Abstract
Periodontitis is a chronic inflammatory disease which leads to destruction of both the soft and hard tissues of the periodontium. Tissue engineering is a therapeutic approach in regenerative medicine that aims to induce new functional tissue regeneration via the synergistic combination of cells, biomaterials, and/or growth factors. Advances in our understanding of the biology of stem cells, including embryonic stem cells and mesenchymal stem cells, have provided opportunities for periodontal tissue engineering. However, there remain a number of limitations affecting their therapeutic efficiency. Due to the considerable proliferation and differentiation capacities, recently described induced pluripotent stem cells (iPSCs) provide a new way for cell-based therapies for periodontal regeneration. This review outlines the latest status of periodontal tissue engineering and highlights the potential use of iPSCs in periodontal tissue regeneration.
Keywords: Induced pluripotent stem cells, Periodontal regeneration, Tissue engineering
Introduction
Periodontitis comprises a set of chronic inflammatory diseases affecting the periodontal supportive tissues (gingiva, alveolar bone, periodontal ligament, and cementum) and, if left untreatred, results in progressive loss of the alveolar bone around the teeth, resulting in loosening and subsequent loss of teeth. Conventional therapy of periodontitis concentrates on reduction of the bacterial load by mechanical and antimicrobial treatment, including scaling and root planing (SRP) or open flap debridement (OFD). These therapeutic procedures can effectively control inflammation and stop disease progression. Moreover, the application of surgical procedures such as guided tissue regeneration (GTR) and/or bioactive materials or molecules has attained some success to regenerate lost periodontal tissues [1••]. However, current regenerative procedures still have limitations in attaining complete and functional tissue regeneration, especially in advanced periodontal defects [2]. Tissue engineering, in which there are three major constituents, (1) biomaterials, (2) stem cells, (3) tissue growth factors, provides a new and better prospective for periodontal tissue regeneration [3]. Embryonic stem cells (ESCs) and mesenchymal stem cells (MSCs), including periodontal ligament stem cells (PDLSCs), bone marrow-derived MSCs (BMSCs), and adipose-derived stem cells (ADSCs) [4], are the most common cells used in periodontal tissue engineering. However, recently developed induced pluripotent stem cells (iPSCs) are increasingly attracting wide interest [5]. The present review will outline the latest status of periodontal tissue engineering and highlight iPSCs in terms of their properties and applications as well as the challenges and future prospects.
Biomaterials
Biomaterials as scaffolds play pivotal roles in periodontal tissue engineering by serving as matrices for cell growth, proliferation, differentiation, and new tissue formation in three dimensions, and as carriers to convey cells and various tissue-inducing substances such as growth factors which are essential for tissue development. Various conventional materials have been investigated as scaffolds in periodontal tissue engineering. They fall into two broad classes: natural materials and synthetic materials. The available natural materials include collagen, gelatin, and modified polyscacchrides (e.g., chitosan). The synthetic materials include calcium phosphate (e.g., hydroxyapatite, tricalcium phosphate), bioactive glass, and some synthetic polymers [e.g., poly(glycolic acid)]. These materials show the characteristics of biocompatibility, controllable degradation, and tunable mechanical properties, but fall short in bioactivity. Recent developments indicate that the application of nanobiomaterials in periodontal tissue engineering improve the mechanical properties and incorporate the nanotopographic features that mimic the natural nanostructure of bone, and show higher bioactivity.
The concept of nanotechnology, raised by the quantum theorist and Nobel laureate Richard Feynman in 1959, is the manipulation of materials at the nanometer scale to fabricate “nanomaterials,” which have “internal or surface structures in one or more dimensions in the size range 1 ∼ 100 nm” [6]. Materials at this scale are endowed with a higher surface to volume ratio, and thereby have superior mechanical, magnetic, optical, and chemical properties to those of the other materials [7]. Their higher surface-to-volume ratio assists in effective adsorbtion of proteins which are beneficial to cell adhesion [8]. Moreover, the geometry of nano-scaffolds, the crystallinity and orientation of the polymer, can affect the affinities of proteins for such materials [9]. Surface roughness is another key characteristic affecting cell response. Obviously, nano-scaffolds possess different scales of surface roughness from conventional scaffolds. The studies demonstrated that cells might be more sensitive to changes in the surface roughness in the nanometer (<100 nm) compared with conventional micro- or macro- (>100 nm) regimes [10]. All these properties reflect superiority of nanostructured scaffolds in bone/periodontal tissue engineering comparing with conventional materials.
Tissue Growth and Chemotactic Factors
The rationale for the use of growth factors for periodontal regeneration relies on the ability of these factors to enhance the proliferation and differentiation of PDLSCs and the other MSCs whether transplanted with tissue engineering construct or derived from host body. Recombinant growth factors can also enhance bone regeneration. For example, fibroblast growth factors (FGF), platelet-derived growth factors (PDGF), insulin-like growth factors (IGF), and bone morphogenetic proteins (BMP) have been used in clinical and pre-clinical trials for the treatment of large periodontal or infra-bony defects [11]. The topical application of enamel matrix derivative (which may contain a mixture of growth factors and other agents able to induce tissue repair and regeneration) with or without use of GTR can partially promote the formation of new periodontal tissues [12–14]. Considering the quick break down and short half-life period of tissue growth factors, controlled release and genetic approaches may overcome these pitfalls [15].
