Abstract
Background:
Hypersomnia of central origin from narcolepsy or idiopathic hypersomnia (IHS) is characterized by pathological levels of excessive daytime sleepiness (EDS). Central hypersomnia has historically been underdiagnosed and poorly understood, especially with respect to its impact on daytime functioning and quality of life in children.
Objective:
Describe the psychosocial adjustment of children treated for narcolepsy or IHS on school performance, quality of life, and physical/extracurricular activities.
Methods:
Using a matched case control design, we compared child self- and parent-reported data from thirty-three 8- to 16-year-olds with an established diagnosis of narcolepsy or IHS, according to ICSD-2 criteria, to that of 33 healthy children matched by age, race/ethnicity, gender, and household income. Assessments evaluated academic performance, quality of life and wellness, sleepiness, and participation in extracurricular activities.
Results:
Compared to healthy controls, children with central hypersomnia had poorer daytime functioning in multiple domains. Children with hypersomnia missed more days of school and had lower grades than healthy controls. Children with hypersomnia had poorer quality of life by both parent and child report. Children with hypersomnia were significantly sleepier, had higher BMI, and were more likely to report a history of recent injury. Finally, children with hypersomnia engaged in fewer after-school activities than healthy controls.
Conclusions:
A range of significant psychosocial consequences are reported in children with hypersomnia even after a diagnosis has been made and treatments initiated. Health care professionals should be mindful of the psychosocial problems that may present in children with hypersomnia over the course of treatment.
Citation:
Avis KT, Shen J, Weaver P, Schwebel DC. Psychosocial characteristics of children with central disorders of hypersomnolence versus matched healthy children. J Clin Sleep Med 2015;11(11):1281–1288.
Keywords: children, daytime sleepiness, hypersomnolence, daytime functioning
Patients with hypersomnia of central origin are unable to stay awake and alert during the day and have unintended lapses into sleep that are not due to disturbed nocturnal sleep, insufficient sleep time, or misaligned circadian rhythms.1 Central hypersomnias include narcolepsy and idiopathic hypersomnia, both of which are characterized by chronic and persistent pathological sleepiness despite obtaining sufficient amounts of sleep of good quality. Patients may not complain of excessive sleepiness, but instead describe a sense of being in a fog, mental dullness, forgetfulness, irritability, declining performance in school, inattentiveness, and even hyperactivity as they attempt to “fight” sleep.2–5 On average, central hypersomnia takes ten years to diagnose, with up to three referrals made before an accurate diagnosis is achieved.6 Prior to diagnosis, individuals with central hypersomnia are often stigmatized as lazy, inattentive, unmotivated, or depressed; over time, the presence of the disorder results in significant psychosocial consequences.2–17
BRIEF SUMMARY
Current Knowledge/Study Rationale: Hypersomnolence may impact children's daytime functioning and quality of life.
Study Impact: Pediatric central disorders of hypersomnolence influences academic functioning, quality of life, and wellness compared to healthy controls matched on age, race/ethnicity, gender, and estimated household income.
Three primary areas of psychosocial difficulty are reported among adults with CH: diminished academic/occupational performance, poor quality of life, and reduced activity/extracurricular activity levels. Within the academic/ occupational domain, the presence of central hypersomnia puts individuals at high risk for academic failure, perhaps due to symptoms of difficulty concentrating in class and mental inefficiency.4,7,8,13,15,18 Similar problems emerge in employment, where adults with central hypersomnia have elevated rates of unemployment, and when employed, lower income levels.18–22 In the quality of life domain, adults with central hypersomnia rate their quality of life as significantly lower than the general population, and at a similar magnitude to individuals with other chronic illnesses such as hypertension and chronic obstructive pulmonary disease.23,24 Central hypersomnia is characterized by difficulty performing everyday tasks and difficulty with marital and other relationships.5,10–17,23–28 Within the health and wellness domain, central hypersomnia is associated with reduced personal mental and physical health, with adults with central hypersomnia reporting elevated levels of depression, sleepiness, weight gain within proximity of onset of symptoms, higher rates of obesity, and higher risk of injury.20,21,23 Adults with central hypersomnia also report reduced participation in activities. They are less physically active and less engaged in social or extracurricular activities than healthier adults, probably due to fatigue and lack of energy, and to greater withdrawal and isolation due to embarrassment of symptoms.12,20,28
Though it may seem intuitive to apply findings from adults with central hypersomnia to the pediatric central hypersomnia population, research in other chronic illnesses suggests developmental processes create differing risks for psychosocial difficulties and different adaptive processes among children with chronic illness compared to adults.29–33 Despite developmental differences, it is reasonable to hypothesize that central hypersomnia might have a significant impact on children's daytime and psychosocial functioning, and existing data, though sparse, provide initial evidence supporting this hypothesis.11,34–36 One study evaluated 70 children with central hypersomnia aged 4 to 18 from centers in the United Kingdom, US, Europe, and Australia using a cross-sectional international survey of patients and healthy controls. Children with central hypersomnia had higher rates of behavioral problems and poorer quality of life based on questionnaire data. Limitations of this study included lack of matching cases with controls and failure to diagnosis children with central hypersomnia uniformly using overnight sleep studies and multiple sleep latency tests.36
A second study evaluated parent-report of psychosocial functioning of 12 children with narcolepsy based on a standardized intelligence battery and parent ratings of child's behavioral functioning.34 Although 11 of 12 (92%) children had average intelligence, 10 of 12 (83%) were rated as having significant behavioral problems and 9 of 12 (75%) as having significant internalizing problems. A few other small studies have been published. A published abstract described moodiness, adjustment problems and increased delinquent behavior in 18 children with onset of narcolepsy symptoms prior to age 14; no control group was reported.36 A case overview of 8 children with central hypersomnia described substantial behavioral and emotional difficulties among the sample, including 2 children with central hypersomnia who had been previously misdiagnosed with a psychiatric disorder.11 Finally, Dahl and others have described obesity as a possible associated health consequence of pediatric central hypersomnia, but other wellness behaviors or health outcomes such as injury history are not well documented.37,38
The present study was designed to extend the current literature by comparing a sizable group of children with pathologic central hypersomnia (n = 33) to a group of healthy children matched by age, gender, race/ethnicity, and household income. We considered a wide range of psychosocial characteristics, categorized into three domains that loosely replicate evidence from the adult literature (school performance, quality of life, and health/wellness). We hypothesized children with central hypersomnia would have lower school performance, poorer quality of life, and lower indicators of wellness compared to healthy matched controls.
