, JA-Ile, SA, JA-SA correlation, JA - JA-Ile correlation, Nicotiana, phytohormone
Abbreviations
- herbivore associated elicitors
HAE
- jasmonic acid
JA
- jasmonic acid isoleucine
JA-Ile
- salicylic acid
SA
- abscisic acid
ABA
In response to herbivore attack, plants induce rapid phytohormonal changes through the perception of chemical cues (herbivore-associated elicitors: HAE) in insect oral secretion (OS).1 The phytohormonal responses include rapid accumulations of jasmonic acid (JA) and its derivatives, JA-Ile, which are the key plant hormones responsible for the activation of defense responses against most insect herbivores in plants.2-4 At same time, HAE can also induce accumulations of other phytohormones, such as SA, ABA and ethylene (ET). These different phytohormones can play either synergistic and/or antagonistic roles in defensive reactions.4 For example, the accumulation of SA, which plays a key role in defending against attack from piercing-sucking insects and biotrophic pathogens,3-6 can antagonize the accumulation of JA and JA induced anti-herbivore defensive functions.7,8 In addition to the antagonistic interaction of JA-SA, ABA was shown to have synergistic effects with JA on anti-herbivore defenses in Nicotiana attenuata.9 Therefore, the specificity of HAE-induced defense responses are thought to be depend on both induced individual phytohormones and their crosstalk.4,10,11 While HAE-induced individual phytohormonal responses were known to be highly variable among plant species and herbivore-dependent,10-12 how conserved and specific the relationships are among induced phytohormones remains unclear. Here, by analyzing our recently published phytohormone data that were collected from the leaves of 6 closely related Nicotiana species,11 we specifically investigated 2 questions: 1) how conserved are the relationships among HAE-induced phytohormones from closely related plant species? 2) can HAE elicitations regulate the relationships among phytohormones within a species?
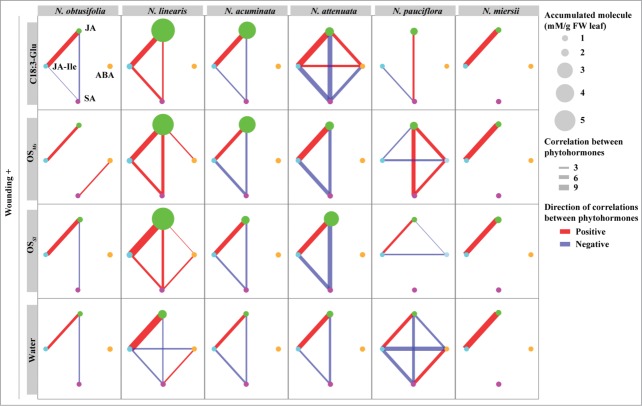
We calculated the pair-wise correlations among the accumulations of 4 phytohormones (JA, JA-Ile, SA and ABA) within 2 hours of being induced by different HAEs in leaves of 6 closely related Nicotiana species.11 Since our aim was to investigate the HAE-induced phytohormonal associations, we excluded the un-induced samples (control samples at 0 h). We found that while ABA did not correlate with other phytohormones in most of species, JA-Ile and SA showed interesting patterns of correlations with JA. JA-Ile was positively correlated with JA among most of the Nicotiana species (Fig. 1) and among different HAE elicitations. The highly conserved positive correlation between HAE-induced endogenous JA and JA-Ile likely reflects their biosynthetic relationship in which JA-Ile is a conjugation product of JA and isoleucine (Ile).13 The only exception was N. pauciflora induced by C18:3-Glu (FAC) and Manduca sexta oral secretion (OSMs), which showed no or slight negative correlation between JA and JA-Ile. This is mainly due to the increased JA levels at later time points (2 h after elicitation), but no increase in JA-Ile levels,11 indicating that the genes related to the metabolisms of JA and JA-Ile, such as jasmonate-resistant (JAR)14 or jasmonoyl-l-isoleucine hydrolase (JIH)15 are regulated by FAC or OSMs in N. pauciflora at later time points. However, further experiments to measure the HAE-induced regulations of these candidate genes are needed to falsify this hypothesis.