The principle of using chemotactic factors in periodontal regeneration relies on recruiting MSCs into periodontal defects [16]. These MSCs are not only derived from periodontitis involved local area but also from systemic circulation [17]. This technique, known as endogenous cell homing, would be less costly and complex than approaches that require substantial ex vivo cell manipulation and that use artificial vehicles for cell delivery, thereby having greater potential to provide new therapeutic options for in situ tissue regeneration [18].
The application of CXCL12 plays an important role in the recruitment of MSCs [19]. In addition, BMPs, basic fibroblast growth factor (bFGF), and PDGF-BB have been also demonstrated to have chemotactic effect on MSCs, of which bFGF possesses wider bioactivities than CXCL12. Schmidt et al. found that the chemotactic effect of bFGF on MSCs was stronger than CXCL12 [20]. Tasso et al. showed that the presence of bFGF in the culture medium during mouse MSCs expansion in vitro is a key factor for the selection of subpopulations inducing host regenerative responses [21]. Moreover, bFGF is related to maintenance of stem cell “stemness,” while preserving its differentiation potential [22]. Additionally, bFGF can exert strong proliferation enhancement on MSCs and osteoblasts [23]. All these activities of bFGF suggest that it may be an optimal choice for in situ periodontal tissue regeneration.
Stem Cells
Stem cells are the foundation cells for every organ and tissue in the body, including the periodontium [24]. Three categories of stem cells, ESCs, MSCs, and the recently developed induced pluripotent stem cells, have been experimentally applied in enhancing bone/periodontal regeneration.
Embryonic stem cells are pluripotent cells which were derived from the inner cell mass of blastocyst-stage embryos. Pluripotency is the potential of one type of cell to differentiate into various kinds of cells, such as muscle cells, neural cells, and even germ cells [25]. Based on this property, ESCs can generate any type of cell to meet the requirements of different applications. ESCs are also capable of self-renewal; they can be semi-permanently cultured on feeder cells, which supply the necessary growth factors for ESCs. The experiments have shown that ESCs possess potential to differentiate towards fibroblastic and osteoblastic lineages in vitro [25, 26] and enhance the regeneration of periodontal furcation defects in a porcine model [27]. However, the number of approved human embryonic stem cell lines for research is limited and this has impacted on the development of these cells for treating human diseases [28]. Indeed, the use of human embryos is controversial, and the ethical considerations, risk of teratoma formation, and immunologic rejection following transplantation in patients all remain significant concerns [29].
MSCs derived from multiple tissue sources have been investigated in preclinical animal studies for periodontal regeneration therapy. The potential of BMSCs, ADSCs, dental pulp stem cells (DPSCs), stem cells from exfoliated deciduous teeth (SCED), PDLSCs, stem cells from apical papilla (SCAP), and progenitor cell populations from the dental follicle and gingiva for periodontal regeneration in a variety of animal models have been comprehensively reviewed by Han et al. [1••]. Furthermore, the application of MSCs in bone tissue engineering has moved to the preclinical stage, and an ex vivo cell manufacturing procedure for obtaining high quality, bioactive MSCs from human bone marrow has been approved by the US Food and Drug Administration (FDA) [30]. Nevertheless, the major obstacles that impede the use of MSCs in clinical practice lie in the heterogeneity of the isolated cell population and the inability to set up optimal growth and differentiation conditions to obtain and maintain required quality and quantity of cells. MSCs have a limited proliferation capacity, reduced the differentiation potential, and decreased protective factors during prolonged ex vivo subculturing and passages [31, 32]. Passage of cells, donor age, and aging-related disorders also significantly impair the survival and differentiation potential of MSCs [33–35]. One study has shown a significant age-related decrease in the growth rate of human-BMSCs from donors older than 50 years old as compared with younger donors [36]. In addition, their ability of self-renewal, osteogenic differentiation, and proliferation decreases due to diseases like osteoporosis and arthritis [37, 38]. There also are increasing concerns that exogenously infused MSCs can home to tumor microenviroment and exert promotive effect on tumor growth [39–41]. Thus, better understanding of the regulation mechanisms of self-renewal and “stemness” retaining is needed in order to sufficiently regulate MSC growth in vitro to produce necessary cell numbers and quality. The interactions between stem cells and the immune system or stem cells and osteoblast-osteoclast balance as well as controlling strategy of MSC malignant transformation in vivo should be further explored [1••].
iPSCs
Generation of iPSCs
Based on the hypothesis that the genes that have important significance in maintaining ESC identity also exert key effects in inducting pluripotency of somatic cells, Takahashi and Yamanaka [42] introduced different combinations of selected 24 genes, which were important transcripts of ESCs and oncogenes, as candidate reprogramming factors into mouse embryonic fibroblasts in order to screen proper reprogramming factors via the Fbx15-Neo reporter system. They found that after introduction of the retroviral mediated factors Oct3/4, Sox2, Klf4, and c-Myc, mouse embryonic fibroblasts were reprogrammed into ES cell-like cells called iPSCs. The generation of iPSCs from adult human dermal fibroblasts was also demonstrated by transfection of the same four factors [43]. Human iPSCs share similar biological characteristics with human ESCs including morphology, proliferation, surface antigens, gene expression, epigenetic status of pluripotent cell-specific genes, and telomerase activity. Moreover, these cells could differentiate to cell types of the three germ layers in vitro and in teratomas [44].