METHODS
Participants and Recruitment
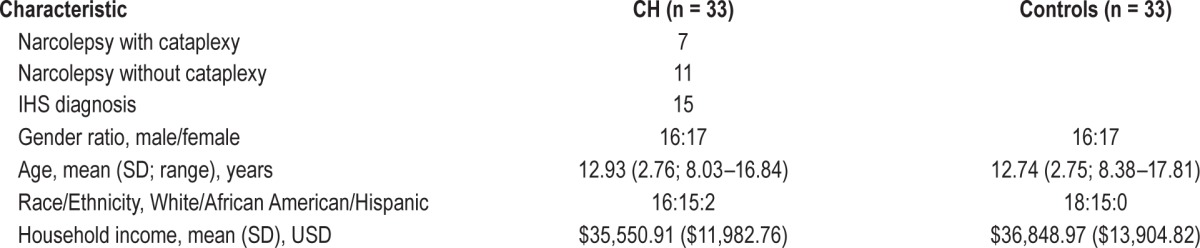
Sixty-six children participated as part of a larger study. Half (n = 33) had been diagnosed with central hypersomnia at the Pediatric Sleep Disorders Center at Children's of Alabama within the past 3 years. Those children met ICSD-2 diagnostic criteria for a hypersomnia of central origin (narcolepsy with or without cataplexy, or idiopathic hypersomnia) based on diagnostic assessments that included overnight polysomnography followed by multiple sleep latency tests the following day, drug screening, and thorough clinical evaluation from board certified sleep specialists. All children with central hypersomnia were treated with wake-promoting medications. Exclusion criteria were minimal. They included cognitive or physical disabilities that prevented full participation in the experimental protocol (e.g., mental retardation, blindness); comorbid medical or neurologic conditions; and antipsychotic medication use. Just one child was excluded, based on recent diagnosis of traumatic brain injury. The sample of children with central hypersomnia had a mean age of 12.93 years old (SD 2.76) and were 51% female, 55% Caucasian, and 45% African American. Two of the Caucasian children were Hispanic. The sample came from families with a mean household income of $35,551 (SD $11,983).
The other half of the sample (n = 33) was composed of healthy children recruited from community sources. The same exclusion criteria were applied, and no children were excluded. The samples were matched by age, gender, race/ethnicity, and average income in the zip code of residence (See Table 1).
Table 1.
Demographic characteristics of children with central hypersomnia (CH) and controls.
The study protocol was approved by the Institutional Review Board of the University of Alabama at Birmingham. All parents provided written informed consent, and all children provided developmentally appropriate assent to participate. Families were compensated for their time.
Measures
Demographics
Parents completed items addressing basic demographic and household information.
Academic Performance and Attendance
Three aspects of school/academic performance were assessed: typical academic grades (on 6-point scale from A, coded as 1, to F, coded as 6), days of home-stay because of sickness, and days of home-stay for reasons other than sickness or attending medical appointments (typically for sleepiness or medical appointments). All 3 items were extracted from the Wolfson and Carskadon's School Sleep Habits Survey.39 Previous research suggests that academic grade reports are a valid reflection of real-world school performance in pediatric sleep disordered populations.29,40
Quality of Life
Quality of life was assessed using both parent-report and child self-report versions of the 23-item Pediatric Quality of Life Inventory 4.0 Generic Core Scales (PedsQL), which assesses health and functioning with 4 scales: physical (8 items), emotional (5 items), social (5 items), and school (5 items).41,42 The parent report format evaluates the parent's perception of their child's quality of life. Scale scores are computed as the sum of the items divided by the number of the items answered, and total scores are transformed to a 100-point scale, with all scores coded such that higher scores indicate higher quality of life. An overall functioning scale is also computed, based on the sum of all items divided by total number of items. The scale is frequently used in both healthy children and pediatric patients with a variety of chronic illnesses and has demonstrated reliability (Cronbach α ≥ 0.80 on all scales) and validity (including construct validity).41,42 Further psychometric information is available in the Appendix.
