Abstract
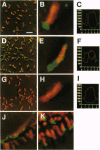
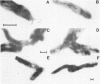
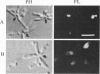
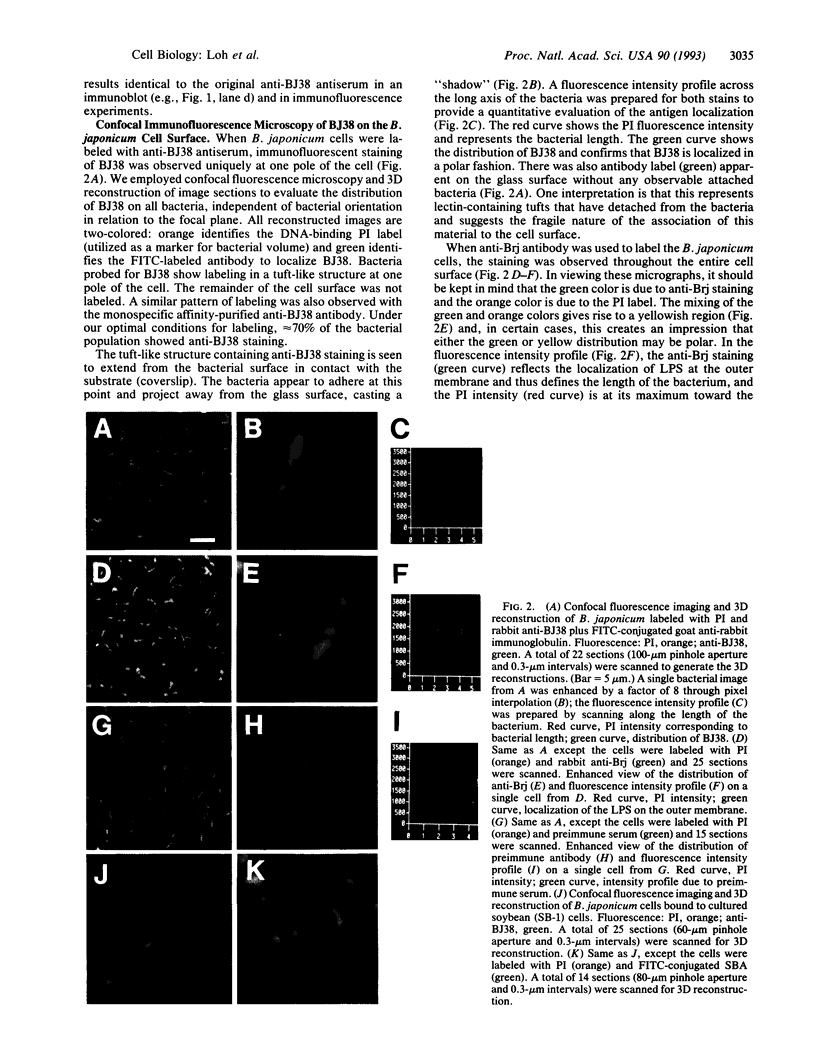
A polyclonal antiserum generated against the Bradyrhizobium japonicum lectin BJ38 was characterized to be specifically directed against the protein. Treatment of B. japonicum cells with this antiserum and subsequent visualization with transmission electron microscopy and both conventional and confocal fluorescence microscopy revealed BJ38 at only one pole of the bacterium. BJ38 appeared to be organized in a tuft-like mass, separated from the bacterial outer membrane. BJ38 localization was coincident with the attachment site for (i) homotypic agglutination to other B. japonicum cells, (ii) adhesion to the cultured soybean cell line SB-1, and (iii) adsorption to Sepharose beads covalently derivatized with lactose. In contrast, the plant lectin soybean agglutinin labeled the bacteria at the pole distant from the bacterial attachment site. These results indicate that the topological distribution of BJ38 is consistent with a suggested role for this bacterial lectin in the polar binding of B. japonicum to other cells and surfaces.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aplin J. D., Hughes R. C. Protein-derivatised glass coverslips for the study of cell-to substratum adhesion. Anal Biochem. 1981 May 1;113(1):144–148. doi: 10.1016/0003-2697(81)90057-9. [DOI] [PubMed] [Google Scholar]
- Bal A. K., Shantharam S., Ratnam S. Ultrastructure of Rhizobium japonicum in relation to its attachment to root hairs. J Bacteriol. 1978 Mar;133(3):1393–1400. doi: 10.1128/jb.133.3.1393-1400.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlool B. B., Schmidt E. L. Immunofluorescent polar tips of Rhizobium japonicum: possible site of attachment or lectin binding. J Bacteriol. 1976 Mar;125(3):1188–1194. doi: 10.1128/jb.125.3.1188-1194.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlool B. B., Schmidt E. L. Lectins: a possible basis for specificity in the Rhizobium--legume root nodule symbiosis. Science. 1974 Jul 19;185(4147):269–271. doi: 10.1126/science.185.4147.269. [DOI] [PubMed] [Google Scholar]
- Calvert H. E., Lalonde M., Bhuvaneswari T. V., Bauer W. D. Role of lectins in plant--microorganism interactions. IV. Ultrastructural localization of soybean lectin binding sites of Rhizobium japonicum. Can J Microbiol. 1978 Jul;24(7):785–793. doi: 10.1139/m78-132. [DOI] [PubMed] [Google Scholar]
- Carlson R. W., Sanders R. E., Napoli C., Albersheim P. Host-Symbiont Interactions: III. Purification and Partial Characterization of Rhizobium Lipopolysaccharides. Plant Physiol. 1978 Dec;62(6):912–917. doi: 10.1104/pp.62.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager D. A., Burgess R. R. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980 Nov 15;109(1):76–86. doi: 10.1016/0003-2697(80)90013-5. [DOI] [PubMed] [Google Scholar]