Cell Sources for Deriving iPSCs and Approaches for Reprogramming
iPSCs have been derived from many different species, such as humans, mice, pigs, rabbits, rats, marmosets, and rhesus monkeys. The successfully reprogrammed cell types contain fibroblasts, marrow mesenchymal cells, gastric/intestinal epithelial cells, keratinocytes, hepatocytes, stomach cells, neural stem cells, pancreatic cells, blood/liver/neural progenitor cells, cord/peripheral blood cells, adipose stem cells, B and T lymphocytes, and so on [45–48], of which fibroblasts are the most commonly used parental somatic cell type for the generation of iPSCs. Between 2010 and 2012, the first reports were published on the production of iPS cell lines from human gingival fibroblasts and periodontal ligament fibroblasts by reprogramming using a retroviral transduction cocktail of OCT3/4, SOX2, KLF4, and c-MYC [49–51]. These induced cell lines expressed the human-ES (hES) cell-associated cell-surface antigens like SSEA3, SSEA4, GCTM-2, TG30 (CD9), and Tra-1-60, as well as the hESC marker genes OCT4, NANOG, and GDF3 [52]. Interestingly, in 2012, another research group established iPSCs from human periodontal ligament fibroblasts by introducing the human-ESC (hESC) markers OCT3/4, SOX2, NANOG, KLF4, and Lin28 through retrovirus transduction, even without the oncogene c-MYC [51], which was found to be responsible for tumors in iPSC chimeric mice [53]. In 2015, Umezaki et al. [54] demonstrated that human gingival integration-free iPSCs could be generated using episomal plasmid vectors without retroviral transduction. Compared with skin, gingival tissue is obtained more easily and gingival wound after sampling heals more quickly. Furthermore, reprogramming efficiency of mouse gingival fibroblasts is higher than that of mouse dermal fibroblasts [55]. These discoveries suggest that iPSCs derived from GFs represent an optimal and more practical cell-based tissue-regenerative treatment for periodontal diseases.
Since the first establishment of iPS cell line by Yamanaka in 2006, many scientists have made efforts to improve the efficiency and safety of the reprogramming process. Generally, the approaches for factor reprogramming include transgene and chemical reprogramming while methods for transgene reprogramming can be classified into three groups: RNA-based, DNA-based, and protein transduction (direct cell transduction). RNA-based reprogramming can be achieved by transfection of synthetic RNA, modified RNA, and micro RNA [56••]. DNA-based ways are most widely used, which include the use of viruses and plasmids. The very first way for cell reprogramming was by retroviral delivery of four transcription factors (Oct4, Sox2, Klf4, and Myc). In 2009, some researchers found direct protein transduction can improve inducing efficiency, but it is easily affected by the quality of recombinant proteins [57–59]. As for chemical cell reprogramming, the use of small-molecule compounds has been developed [51], and several reviews on small molecule drugs used for improving the generation of iPSCs are available [60, 61]. A variety of reprogramming methods to derive iPSCs and their advantages and disadvantages are shown in Table 1. Among available reprogramming methods, the easiest and most efficient method by now is the integration of reprogramming factors into the genome by retroviral or lentiviral transduction [43, 75].
Table 1.
Advantages and disadvantages reprogramming methods to derive iPSCs
| Approach | Cell type | Advantage | Disadvantage | Reference | ||
|---|---|---|---|---|---|---|
| Forms | Vectors | Fibroblasts, neural stem cells, stomach cells, liver cells, keratinocytes, amniotic cells, blood cells, adipose cells, melanocytes, human T cells, β cells | No genomic integration, no premature silencing, inexpensive | Low efficiency, sequence-sensitive RNA replicase, difficulty in purging cells of replicating virus Genomic recombination, insertional mutagenesis |
[62–65] | |
| Transgene (OSKM) | DNA-based | Viruses (lentivirus, adenovirus, Sendai virus ) | ||||
| Transposon | Fibroblasts, mouse ESCs | Host-factor independent, wide chromosomal distribution, high efficiency | Gene mutations, genomic rearrangements | [66–68] | ||
| Minicircle DNA | Human ASCs | High expression in mammalian cells, high transfection efficiency, stable ectopic transgene expression | Low expressions for transcription factors | [69] | ||
| RNA-based | Micro RNA | Mouse fibroblasts, Human fibroblasts | Nonviral, nontranscription-factor | Multiple transfection, low efficiency | [70, 71] | |
| Modified RNAs | Human fibroblasts | Avoid the endogenous antiviral cell defense, very high efficiency | Technically complex | [65] | ||
| Synthetic RNAs | Murine EFs, human epidermal keratinocytes | High efficiency | High and dose-dependent cytotoxicity | [65, 72] | ||
| Recombinant Proteins | Human fibroblasts, mouse fibroblasts | Nongenome integration, easily controlled | Very low efficiency, unstable, easily affected by quality of proteins | [57–59] | ||
| Chemical approaches (small molecule compounds) | Mouse EFs | Nonimmunogenic, cost-effective, easy to handle, structural versatility, faster, more efficient, self-renewal promotion, controllable microenvironment | Time and dosage of specific biochemicals need to be optimized | [44, 61, 73, 74] | ||
OSKM and similar factor names represent combinations of reprogramming factors: O, OCT4; S, SOX2; K, KLF4; M, c-MYC
ESCs embryonic stem cells, EFs embryonic fibroblasts
Advantages of iPSCs Over Other Stem Cells
The serious ethical concerns associated with ESC application, and limited proliferation and differentiation potential of MSCs, has prompted efforts to genetically reprogram somatic cells to generate iPSCs. On the first hand, these cells have similar biological features to ESCs without any ethical concerns associated with ESCs [43, 76–78]. Although ESCs and iPSCs both carry tumorigenic properties, raising a significant safety challenge in the use of these cells for regenerative therapies, the most important advantage of iPSCs compared to ESCs is the possibility to use mature somatic cells from patients who suffer from defined diseases genetically [79–81]. In addition, human-iPSCs can differentiate into all cell types of three germ layers in vitro and in vivo [43]. Overall, iPSCs overcome major ethical concerns about hESCs such as the destruction of human embryos and oocytes. In particular, the clinical use of iPSCs is expected to solve the problems of immune rejection. On the other hand, iPSCs have a greater proliferation capacity than traditional MSCs so that they can expand into large numbers before in vitro differentiation and transplantation while remaining similar or superior multipotent differentiation potential to their parental MSCs [82, 83].