Wellness
Six constructs assessed general wellness and health: sleepiness, body mass index (BMI), injury history, participation in both physical and extracurricular activities, and studying/homework time after school. To evaluate sleepiness, we sampled level of sleepiness on the day of evaluation using the Epworth Sleepiness Scale-modified for children (ESS).43 Well validated in adults, reliability and validity of this measure were recently rated as “approaching well-established” in the latest review of pediatric sleep measures.44 Internal reliability is adequate (Cronbach α = 0.75).45 Further psychometric information is available in the Appendix. To compute BMI, children's height and weight were measured using standard anthropometric techniques in a private room and BMI was calculated using the web-based CDC BMI calculator. Injury history was assessed by parent-report concerning whether the child had experienced an injury requiring professional medical attention in the past year, a standard measure in the injury prevention literature with adequate reliability and validity.46 Responses were dichotomous (yes vs no). Participation in physical and extracurricular activities and studying/homework time after school was evaluated based on report of children's time spent during the past 2 weeks in (a) organized sports or a regularly scheduled physical activity, (b) organized extracurricular activities, and (c) study/homework outside of school. These items were extracted from Wolfson and Carskadon's School Sleep Habits Survey.39
Data Analysis
Descriptive data were examined first, including a comparison of case vs. control groups. Primary hypotheses were addressed using χ2 (categorical data) and independent samples t-tests (continuous data) to examine differences between the children with central hypersomnia and control children on available measures within the 3 primary domains of interest: school performance, quality of life, and wellness.
RESULTS
Demographic data are reported in Table 1, including gender, age, ethnic groups, and household income. As expected given recruitment strategies to match the samples, no statistically significant differences emerged between children with central hypersomnia and controls on gender ratio, age, racial/ethnic group or household income.
Academic performance and attendance were assessed with 3 outcome measures: academic grades, days of home-stay because of sickness, and days of home-stay for reasons other than sickness. As shown in Table 2, children with central hypersomnia scored significantly lower on academic grades at school than healthy controls (t59.4 = 2.1, p < 0.05). Children with central hypersomnia also stayed at home for more days because of reasons other than sickness or attending medical appointments, compared to controls (t30.6 = 2.7, p < 0.05). There was no significant difference between groups on the days of home stay because of sickness (t52 = 0.7, ns).
Table 2.
Independent samples t-tests between children with CH and controls.
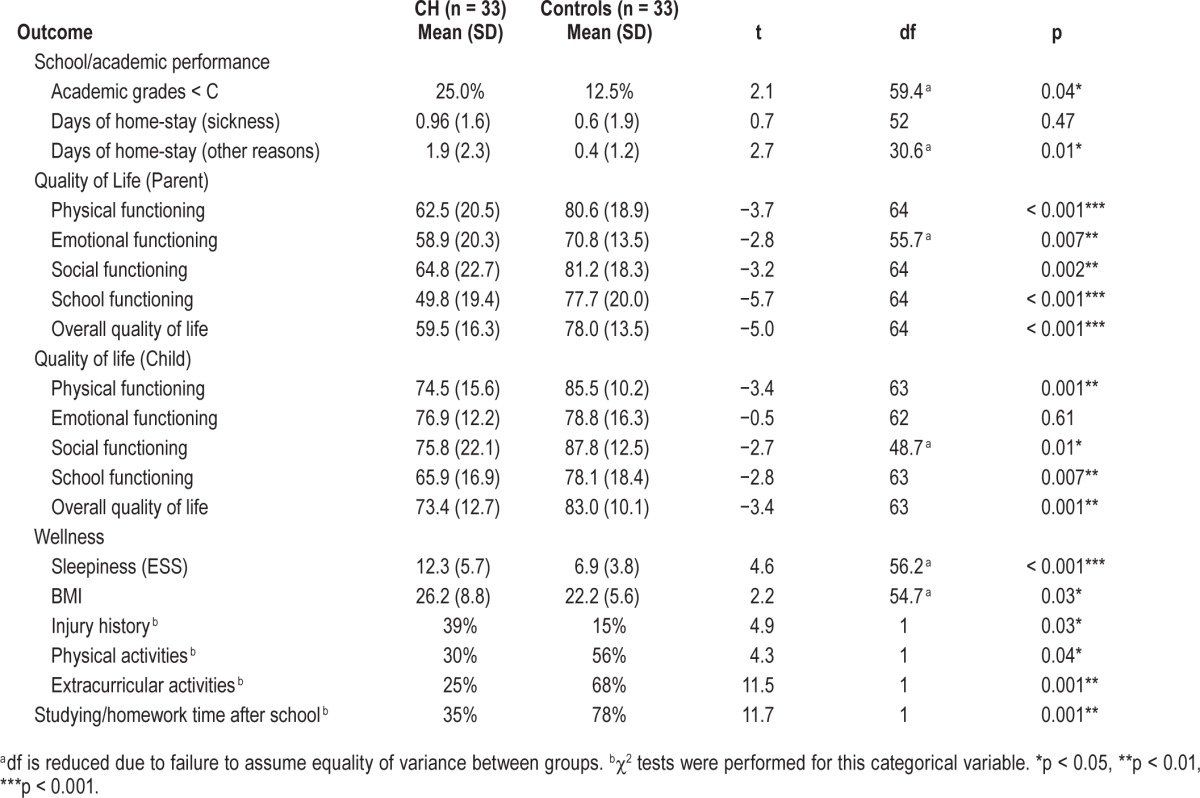
The PedsQL quality of life instrument yields 4 scale scores (physical, emotional, social, and school functioning) and an overall scale score from both parent- and child-report. As shown in Table 2, parents reported children with central hypersomnia to have significantly lower quality of life than parents reported of children in the control group on physical (t64 = −3.7, p < 0.001), emotional (t55.7 = −2.8, p < 0.01), so -cial (t64 = −3.2, p < 0.01), and school functioning (t64 = −5.7, p < 0.001). Children with central hypersomnia also scored significantly lower on parent-reported overall quality of life scale (t64 = −5.0, p < 0.001). Children's self-report on quality of life generally matched those of their parents. Children with central hypersomnia reported lower quality of life than matched control children on physical (t63 = −3.4, p < 0.01), social (t48.7 = −2.7, p < 0.01), and school functioning (t63 = −2.8, p < 0.01), as well as for overall quality of life (t63 = −3.4, p < 0.01). No significant difference emerged between children with central hypersomnia and controls on child-reported emotional functioning quality of life (t62 = −0.5, ns).