- Halverson L. J., Stacey G. Effect of lectin on nodulation by wild-type Bradyrhizobium japonicum and a nodulation-defective mutant. Appl Environ Microbiol. 1986 Apr;51(4):753–760. doi: 10.1128/aem.51.4.753-760.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson L. J., Stacey G. Signal exchange in plant-microbe interactions. Microbiol Rev. 1986 Jun;50(2):193–225. doi: 10.1128/mr.50.2.193-225.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S. C., Schindler M., Wang J. L. Carbohydrate binding activities of Bradyrhizobium japonicum. II. Isolation and characterization of a galactose-specific lectin. J Cell Biol. 1990 Oct;111(4):1639–1643. doi: 10.1083/jcb.111.4.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S. C., Wang J. L., Schindler M. Carbohydrate binding activities of Bradyrhizobium japonicum. I. Saccharide-specific inhibition of homotypic and heterotypic adhesion. J Cell Biol. 1990 Oct;111(4):1631–1638. doi: 10.1083/jcb.111.4.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S. C., Ye W. Z., Schindler M., Wang J. L. Quantitative assay for binding of Bradyrhizobium japonicum to cultured soybean cells. J Bacteriol. 1988 Sep;170(9):3882–3890. doi: 10.1128/jb.170.9.3882-3890.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mort A. J., Bauer W. D. Composition of the Capsular and Extracellular Polysaccharides of Rhizobium japonicum: CHANGES WITH CULTURE AGE AND CORRELATIONS WITH BINDING OF SOYBEAN SEED LECTIN TO THE BACTERIA . Plant Physiol. 1980 Jul;66(1):158–163. doi: 10.1104/pp.66.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pueppke S. G. Adsorption of slow- and fast-growing rhizobia to soybean and cowpea roots. Plant Physiol. 1984 Aug;75(4):924–928. doi: 10.1104/pp.75.4.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. E., Fisher P. A. Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984 Jul;99(1 Pt 1):20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey G., Paau A. S., Brill W. J. Host recognition in the Rhizobium-soybean symbiosis. Plant Physiol. 1980 Oct;66(4):609–614. doi: 10.1104/pp.66.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien H. C., Schmidt E. L. Polarity in the exponential-phase Rhizobium japonicum cell. Can J Microbiol. 1977 Sep;23(9):1274–1284. doi: 10.1139/m77-191. [DOI] [PubMed] [Google Scholar]
- Vesper S. J., Bauer W. D. Role of Pili (Fimbriae) in Attachment of Bradyrhizobium japonicum to Soybean Roots. Appl Environ Microbiol. 1986 Jul;52(1):134–141. doi: 10.1128/aem.52.1.134-141.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Voort H. T., Brakenhoff G. J., Baarslag M. W. Three-dimensional visualization methods for confocal microscopy. J Microsc. 1989 Feb;153(Pt 2):123–132. doi: 10.1111/j.1365-2818.1989.tb00553.x. [DOI] [PubMed] [Google Scholar]