iPSCs Used in Periodontal Regeneration
iPSCs now provide a novel approach to the field of tissue engineering. Recently, some studies have shown that iPSCs are a promising source of stem cells to be used for periodontal tissue regeneration therapy. In general, the rationale of applying iPSCs in periodontal regeneration includes the following aspects: (1) iPSCs can be induced from dental derived cells, such as gingival fibroblasts and periodontal ligament fibroblasts; (2) iPSCs can differentiate to osteogenic cells after stimulated by certain factors; (3) combining with or without the scaffolds, iPSCs can facilitate the healing of man-made periodontal bone defect and form new periodontal tissues like alveolar bone, cementum, and periodontal ligament. The development of iPSCs in the field of periodontal regeneration was summarized in Fig. 1.
Fig. 1.
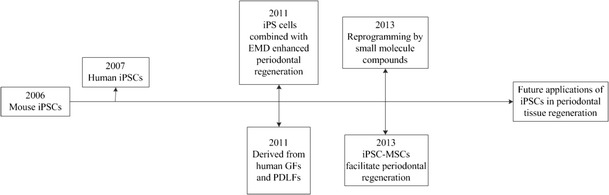
History of development for iPSCs in the field of periodontal regeneration
iPSC Differentiation Toward Osteoblasts
In 2012, it was reported the murine iPSC-derived cells can differentiate to osteoblasts with the help of TGF-beta family, bFGF or BMP-2 [84]. Recent studies have reported that functional MSCs which were derived from human iPSCs could express characteristic MSC markers, differentiate into osteoblasts, adipocytes, and chondrocytes, and promote vascular and muscle regeneration [32]. Our group previously found that when iPSCs were stimulated with enamel matrix-derived (EMD) gel, the mRNA expression level of Runt-related transcription factor 2 (Runx2), a key transcription factor expressed during osteogenic differentiation, greatly increased in EMD-stimulated media; thus, EMD gel can promote the differentiation of iPSCs to osteogenic cells [85•]. Similarly, another study showed that iPSC-MSCs had good ability for attachment, proliferation, and osteogenic differentiation when attached on calcium phosphate cement (CPC) scaffold, showing high gene expressions of osteogenic markers including osteocalcin, alkaline phosphatase (ALP), collagen type I, and Runx2; hence, the iPSC-MSC-CPC construct is predicted promising to promote bone regeneration in periodontal or craniofacial repairs [86]. Overall, iPSCs have the potential to differentiate into osteogenic cells.
iPSCs Promote Periodontal Regeneration
The development of new cementum with periodontal ligament fibers connected to alveolar bone is the main goal of periodontal regeneration [86, 87]. Ideally, regenerated periodontal ligament fibers are inserted into the newly formed cementum to connect the root surface and alveolar bone. Since iPSCs can be used as seed cells in tissue engineering, some researchers have revealed that mouse iPSCs combined with scaffolds such as EMD gel can promote the formation of alveolar bone, cementum, and periodontal ligament, thus enhancing the repair and healing of mouse periodontal defects [85•]. Another study has demonstrated that iPSCs can be induced to differentiate into MSC-like cells [88•]. These cells fulfill the International Society of Cellular Therapy’s minimal criteria for defining multipotent MSCs: they had plastic adherent properties, expressed key MSC-associated markers, and had the capacity to undergo tri-lineage differentiation. Furthermore, the generated human iPSC-MSC-like cells had the capacity to facilitate periodontal regeneration in a rat periodontal defect model, including newly formed fibrous tissue, newly formed mineralized tissue, and newly formed PDL-like tissues [88•]. Similarly, Yang et al. demonstrated that rat iPSCs could be induced to differentiate to MSCs, and intravenous and topical administration of these cells that had been transfected with tumor necrosis factor alpha-stimulated gene-6 (TSG-6), which has strong anti-inflammatory effect, was capable to decrease inflammation and inhibit alveolar bone resorption in rat experimental periodontitis [89].
The Challenges and Future Perspectives Regarding iPSCs
At present, many limitations still affect the possible applications of iPSCs in clinical medicine. The main hindrances are related to the reprogramming efficiency, biological safety, and large-scale expansion and directed differentiations.
No matter what the reprogramming methods are, one of the main obstacles in reprogramming iPSCs is the inherent low efficiency of complete reprogramming [90]. Generally speaking, there are gene expression differences between human-iPSCs (hiPSCs) and hESCs or among different iPSC lines. One report has shown that gene expression of the donor cell type significantly contributes to the differences among hiPSCs and hESCs. Specifically, further analysis reveals that gene expression of fetal fibroblast-derived hiPSCs is closer to that of hESCs, followed by adipose, neonatal fibroblast, and keratinocyte-derived hiPSCs [91]. Therefore, the optimal choice of original cell type for iPSC reprogramming and the ideal protocols for high efficient complete reprogramming are the prerequisites for future clinical transformational study.