Wellness was assessed with 6 constructs: sleepiness (as measured by the modified ESS), BMI, injury history, and participation in physical and extracurricular activities, and studying/homework time after school. Children with central hypersomnia were clinically sleepier, as measured by the ESS, (t56.2 = 4.6, p < 0.01). Children with central hypersomnia had significantly higher BMI scores than controls (t54.7 = 2.2, p < 0.05). Relative to injury history, χ2 analysis revealed a significant difference, with 39% of children with central hypersomnia experiencing an injury requiring professional medical attention in the past year but only 15% of controls experiencing one, χ2 (1) = 4.9, p < 0.05. Finally, evaluation of physical and extracurricular activity found that children with central hypersomnia were significantly less engaged in all activities: organized sports or regularly scheduled physical activities (χ2 (1) = 4.3, p < 0.05), organized extracurricular activities (χ2 (1) = 11.5, p < 0.01), and in studying/homework time after school (χ2 (1) = 11.7, p < 0.01), compared to children in the control group.
DISCUSSION
Our findings document significant psychosocial difficulties in children with central hypersomnia in all areas we assessed: school performance, quality of life, and health and wellness. These findings extend what has been reported in the adult literature7,24 and in scattered small studies with children11,34–36 and lend weight to the hypothesis that children with central hypersomnia are at high risk for a range of psychosocial difficulties.
Our results are consistent with the broader literature, including reports of psychosocial consequences from other pediatric sleep conditions. For example, similar negative psychosocial consequences are reported in studies of children with untreated nocturnal sleep disorders such as obstructive sleep apnea29,40,43,47–53 and periodic limb movement disorder.54 Whether the current findings are due to excessive sleepiness as in other sleep disorders or a particular manifestation of this particular sleep disorder remains inconclusive and might be examined in future research.
We also replicated a trend from other pediatric chronic illness populations that parents of children with chronic illness rate children's quality of life as lower than the children themselves rate it.55–58 A likely explanation for this result is that sleepiness (and other illness symptoms) experienced by the child is internalized, such that over time the children accept it as “normal.” Perhaps parents notice and recognize the abnormality of children's symptoms, for example by witnessing children actively fall asleep at inappropriate times or by receiving reports from teachers about the impact of sleepiness on children's school performance. This result highlights the importance of multi-informant assessment and addressing relevant issues in clinical settings with both children and parents.
The present study has other implications for practice also. Once a child is diagnosed, clinicians generally have three primary aims of treatment for central hypersomnia: decrease sleepiness, improve functionality, and improve quality of life.2–5,59–61 For children and adults alike, treatment focuses on education, healthy sleep habits, and pharmacologic interventions, with the most substantial clinical focus usually centering around symptom reduction of sleepiness.11,60 The effect of nonpharmacological behavioral interventions on improving psychosocial outcomes beyond sleepiness has not been evaluated in published trials, but our results imply there are significant psychosocial impacts from central hypersomnia, and clinical focus on the broad and associated symptom patterns should be considered. In fact, the sample of children with central hypersomnia we studied was being treated with medication and other educational and behavioral strategies such as recommendations to increase physical activity, take short refreshing naps, change tasks frequently, and sit near the teacher in class,59–61 but still reported substantial psychosocial consequences. Those behavioral approaches were apparently insufficient to achieve psychosocial functioning equivalent to matched controls, suggesting further research may be needed to identify barriers to the current treatment recommendations and to shed light on alternative treatment mechanisms.
Development of psychosocial treatment strategies will benefit greatly from uncovering the causal mechanism behind reduced psychosocial functioning among children with chronic illnesses such as central hypersomnia. Many causal mechanisms may be considered, and we did not carefully evaluate specific severity factors such as severity/frequency of cataplexy or level of cognitive fatigue in this study. One possible explanation is that experiencing sleepiness in and of itself significantly contributes to reduced psychosocial functioning. Findings in the adult literature have been somewhat inconsistent.62,63 In a post hoc analysis, we did find that sleepiness, as measured by the ESS, was significantly correlated with school functioning by both parent (r = −0.30, p = 0.02) and child report (r = −0.28, p = 0.02). By child report only, sleepiness was significantly correlated with social functioning as well (r = −0.25, p = 0.048). As most peer relationships are formed at school, perhaps the child directly experiences the impact of sleepiness relative to their forming peer relationships. No other measures of QOL were significantly associated with sleepiness.
Another plausible hypothesis is that barriers in opportunity for normal development mediates the relation.29–33 Childhood is a critical time for development of social relationships at school, in the community and with a family, and also for the development of metacognitive skills necessary for learning and school success. Failure to experience those developmental milestones due to chronic illness may lead to inferior metacognition and development of social skills.