Figure 1.
Correlations among the accumulations of 4 HAE-induced phytohormones. Each column represents different species, and each row represents treatments with different HAEs. FAC: C18:3-Glu, the most active fatty acid conjugate found in Manduca sexta oral secretion; OSMs: M. sexta oral secretion: OSSl: Spodoptera littoralis oral secretion. Within each plot, the circles represent different phytohormone molecules. Green: JA; light blue: JA-Ile; pink: SA; orange: ABA. The size of the circle indicates the total amount of accumulated JA within 2 h of elicitation. The correlated phytohormones (P < 0.05, linear regression) were connected with lines, with color and thickness referring to the direction and significance of the correlation, respectively. Blue color indicates negative correlations and red color indicates positive correlations. The thickness of the lines indicates the value of -log10 (P-value), while the P-value was calculated using linear regression on 2 different phytohormones that were logarithmic transformed as f(x) = ln(x + 1).
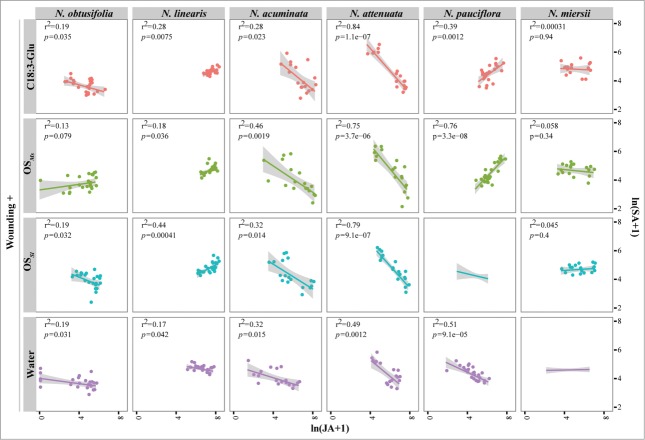
SA's correlation with JA was found to be highly variable. When treated with only wounding and water, all species except N. miersii showed a strong antagonistic JA-SA relationship (Fig. 2, bottom row). Interestingly, HAE inductions regulated the correlation of JA-SA differently among the different species. After HAE elicitation, while the correlation between JA and SA remained negative in N. obtusifolia, N. acuminata and N. attenuata, results consistent with previous studies using trangenetic approaches,7,16 this negative relationship was reversed in N. lineariz and N. pauciflora (Fig. 2, second and fourth column). Interestingly, the regulations on JA-SA correlation within each species were largely consistent among different HAEs (Fig. 2).
Figure 2.
The HAE-induced JA-SA correlations among 6 closely related Nicotiana species. Each column represents different species, and each row represents treatments with different HAEs. The color indicates the different HAEs. Vermillion: C18:3-Glu, the most active fatty acid conjugate found in OSMs; green: Manduca sexta oral secretion (OSMs); light blue: Spodoptera littoralis oral secretion (OSSl); purple: water; The amount of JA and SA were f(x) = ln(x + 1) transformed. Linear regression and r-square were shown in each plot; the ribbon represents 95% confidence interval.
The observed variations on JA-SA relationships have 3 implications. 1) Consistent with the patterns found among different Asclepias spp.,12 HAE-induced JA and SA accumulations in Nicotiana can be regulated independently. Although at a qualitative level, the induction of JA and SA were mostly positively correlated - when JA is induced, SA is usually also induced11 - at quantitative level, however, no consistent relationships between JA and SA were found among species (Fig. 1). This is likely due to the fact that JA and SA were induced at different times. For example, after HAE elicitation, the highest JA accumulated was at 30 minutes in most of studied Nicotiana species, except N. pauciflora, however, from our data the highest SA accumulation was at 2 h in most of species.11 2) The antagonistic associations between wound-induced JA and SA elicitation can be rewired when HAE are introduced into wounds. The fact that the negative correlations between JA and SA, representing the general JA-SA antagonism frequently reported in plants,8 can be either enhanced or reversed in different Nicotiana species after HAE elicitations (Fig. 1 and 2) indicates that other factors induced by HAE in Nicotiana, such as ethylene might contribute to the regulations of the antagonistic effects between JA and SA.17-19 Further investigations on HAE induced ethylene levels would provide insights into how did HAE regulate the association between JA and SA. 3) The HAE-induced JA and SA association can evolve rapidly among closely related plant species, since closely related Nicotiana species can have different induced JA-SA associations (Fig. 2). Although the antagonism between JA and SA are thought to have evolved early in the evolutionary history of angiosperm,8 the breakdown of antagonisms was also reported in Zea mays20 and milkweed.8,12 Our study further suggests that such deconstructions of the antagonistic relationships between JA and SA might be more frequent than previously thought.