Biological safety is another main concern in relation to the application of iPSCs in tissue regeneration, including periodontal regeneration. First, the integration of reprogramming factors into the genome by retroviral or lentiviral transduction is the easiest and most efficient method by now for reprogramming iPSCs. However, the use of cells containing viruses brings up the possibility that viruses integrate into host chromosomes and lead to replication-induced DNA mutation [92] and potentially malignant transformations [93]. Additionally, the existence of viruses may stimulate immunological reaction [94]. Second, the principles of iPSCs in regenerative medicine rely on their ability to self-renew and to differentiate to cells of the three germ layers. However, it is these properties that predispose iPS to be tumorigenic and therefore hinder the clinical applications of these cells [95]. These issues highlight the need for generating virus-free iPSCs that are functionally identical to true ESCs [96].
Another handicap for using iPSCs in regenerative medicine is the deficiency of large-scale expansion and directed differentiation approaches. Obtaining sufficient iPSCs and directing them to differentiate into osteoblasts, cementoblasts, and periodontal fibroblasts are prerequisites for successful periodontal regeneration, and although there have been some evidences showing the strong potentials of iPSCs in cell-based periodontal regenerative therapy [85•, 88•], the ability of iPSC differentiation to cementoblasts, most key cells for periodontal regeneration, remains to be explored.
Finally, whether or not iPSCs possess immunoregulatory properties, which is regarded as an important aspect of mechanisms of MSCs, has not been explored and challenge also remains to identify the best combination of iPSCs, biomaterials, and growth factors for various clinical situations.
Conclusion
The significant ethical concerns accompanied with the clinical use ESC in humans and limited proliferation and differentiation abilities of MSCs suggest that iPSCs may be a good alternative cell source for use in regenerative medicine. Although studies on the use of iPSCs for periodontal regeneration are in their early stages, iPSCs-based therapy strategies will have solid background and good prospectives for clinical periodontal regenerative treatment. The main endeavors should be to strengthen the reprogramming efficiency, assure biological safety, and optimize the strategies of large-scale expansion and directed differentiations.
Acknowledgments
This study was supported by National Natural Science Foundation of China (No. 81271141 and No. 81200789).
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Mi Du, Xuejing Duan, and Pishan Yang declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Stem-Cell Biology for Tooth and Periodontal Regeneration
Contributor Information
Mi Du, Email: dumi8211@163.com.
Xuejing Duan, Email: miqiu410@126.com.
Pishan Yang, Email: yangps@sdu.edu.cn.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.••.Han J, et al. Stem cells, tissue engineering and periodontal regeneration. Aust Dent J. 2014;59(Suppl 1):117–130. doi: 10.1111/adj.12100. [DOI] [PubMed] [Google Scholar]
- 2.Sander L, Karring T. Healing of periodontal lesions in monkeys following the guided tissue regeneration procedure. A histological study. J Clin Periodontol. 1995;22(4):332–337. doi: 10.1111/j.1600-051X.1995.tb00156.x. [DOI] [PubMed] [Google Scholar]
- 3.Bartold PM, et al. Principles and applications of cell delivery systems for periodontal regeneration. Periodontol 2000. 2006;41:123–135. doi: 10.1111/j.1600-0757.2006.00156.x. [DOI] [PubMed] [Google Scholar]
- 4.Tobita M, et al. Periodontal tissue regeneration with adipose-derived stem cells. Tissue Eng A. 2008;14:945–953. doi: 10.1089/ten.tea.2007.0048. [DOI] [PubMed] [Google Scholar]
- 5.Hynes K, Gronthos S, Bartold PM. iPSC for dental tissue regeneration. Curr Oral Health Rep. 2014;1(1):9–15. doi: 10.1007/s40496-013-0001-8. [DOI] [Google Scholar]
- 6.Maynard AD. Don’t define nanomaterials. Nature. 2011;475(7354):31. doi: 10.1038/475031a. [DOI] [PubMed] [Google Scholar]
- 7.Shakir S, et al. Transforming growth factor beta 1 augments calvarial defect healing and promotes suture regeneration. Tissue Eng A. 2015;21(5–6):939–47. doi: 10.1089/ten.tea.2014.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woo KM, et al. Nano-fibrous scaffolding promotes osteoblast differentiation and biomineralization. Biomaterials. 2007;28(2):335–43. doi: 10.1016/j.biomaterials.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Woo KM, Chen VJ, Ma PX. Nano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachment. J Biomed Mater Res A. 2003;67(2):531–7. doi: 10.1002/jbm.a.10098. [DOI] [PubMed] [Google Scholar]
- 10.Mustafa K, et al. The influence of surface topography of ceramic abutments on the attachment and proliferation of human oral fibroblasts. Biomaterials. 2005;26(4):373–81. doi: 10.1016/j.biomaterials.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 11.Christoph A, et al. Advanced regenerative technologies for periodontal tissue repair. Periodontol 2000. 2012;59(1):185–202. doi: 10.1111/j.1600-0757.2011.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonçalves PF, et al. Effect of two different approaches for root decontamination on new cementum formation following guided tissue regeneration: a histomorphometric study in dogs. J Periodontal Res. 2006;41(6):535–40. doi: 10.1111/j.1600-0765.2006.00902.x. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann T, et al. A randomized clinical multi-centre trial comparing enamel matrix derivative and membrane treatment of buccal class II furcation involvement in mandibular molars. Part III: patient factors and treatment outcome. J Clin Periodontol. 2006;33(8):575–83. doi: 10.1111/j.1600-051X.2006.00947.x. [DOI] [PubMed] [Google Scholar]