As a parallel, consider the more established literature of psychosocial functioning and treatment of children with attention deficit hyperactivity disorder (ADHD). Although we recognize differences between the disorders, like central hypersomnia, ADHD is primarily treated with medication but children with ADHD also benefit greatly from psychosocial and educational interventions designed to improve cognitive development, and metacognition.64 Such behavioral interventions reduce cognitive impairment and improve health, social development, school performance, quality of life, and success in adulthood among children with ADHD. The large randomized MTA study found that both treatment with medication alone and the combined treatment with medication and behavioral interventions result in the greatest improvement in ADHD symptoms.62,63 However, the combination of medication and behavioral intervention resulted in the greatest improvement in areas of associated psychosocial functioning such as anxiety, academic performance, behavioral difficulties, and parent child relations and relationships with peers. Receiving only pharmacological treatment did not achieve improvement in those domains for children with ADHD.65 Drawing a parallel, it may be that similar interventions for children with central hypersomnia—those combining pharmacological and behavioral interventions—may yield similar results that reduce symptoms and also improve psychosocial functioning. Perhaps interpersonal and educational interventions (for example, peer tutoring to increase attention and encourage social interactions; shortened tasks with breaks, support groups, family interaction supportive interventions, structured regular activities self-monitoring of symptoms) that are effective with children with ADHD would improve academic, neurobehavioral, and psychosocial functioning among children with central hypersomnia also.
The current study had several strengths. We evaluated a relatively large sample of children with central hypersomnia, especially compared to prior research. We used a matched case control design to compare children with central hypersomnia to healthy controls and evaluated children with central hypersomnia based on strict ICSD-2 clinical criteria, including results of overnight sleep study and multiple sleep latency test. Finally, our study is unique in that we evaluated previously unreported aspects of psychosocial functioning. The research also had limitations. It was conducted in previously diagnosed children whose length of treatment varied widely; exact time of diagnosis was not known and our analysis was not prospective in design. In addition, although we included both child-and parent-report measures, we relied solely on self-report of psychosocial functioning, and such instruments may suffer from various biases, including recall bias. Further, some instruments (e.g., the Epworth Sleepiness Scale) are not fully established as valid when used with children.
Future research should use longitudinal and prospective designs to understand how psychosocial difficulties emerge, develop, and perhaps resolve among children with central hypersomnia, and how they persist and change as individuals with central hypersomnia develop into adulthood. Moderating, mediating, and potentially alterable medical and psychosocial influences should be examined in greater detail, including the influence of delays in diagnosis and misdiagnosis; adverse medication effects; pharmacological and behavioral treatment programs; reactions to the child's symptoms by peers, parents, teachers, and others; and other potential mediating factors such as cataplexy, mental fogginess, or residual fatigue. Similarly, although comparing children with central hypersomnia to healthy children provides information about difficulties associated with central hypersomnia, research is needed to identify individual difference factors that predict psychosocial difficulties. Some children are likely more resilient than others.
In conclusion, study findings highlight ongoing psychosocial difficulties in children with central hypersomnia. Children with central hypersomnia were found to have significant impairment in all three domains investigated: school performance, quality of life, and wellness, even after receiving the standard of care treatment recommendations of medication, academic and behavioral modifications, and participation in exercise and activities. There is clear need for ongoing monitoring of a breadth of symptoms once treatments are initiated and for further investigation in how the field of sleep medicine may better understand and address persistent psychosocial difficulties among children with central hypersomnia.
DISCLOSURE STATEMENT
This was not an industry supported study. This project was supported financially by the Kaul Pediatric Research Institute of the Children's of Alabama Foundation. It was also partly supported by Award Number R01HD058573 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The authors have indicated no financial conflicts of interest. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health. Author Contributions: Dr. Avis conceptualized and designed the study, drafted the initial manuscript, and approved the final manuscript as submitted. Dr. Shen carried out the initial analyses, reviewed and revised the manuscript, and approved the final manuscript as submitted. Mr. Weaver carried out analyses, reviewed and revised the manuscript, and approved the final manuscript as submitted. Dr. Schwebel conceptualized and designed the study, reviewed and revised the initial manuscript, and approved the final manuscript as submitted.
ACKNOWLEDGMENTS
The authors are grateful to the children and their parents who participated in the study. We also acknowledge Anna Johnston and the students of the UAB Youth Safety Lab for their endless time and energy in the recruitment of participants and execution of this project.
ABBREVIATIONS
- EDS
excessive daytime sleepiness
- BMI
body mass index
- CH
central hypersomnia
- ESS
Epworth Sleepiness Scale
- IHS
idiopathic hypersomnia
- PedsQL
Pediatric Quality of Life Inventory
APPENDIX
PedsQL Pediatric Quality of Life Inventory
The PedsQL Pediatric Quality of Life Inventory is a 23-item measure for assessment of the quality of life of children ages 2–18. Parent-proxy report forms are available for all ages and self-report measures are available for ages 5–18. Forms are available for the following age groups: toddler (age 2–4), young child (5–7), children (8–12), and teen (13–18).
The items are structured to assess the following 4 scales: physical, emotional, social, and school functioning (Varni, 2001). Each item is rated on a 5-point Likert scale referring to how much of a problem each item has been for the child during the past one month, ranging from 0 (never a problem) to 4 (almost always a problem). These items are reverse scored and then transformed to reflect a zero to one hundred rating (0 = 100, 1 = 75, 2 = 50, 3 = 25, 4 = 0).
Scores yield an overall health related quality of life score, computed as the sum of the items divided by the number of items answered on the appropriate subscales. Higher scores reflect better health related quality of life.