In summary, our data showed that among closely related Nicotiana species, while HAE-induced positive association between JA and JA-Ile is mostly conserved, the induced association between JA and SA can be regulated by HAE elicitation and can evolve rapidly among closely related species.
Funding
We are grateful for funding by Swiss National Science Foundation (Project number PEBZP3-142886 to SX), the Marie Curie Intra-European Fellowships (IEF) (Project Number 328935 to SX), the Max Planck Society and European Research Council advanced grant ClockworkGreen (Project number 293926 to ITB).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank T. Krügel for providing the seeds of different Nicotiana species, M. Kallenbach for providing the synthetic C18:3-Glu and an anonymous reviewer for constructive suggestions on the manuscript.
References
- 1.Bonaventure G, VanDoorn A, Baldwin IT. Herbivore-associated elicitors: FAC signaling and metabolism. Trends Plant Sci 2011; 16:294-9; PMID:21354852; http://dx.doi.org/ 10.1016/j.tplants.2011.01.006 [DOI] [PubMed] [Google Scholar]
- 2.Farmer EE, Ryan CA. Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase-inhibitors. Plant Cell 1992; 4:129-34; PMID:12297644; http://dx.doi.org/ 10.1105/tpc.4.2.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu J, Baldwin IT. New insights into plant responses to the attack from insect herbivores. Annu Rev Genet 2010; 44:1-24; PMID:20649414; http://dx.doi.org/ 10.1146/annurev-genet-102209-163500 [DOI] [PubMed] [Google Scholar]
- 4.Erb M, Meldau S, Howe GA. Role of phytohormones in insect-specific plant reactions. Trends Plant Sci 2012; 17:250-9; PMID:22305233; http://dx.doi.org/ 10.1016/j.tplants.2012.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaffney T, Friedrich L, Vernooij B, Negrotto D, Nye G, Uknes S, Ward E, Kessmann H, Ryals J. Requirement of salicylic-acid for the induction of systemic acquired-resistance. Science 1993; 261:754-6; PMID:17757215; http://dx.doi.org/ 10.1126/science.261.5122.754 [DOI] [PubMed] [Google Scholar]
- 6.Delaney TP, Uknes S, Vernooij B, Friedrich L, Weymann K, Negrotto D, Gaffney T, Gut-Rella M, Kessmann H, Ward E, et al.. A central role of salicylic-acid in plant-disease resistance. Science 1994; 266:1247-50; PMID:17810266; http://dx.doi.org/ 10.1126/science.266.5188.1247 [DOI] [PubMed] [Google Scholar]
- 7.Rayapuram C, Baldwin IT. Increased SA in NPR1-silenced plants antagonizes JA and JA-dependent direct and indirect defenses in herbivore-attacked Nicotiana attenuata in nature. Plant J 2007; 52:700-15; PMID:17850230; http://dx.doi.org/ 10.1111/j.1365-313X.2007.03267.x [DOI] [PubMed] [Google Scholar]
- 8.Thaler JS, Humphrey PT, Whiteman NK. Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci 2012; 17:260-70; PMID:22498450; http://dx.doi.org/ 10.1016/j.tplants.2012.02.010 [DOI] [PubMed] [Google Scholar]