- 14.Kaigler D, Cirelli JA, Giannobile WV. Growth factor delivery for oral and periodontal tissue engineering. Expert Opin Drug Deliv. 2006;3(5):647–62. doi: 10.1517/17425247.3.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vo TN, Kasper FK, Mikos AG. Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv Drug Deliv Rev. 2012;64(12):1292–309. doi: 10.1016/j.addr.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du L, Yang P, Ge S. Stromal cell-derived factor-1 significantly induces proliferation, migration, and collagen type I expression in a human periodontal ligament stem cell subpopulation. J Periodontol. 2012;83(3):379–88. doi: 10.1902/jop.2011.110201. [DOI] [PubMed] [Google Scholar]
- 17.Knight MN, Hankenson KD. Mesenchymal stem cells in bone regeneration. Adv Wound Care (New Rochelle) 2013;2(6):306–316. doi: 10.1089/wound.2012.0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen FM, et al. Homing of endogenous stem/progenitor cells for in situ tissue regeneration: promises, strategies, and translational perspectives. Biomaterials. 2011;32(12):3189–209. doi: 10.1016/j.biomaterials.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 19.Lau TT, Wang DA. Stromal cell-derived factor-1 (SDF-1): homing factor for engineered regenerative medicine. Expert Opin Biol Ther. 2011;11(2):189–97. doi: 10.1517/14712598.2011.546338. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt A, et al. Basic fibroblast growth factor controls migration in human mesenchymal stem cells. Stem Cells. 2006;24(7):1750–8. doi: 10.1634/stemcells.2005-0191. [DOI] [PubMed] [Google Scholar]
- 21.Tasso R, et al. The role of bFGF on the ability of MSC to activate endogenous regenerative mechanisms in an ectopic bone formation model. Biomaterials. 2012;33(7):2086–96. doi: 10.1016/j.biomaterials.2011.11.043. [DOI] [PubMed] [Google Scholar]
- 22.Bianchi G, et al. Ex vivo enrichment of mesenchymal cell progenitors by fibroblast growth factor 2. Exp Cell Res. 2003;287(1):98–105. doi: 10.1016/S0014-4827(03)00138-1. [DOI] [PubMed] [Google Scholar]
- 23.Rodan SB, et al. Effects of acidic and basic fibroblast growth factors on osteoblastic cells. Connect Tissue Res. 1989;20(1–4):283–8. doi: 10.3109/03008208909023898. [DOI] [PubMed] [Google Scholar]
- 24.Thesleff I, Tummers M. Stem cells and tissue engineering: prospects for regenerating tissues in dental practice. Med Princ Pract. 2003;12(Suppl 1):43–50. doi: 10.1159/000069840. [DOI] [PubMed] [Google Scholar]
- 25.Inanç B, Elçin AE, Elçin YM. In vitro differentiation and attachment of human embryonic stem cells on periodontal tooth root surfaces. Tissue Eng A. 2009;15(11):3427–35. doi: 10.1089/ten.tea.2008.0380. [DOI] [PubMed] [Google Scholar]
- 26.Elçin YM, Inanç B, Elçin AE. Human embryonic stem cell differentiation on periodontal ligament fibroblasts. Methods Mol Biol. 2010;584:269–281. doi: 10.1007/978-1-60761-369-5_14. [DOI] [PubMed] [Google Scholar]
- 27.Yang YR, et al. Transplantation of embryonic stem cells improves the regeneration of periodontal furcation defects in a porcine model. J Clin Periodontol. 2013;40(4):364–371. doi: 10.1111/jcpe.12069. [DOI] [PubMed] [Google Scholar]
- 28.Mosher JT, et al. Lack of population diversity in commonly used human embryonic stem-cell lines. N Engl J Med. 2010;362(2):183–5. doi: 10.1056/NEJMc0910371. [DOI] [PubMed] [Google Scholar]
- 29.Swijnenburg RJ, et al. In vivo imaging of embryonic stem cells reveals patterns of survival and immune rejection following transplantation. Stem Cells Dev. 2008;17(6):1023–9. doi: 10.1089/scd.2008.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robey PG, et al. Generation of clinical grade human bone marrow stromal cells for use in bone regeneration. Bone. 2015;70:87–92. doi: 10.1016/j.bone.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner W, et al. Aging and replicative senescence have related effects on human stem and progenitor cells. PLoS One. 2009;4(6) doi: 10.1371/journal.pone.0005846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lian Q, et al. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121(9):1113–23. doi: 10.1161/CIRCULATIONAHA.109.898312. [DOI] [PubMed] [Google Scholar]
- 33.Kretlow JD, et al. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 2008;9:60. doi: 10.1186/1471-2121-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katsara O, et al. Effects of donor age, gender, and in vitro cellular aging on the phenotypic, functional, and molecular characteristics of mouse bone marrow-derived mesenchymal stem cells. Stem Cells. 2011;20(9):1549–61. doi: 10.1089/scd.2010.0280. [DOI] [PubMed] [Google Scholar]
- 35.Mueller SM, Glowacki J. Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J Cell Biochem. 2001;82(4):583–90. doi: 10.1002/jcb.1174. [DOI] [PubMed] [Google Scholar]
- 36.Mendes SC, et al. Bone tissue-engineered implants using human bone marrow stromal cells: effect of culture conditions and donor age. Tissue Eng. 2002;8(6):911–20. doi: 10.1089/107632702320934010. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez JP, et al. Mesenchymal stem cells from osteoporotic patients produce a type I collagen-deficient extracellular matrix favoring adipogenic differentiation. J Cell Biochem. 2000;79(4):557–65. doi: 10.1002/1097-4644(20001215)79:4<557::AID-JCB40>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki Y, et al. Stromal cell activity in bone marrow from the tibia and iliac crest of patients with rheumatoid arthritis. J Bone Miner Metab. 2001;19(1):56–60. doi: 10.1007/s007740170061. [DOI] [PubMed] [Google Scholar]