The measure has very good feasibility (1.54% missing on self-report measures, 1.95% missing from parent proxy reports) (Varni, 2001). High internal consistency reliability data has been found for all scores (total scale score: α = 0.88 child and 0.90 parent-proxy; physical health summary score: α = 0.80 child and 0.88 parent-proxy; psychosocial health summary score: α = 0.83 child and 0.86 parent-proxy). The measure also has been shown to have good construct validity using the known-groups method of comparing healthy controls to both acutely and chronically ill children. The child self-report and parent proxy report measures have moderate to good agreement across the scores. The highest agreements were found in the total score, psychosocial score, and emotional functioning scores (Varni, 2007).
References
- Varni JW, Limbers CA, Burwinkle TM. How young can children reliably and validly self-report their health-related quality of life? An analysis of 8,591 children across age subgroups with the PEDSQL 4.0 Generic Core Scales. Health Qual Life Outcomes. 2007:5. doi: 10.1186/1477-7525-5-1. doi: 10.1186/1477-7525-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varni JW, Seid M, Rode CA. The PedsQL: Measurement model for the Pediatric Quality of Life Inventory. Med Care. 1999;37:126–39. doi: 10.1097/00005650-199902000-00003. [DOI] [PubMed] [Google Scholar]
- Varni JW, Seid M, Kurtin PS. The PedsQL 4.0: Reliability and validity of the Pediatric Quality of Life Inventory Version 4.0 Generic Core Scales in healthy and patient populations. Medical Care. 2001;39:800–12. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
Epworth Sleepiness Scale
The Epworth Sleepiness Scale (Johns 1991) is a measure used to assess daytime sleepiness, and is modified for children to rate the propensity to fall asleep in 8 situations in which a child may be likely to fall asleep (Moore 2009; Melendres 2004). Response options are “would never doze'” slight chance of dozing,” “moderate chance of dozing,” and “high chance of dozing.” The questionnaire, originally designed for adults, is used in studies with children with slight variations in terminology on 2 questions. First, the mention of alcohol is removed in question 7. Second, question 8 was modified in different ways across studies. In this study, question 8 was modified to indicate the child is a passenger in a car. In others, question 8 is changed to indicate sleepiness in the event of taking a test or doing homework.
For children, Moore et al. (2009) reported Cronbach α = 0.75. In the latest review of pediatric sleep measures, the ESS was rated as “approaching well-established” for reliability and validity due to the fact that question 8 has been modified differently across studies (Lewandowski, Toliver-Sokol, and Palermo, 2011).
References
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- Moore M. Relationships among sleepiness, sleep time, and psychological functioning in adolescents. J Ped Psych. 2009;34:1175–83. doi: 10.1093/jpepsy/jsp039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendres CS, Lutz JM, Rubin ED, Marcus CL. Daytime sleepiness and hyperactivity in children with suspected sleep-disordered breathing. Pediatrics. 2004;114:768–75. doi: 10.1542/peds.2004-0730. [DOI] [PubMed] [Google Scholar]
- Lewandowski AS, Toliver-Sokol M, Palermo TM. Evidence-based review of subjective pediatricsleep measures. J Ped Psych. 2011;36:780–793. doi: 10.1093/jpepsy/jsq119. [DOI] [PMC free article] [PubMed] [Google Scholar]
REFERENCES
- 1.American Academy of Sleep Medicine. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. International classification of sleep disorders: diagnostic and coding manual. [Google Scholar]
- 2.Nevsimalova S. Narcolepsy in childhood. Sleep Med Rev. 2009;132:169–80. doi: 10.1016/j.smrv.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Ali M, Anger RR, Slocumb NL, Morgenthaler TI. Idiopathic hypersomnia: clinical features and response to treatment. J Clin Sleep Med. 2009;5:562–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Mindell JA, Owens JA. Excessive daytime sleepiness. In: Mindell JA, Owens JA, editors. A clinical guide to pediatric sleep: diagnosis and management of sleep problems. 2nd ed. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 5.Quinell TG, Smith IE. Narcolepsy, idiopathic hypersomnia, and related conditions. Clin Med. 2011;11:282–6. doi: 10.7861/clinmedicine.11-3-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrish E, King MA, Smith IE, Shneerson JM. Factors associated with a delay in the diagnosis of narcolepsy. Sleep Med. 2004;5:37–41. doi: 10.1016/j.sleep.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Peterson PC, Husain AM. Pediatric narcolepsy. Brain Dev. 2008;30:609–23. doi: 10.1016/j.braindev.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Teixeira VG, Faccenda JF, Douglas NJ. Functional status in patients with narcolepsy. Sleep Med. 1004;5:477–83. doi: 10.1016/j.sleep.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Kryger MH, Walid R, Manfreda J. Diagnoses received by narcolepsy patients in the year prior to diagnosis by a sleep specialist. Sleep. 2002;25:36–41. doi: 10.1093/sleep/25.1.36. [DOI] [PubMed] [Google Scholar]