- 9.Dinh ST, Baldwin IT, Galis I. The HERBIVORE ELICITOR-REGULATED1 gene enhances abscisic acid levels and defenses against herbivores in Nicotiana attenuata plants. Plant Physiol 2013; 162:2106-24; PMID:23784463; http://dx.doi.org/ 10.1104/pp.113.221150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmelz EA, Engelberth J, Alborn HT, Tumlinson JH, Teal PE. Phytohormone-based activity mapping of insect herbivore-produced elicitors. Proc Natl Acad Sci U S A 2009; 106:653-7; PMID:19124770; http://dx.doi.org/ 10.1073/pnas.0811861106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu S, Zhou W, Pottinger S, Baldwin IT. Herbivore associated elicitor-induced defences are highly specific among closely related Nicotiana species. BMC Plant Biol 2015; 15:2; PMID:25592329; http://dx.doi.org/ 10.1186/s12870-014-0406-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agrawal AA, Hastings AP, Patrick ET, Knight AC. Specificity of herbivore-induced hormonal signaling and defensive traits in five closely related milkweeds (Asclepias spp.). J Chem Ecol 2014; 40:717-29; PMID:24863490; http://dx.doi.org/ 10.1007/s10886-014-0449-6 [DOI] [PubMed] [Google Scholar]
- 13.Staswick PE, Tiryaki I. The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 2004; 16:2117-27; PMID:15258265; http://dx.doi.org/ 10.1105/tpc.104.023549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang JH, Wang L, Giri A, Baldwin IT. Silencing threonine deaminase and JAR4 in Nicotiana attenuata impairs jasmonic acid-isoleucine-mediated defenses against Manduca sexta. Plant Cell 2006; 18:3303-20; PMID:17085687; http://dx.doi.org/ 10.1105/tpc.106.041103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woldemariam MG, Onkokesung N, Baldwin IT, Galis I. Jasmonoyl-l-isoleucine hydrolase 1 (JIH1) regulates jasmonoyl-l-isoleucine levels and attenuates plant defenses against herbivores. Plant J 2012; 72:758-67; PMID:22860609; http://dx.doi.org/ 10.1111/j.1365-313X.2012.05117.x [DOI] [PubMed] [Google Scholar]
- 16.Diezel C, von Dahl CC, Gaquerel E, Baldwin IT. Different lepidopteran elicitors account for cross-talk in herbivory-induced phytohormone signaling. Plant Physiol 2009; 150:1576-86; PMID:19458114; http://dx.doi.org/ 10.1104/pp.109.139550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diezel C, Allmann S, Baldwin IT. Mechanisms of optimal defense patterns in Nicotiana attenuata: flowering attenuates herbivory-elicited ethylene and jasmonate signaling. J Integr Plant Biol 2011; 53:971-83; PMID:22054509; http://dx.doi.org/ 10.1111/j.1744-7909.2011.01086.x [DOI] [PubMed] [Google Scholar]
- 18.Leon-Reyes A, Du YJ, Koornneef A, Proietti S, Korbes AP, Memelink J, Pieterse CM, Ritsema T. Ethylene signaling renders the jasmonate response of Arabidopsis insensitive to future suppression by salicylic acid. Mol Plant Microbe Interact 2010; 23:187-97; PMID:20064062; http://dx.doi.org/ 10.1094/MPMI-23-2-0187 [DOI] [PubMed] [Google Scholar]
- 19.Leon-Reyes A, Spoel SH, De Lange ES, Abe H, Kobayashi M, Tsuda S, Millenaar FF, Welschen RA, Ritsema T, Pieterse CM. Ethylene modulates the role of NONEXPRESSOR OF PATHOGENESIS-RELATED GENES1 in cross talk between salicylate and jasmonate signaling. Plant Physiol 2009; 149:1797-809; PMID:19176718; http://dx.doi.org/ 10.1104/pp.108.133926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engelberth J, Viswanathan S, Engelberth MJ. Low concentrations of salicylic acid stimulate insect elicitor responses in Zea mays seedlings. J Chem Ecol 2011; 37:263-6; PMID:21360274; http://dx.doi.org/ 10.1007/s10886-011-9926-3 [DOI] [PubMed] [Google Scholar]