- 39.Bo Q, et al. Human mesenchymal stem cells as delivery of osteoprotegerin gene: homing and therapeutic effect for osteosarcoma. Drug Des Devel Ther. 2015;9:969–76. doi: 10.2147/DDDT.S77116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diptiman C, et al. Therapeutic potential of adult bone marrow-derived mesenchymal stem cells in diseases of the skeleton. J Cell Biochem. 2010;111(2):249–57. doi: 10.1002/jcb.22701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krishna KK, et al. Mesenchymal stem cells as vectors for lung cancer therapy. Respiration. 2013;85(6):443–51. doi: 10.1159/000351284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 44.Hirschi KK, Li S, Roy K. Induced pluripotent stem cells for regenerative medicine. Annu Rev Biomed Eng. 2014;16:277–94. doi: 10.1146/annurev-bioeng-071813-105108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 46.Ebert AD, et al. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457(7227):277–80. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marchetto MC, et al. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell. 2010;143(4):527–39. doi: 10.1016/j.cell.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown ME, et al. Derivation of induced pluripotent stem cells from human peripheral blood T lymphocytes. PLoS One. 2010;5(6) doi: 10.1371/journal.pone.0011373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tamaoki N, et al. Dental pulp cells for induced pluripotent stem cell banking. J Dent Res. 2010;89(8):773–8. doi: 10.1177/0022034510366846. [DOI] [PubMed] [Google Scholar]
- 50.Wada N, et al. Induced pluripotent stem cell lines derived from human gingival fibroblasts and periodontal ligament fibroblasts. J Periodontal Res. 2011;46(4):438–47. doi: 10.1111/j.1600-0765.2011.01358.x. [DOI] [PubMed] [Google Scholar]
- 51.Nomura Y, et al. Human periodontal ligament fibroblasts are the optimal cell source for induced pluripotent stem cells. Histochem Cell Biol. 2012;137(6):719–32. doi: 10.1007/s00418-012-0923-6. [DOI] [PubMed] [Google Scholar]
- 52.Hou P, et al. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 2013;341(6146):651–4. doi: 10.1126/science.1239278. [DOI] [PubMed] [Google Scholar]
- 53.Nakagawa M, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26(1):101–6. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 54.Umezaki Y, et al. Human gingival integration-free iPSCs; a source for MSC-like cells. Int J Mol Sci. 2015;16(6):13633–48. doi: 10.3390/ijms160613633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Egusa H, et al. Gingival fibroblasts as a promising source of induced pluripotent stem cells. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0012743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.••.Hu KJ. All roads lead to induced pluripotent stem cells: the technologies of iPSC generation. Stem Cells Dev. 2014;23(12):1285–300. doi: 10.1089/scd.2013.0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim D, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4(6):472–6. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou H, et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4(5):381–4. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cho HJ, et al. Induction of pluripotent stem cells from adult somatic cells by protein-based reprogramming without genetic manipulation. Blood. 2010;116(3):386–95. doi: 10.1182/blood-2010-02-269589. [DOI] [PubMed] [Google Scholar]
- 60.Ru Z, et al. iPSCs and small molecules: a reciprocal effort towards better approaches for drug discovery. Acta Pharmacol Sin. 2013;34(6):765–76. doi: 10.1038/aps.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feng B, et al. Molecules that promote or enhance reprogramming of somatic cells to induced pluripotent stem cells. Cell Stem Cell. 2009;4(4):301–12. doi: 10.1016/j.stem.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 62.Zhou W, Freed CR. Adenoviral gene delivery can reprogram human fibroblasts to induced pluripotent stem cells. Stem Cells. 2009;27(11):2667–74. doi: 10.1002/stem.201. [DOI] [PubMed] [Google Scholar]
- 63.Stadtfeld M, et al. Induced pluripotent stem cells generated without viral integration. Science. 2008;322(5903):945–9. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fusaki N, et al. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85(8):348–62. doi: 10.2183/pjab.85.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Warren L, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7(5):618–30. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Woltjen K, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458(7239):766–70. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ding S, et al. Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell. 2005;122(3):473–83. doi: 10.1016/j.cell.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 68.Geurts AM, et al. Gene mutations and genomic rearrangements in the mouse as a result of transposon mobilization from chromosomal concatemers. PLoS Genet. 2006;2(9) doi: 10.1371/journal.pgen.0020156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kazim H, et al. Generation of adult human induced pluripotent stem cells using non-viral minicircle DNA vectors. Nat Protoc. 2011;6(1):78–88. doi: 10.1038/nprot.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anokye-Danso F, et al. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell. 2011;8(4):376–88. doi: 10.1016/j.stem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miyoshi N, et al. Reprogramming of mouse and human cells to pluripotency using mature microRNAs. Cell Stem Cell. 2011;8(6):633–8. doi: 10.1016/j.stem.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 72.Warren L, et al. Feeder-free derivation of human induced pluripotent stem cells with messenger RNA. Sci Rep. 2012;2:657. doi: 10.1038/srep00657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang R, Zhang LH, Xie X. iPSCs and small molecules: a reciprocal effort towards better approaches for drug discovery. Acta Pharmacol Sin. 2013;34(6):765–76. doi: 10.1038/aps.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huangfu D, et al. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol. 2008;26(7):795–7. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Novak A, et al. Enhanced reprogramming and cardiac differentiation of human keratinocytes derived from plucked hair follicles, using a single excisable lentivirus. Cell Reprogramming. 2010;12(6):665–78. doi: 10.1089/cell.2010.0027. [DOI] [PubMed] [Google Scholar]
- 76.Liu H, et al. Generation of endoderm-derived human induced pluripotent stem cells from primary hepatocytes. Hepatology. 2010;51(5):1810–9. doi: 10.1002/hep.23626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou T, et al. Generation of induced pluripotent stem cells from urine. J Am Soc Nephrol. 2011;22(7):1221–8. doi: 10.1681/ASN.2011010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aasen T, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26(11):1276–84. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- 79.Maetzel D, et al. Genetic and chemical correction of cholesterol accumulation and impaired autophagy in hepatic and neural cells derived from Niemann-Pick type C patient-specific iPS cells. Stem Cell Rep. 2014;2(6):866–80. doi: 10.1016/j.stemcr.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eggenschwiler R, et al. Sustained knockdown of a disease-causing gene in patient-specific induced pluripotent stem cells using lentiviral vector-based gene therapy. Stem Cells Transl Med. 2013;2(9):641–54. doi: 10.5966/sctm.2013-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reinhardt P, et al. Genetic correction of a lrrk2 mutation in human iPSCs links parkinsonian neurodegeneration to ERK-dependent changes in gene expression. Cell Stem Cell. 2013;12(3):354–67. doi: 10.1016/j.stem.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 82.Egusa H, et al. Comparative analysis of mouse-induced pluripotent stem cells and mesenchymal stem cells during osteogenic differentiation in vitro. Stem Cells Dev. 2014;23(18):2156–69. doi: 10.1089/scd.2013.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Diederichs S, Tuan RS. Functional comparison of human-induced pluripotent stem cell-derived mesenchymal cells and bone marrow-derived mesenchymal stromal cells from the same donor. Stem Cells Dev. 2014;23(14):1594–610. doi: 10.1089/scd.2013.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li F, Niyibizi C. Cells derived from murine induced pluripotent stem cells (iPSC) by treatment with members of TGF-beta family give rise to osteoblasts differentiation and form bone in vivo. BMC Cell Biol. 2012;13:35. doi: 10.1186/1471-2121-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.•.Duan XJ, et al. Application of induced pluripotent stem (iPS) cells in periodontal tissue regeneration. J Cell Physiol. 2011;226(1):150–7. doi: 10.1002/jcp.22316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Polimeni G, et al. Histopathological observations of a polylactic acid-based device intended for guided bone/tissue regeneration. Clin Implant Dent Relat Res. 2008;10(2):99–105. doi: 10.1111/j.1708-8208.2007.00067.x. [DOI] [PubMed] [Google Scholar]
- 87.Sculean A, Nikolidakis D, Schwarz F. Regeneration of periodontal tissues: combinations of barrier membranes and grafting materials—biological foundation and preclinical evidence: a systematic review. J Clin Periodontol. 2008;35(Suppl):106–16. doi: 10.1111/j.1600-051X.2008.01263.x. [DOI] [PubMed] [Google Scholar]
- 88.•.Hynes K, et al. Mesenchymal stem cells from iPS cells facilitate periodontal regeneration. J Dent Res. 2013;92(9):833–9. doi: 10.1177/0022034513498258. [DOI] [PubMed] [Google Scholar]
- 89.Yang H, et al. Therapeutic effect of TSG-6 engineered iPSC-derived MSCs on experimental periodontitis in rats: a pilot study. PLoS One. 2014;9(6) doi: 10.1371/journal.pone.0100285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao Y, et al. Two supporting factors greatly improve the efficiency of human iPSC generation. Cell Stem Cell. 2008;3(5):475–9. doi: 10.1016/j.stem.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 91.Zhumur G, et al. Persistent donor cell gene expression among human induced pluripotent stem cells contributes to differences with human embryonic stem cells. PLoS One. 2010;5(2) doi: 10.1371/journal.pone.0008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gore A, et al. Somatic coding mutations in human induced pluripotent stem cells. Nature. 2011;471(7336):63–7. doi: 10.1038/nature09805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448(7151):313–7. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 94.Zhao T, et al. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474(7350):212–5. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- 95.Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer. 2011;11(4):268–77. doi: 10.1038/nrc3034. [DOI] [PubMed] [Google Scholar]
- 96.Lengner CJ, et al. iPS cell technology in regenerative medicine. Ann N Y Acad Sci. 2010;1192:38. doi: 10.1111/j.1749-6632.2009.05213.x. [DOI] [PubMed] [Google Scholar]