- 10.Stores G. Misdiagnosing sleep disorders as primary psychiatric conditions. Adv Psychiatr Treat. 2013;9:69–77. [Google Scholar]
- 11.Dahl RE, Holttum J, Trubnick L. A clinical picture of child and adolescent narcolepsy. J Am Acad Child Adolesc Psychiatry. 1994;33:834–41. doi: 10.1097/00004583-199407000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Vendrame M, Havagli N, Matadeen-Ali C, Adams R, Kothare KV. Narcolepsy in children: a single-center clinical experience. Pediatr Neurol. 2008;38:314–20. doi: 10.1016/j.pediatrneurol.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Masri TJ, Gonzales CG, Kushida CA. Idiopathic hypersomnia. Sleep Med Clin. 2012;7:283–289. [Google Scholar]
- 14.Morgenthaler TI, Kapur VK, Brown T, et al. Standards of Practice Committee of the America Academy of Sleep Medicine. Practice parameters for the treatment of nacolepsy and other hypersomnias of central origin. Sleep. 2007;30:1705–11. doi: 10.1093/sleep/30.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kothare S, Kaleyias J. Narcolepsy and other hypersomnias in chidren. Curr Opin Pediatr Neurol. 2008;20:666–75. doi: 10.1097/mop.0b013e328316bd85. [DOI] [PubMed] [Google Scholar]
- 16.Kothare S, Kaleyias J. The clinical and laboratory assessment of the sleepy child. Semin Ped Neurol. 2008;15:551–69. doi: 10.1016/j.spen.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Millman RP Working Group on Sleepiness in Adolescents/Young Adults, and AAP Committee on Adolescence. Excessive sleepiness in adolescents and young adults: causes, consequences, and treatment strategies. Pediatrics. 2005;115:1774–86. doi: 10.1542/peds.2005-0772. [DOI] [PubMed] [Google Scholar]
- 18.Daniels E, King MA, Smith IE, Shneerson JM. Health-related quality of life in narcolepsy. J Sleep Res. 2001;10:75–81. doi: 10.1046/j.1365-2869.2001.00234.x. [DOI] [PubMed] [Google Scholar]
- 19.Anderson KN, Pilswroth S, Shaples LD, Smith IE, Shneerson JM. Idiopathic hypersomnia: a study of 77 cases. Sleep. 2007;30:1274–81. doi: 10.1093/sleep/30.10.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jennum P, Ibsen R, Petersen ER, Knudsen S, Kjeilberg J. Health, social, and economic consequences of narcolepsy: a controlled national study evaluating the societal effect on patients and their partners. Sleep Med. 2012;13:1086–93. doi: 10.1016/j.sleep.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Jennum P, Knudsen S, Kjellberg J. The economic consequences of narcolepsy. J Clin Sleep Med. 2009;5:240–5. [PMC free article] [PubMed] [Google Scholar]
- 22.Dodel R, Peter H, Walbert T, et al. The socioeconomic impact of narcolepsy. Sleep. 2004;27:1123–8. doi: 10.1093/sleep/27.6.1123. [DOI] [PubMed] [Google Scholar]
- 23.Reimer MA, Flemmons WW. Quality of life in sleep disorders. Sleep Med Rev. 2003;7:335–49. doi: 10.1053/smrv.2001.0220. [DOI] [PubMed] [Google Scholar]
- 24.Goswami M. The influence of clinical symptoms on quality of life in patients with narcolepsy. Neurology. 1998;50:S31–S36. doi: 10.1212/wnl.50.2_suppl_1.s31. [DOI] [PubMed] [Google Scholar]
- 25.Dodel R, Peter H, Spottke A, et al. Health related quality of life in patients with narcolepsy. Sleep Med. 2007;8:733–41. doi: 10.1016/j.sleep.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 26.Bruck D. The impact of narcolepsy on psychological health and role behaviours: negative effects and comparisons with other illness groups. Sleep Med. 2001;2:437–46. doi: 10.1016/s1389-9457(01)00067-3. [DOI] [PubMed] [Google Scholar]
- 27.Ervick S, Abdelnoor M, Haier MS, Ramberg M, Strand G. Health-related quality of life in narcolepsy. J Sleep Res. 2001;10:75–81. doi: 10.1046/j.1365-2869.2001.00234.x. [DOI] [PubMed] [Google Scholar]
- 28.Douglas NJ. The psychosocial aspects of narcolepsy. Neurology. 1998;50(suppl):527–30. doi: 10.1212/wnl.50.2_suppl_1.s27. [DOI] [PubMed] [Google Scholar]
- 29.Beebe DW, Ris MD, Kramer ME, Long E, Amin R. The association between sleep disordered breathing, academic grades, and cognitive and behavioral functioning among overweight subjects during middle to late childhood. Sleep. 2010;33:1447–56. doi: 10.1093/sleep/33.11.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Falvo D. Psychosocial and functional aspects of chronic illness and disability. In: Falvo D, editor. Medical and psychosocial aspects of chronic illness and disability. 4th ed. Jones & Bartlett Learning; 2008. pp. 9–33. [Google Scholar]
- 31.Stam H, Hartman EE, Deurlow JA, Groothoff J. Young adult patients with a history of pediatric disease: Impact on course of life and transition into adulthood. J Adol Health. 2006;39:4–13. doi: 10.1016/j.jadohealth.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 32.Gledhill, Rangel L, Garraida E. Surviving chronic physical illness: psychosocial outcome in adult life. Arch Dis Child. 2000;83:104–10. doi: 10.1136/adc.83.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livneh H. Psychosocial adaptation to chronic illness and disability: a conceptual framework. Rehab Couns Bull. 2001;44:194–208. [Google Scholar]
- 34.Dorris L, Zuberi SM, Scott N, Moffat C, MacArthur I. Psychosocial and intellectual functioning in childhood narclepsy. Dev Neurorehabil. 2008;11:187–94. doi: 10.1080/17518420802011493. [DOI] [PubMed] [Google Scholar]
- 35.Stores G, Montgomery P, Wiggs L. The psychosocial problems of children with narcolepsy and those with excessive daytime sleepiness of unknown origin. Pediatrics. 2006;118:1116–23. doi: 10.1542/peds.2006-0647. [DOI] [PubMed] [Google Scholar]
- 36.Kashden J, Wise M, Alvarado I, Gillespie S, Bell T. Psychosocial aspects of childhood narcolepsy [abstract] Sleep Res. 1996;25:261. [Google Scholar]
- 37.Kotagal S, Krahn LE, Slocumb N, et al. A putative link between childhood narcolepsy and obesity. Sleep Med. 2004;5:147–50. doi: 10.1016/j.sleep.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Aran A, Einen M, Lin L, Plazzi G, Nishino S, Mignot E. Clinical and therapeutic aspects of childhood narcolepsy-cataplexy: a retrospective study of 51 children. Sleep. 2010;33:1457–64. doi: 10.1093/sleep/33.11.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Dev. 1998;69:875–87. [PubMed] [Google Scholar]
- 40.Beebe DW. Neurobehavioral effects of childhood sleep-disordered breathing (SDB): a comprehensive review. Sleep. 2006;29:1115–34. doi: 10.1093/sleep/29.9.1115. [DOI] [PubMed] [Google Scholar]
- 41.Varni JW, Seid M, Rode CA. The PedsQL: Measurement model for the Pediatric Quality of Life Inventory. Med Care. 1999;37:126–39. doi: 10.1097/00005650-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Varni JW, Seid M, Kurtin PS. The PedsQL 4.0: Reliability and validity of the Pediatric Quality of Life Inventory Version 4.0 Generic Core Scales in healthy and patient populations. Med Care. 2001;39:800–12. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Melendres CS, Lutz JM, Rubin ED, Marcus CL. Daytime sleepiness and hyperactivity in children with suspected sleep-disordered breathing. Pediatrics. 2004;114:768–75. doi: 10.1542/peds.2004-0730. [DOI] [PubMed] [Google Scholar]
- 44.Lewandowski AS, Toliver-Sokol M, Palermo TM. Evidence-based review of subjective pediatric sleep measures. J Ped Psych. 2011;36:780–793. doi: 10.1093/jpepsy/jsq119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moore M. Relationships among sleepiness, sleep time, and psychological functioning in adolescents. J Pediatr Psychol. 2009;34:1175–83. doi: 10.1093/jpepsy/jsp039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwebel DC, Barton BK. Temperament and children's unintentional injuries. In: Vollrath M, editor. Handbook of personality and health. New York: Wiley; 2006. pp. 51–7. [Google Scholar]
- 47.Beebe DW. Neurobehavioral effects of obstructive sleep apnea: an overview and heuristic model. Curr Opin Pulm Med. 2005;11:494–500. doi: 10.1097/01.mcp.0000183059.52924.39. [DOI] [PubMed] [Google Scholar]
- 48.Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res. 2002;11:1–16. doi: 10.1046/j.1365-2869.2002.00289.x. [DOI] [PubMed] [Google Scholar]
- 49.Baldassari CM, Mitchell RB, Schubert C, Rudnick EF. Pediatric obstructive sleep apnea and quality of life: a meta-analysis. Otolaryngol Head Neck Surg. 2008;138:265–73. doi: 10.1016/j.otohns.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 50.Mitchell RB. Adenotonsillectomy for obstructive sleep apnea in children: outcome evaluated by pre- and postoperative polysomnography. Laryngoscope. 2007;117:1844–54. doi: 10.1097/MLG.0b013e318123ee56. [DOI] [PubMed] [Google Scholar]
- 51.Mitchell RB, Kelly J. Outcome of adenotonsillectomy for severe obstructive sleep apnea in children. Int J Pediatr Otorhinolaryngol. 2004;68:1375–9. doi: 10.1016/j.ijporl.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 52.Crabtree VM, Varni JW, Gozal D. Health-related quality of life and depressive symptoms in children with suspected sleep-disordered breathing. Sleep. 2004;27:1131–8. doi: 10.1093/sleep/27.6.1131. [DOI] [PubMed] [Google Scholar]
- 53.Rosen CL, Palermo TM, Larkin EK, Redline S. Health-related quality of life and sleep-disordered breathing in children. Sleep. 2002;25:657–66. [PubMed] [Google Scholar]
- 54.Gaultney JF, Merchant K, Gingras JL. Parents of children with periodic limb movement disorder versus sleep -disordered breathing report greater daytime mood and behavior difficulties in their child: the importance of using the ICSD-2 edition criteria to define a PLMD study group. Behav Sleep Med. 2009;7:119–35. doi: 10.1080/15402000902976655. [DOI] [PubMed] [Google Scholar]
- 55.Ingerski LM, Modi AC, Hood KK, et al. Health related quality of life across pediatric chronic conditions. J Pediatr. 2010;156:639–44. doi: 10.1016/j.jpeds.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Upton P, Lawford J, Eiser C. Parent-child agreement across child health-related quality of life instruments: a review of the literature. Qual Life Res. 2008;17:895–913. doi: 10.1007/s11136-008-9350-5. [DOI] [PubMed] [Google Scholar]
- 57.Cremeens J, Eiser C, Blades M. Factors influencing agreement between child self-report and parent proxy-reports on the Pediatric Quality of Life Inventory 4.0 (PedsQL) generic core scales. Health Qual Life Outcomes. 2006;4:1–8. doi: 10.1186/1477-7525-4-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eiser C, Morse R. Can parents rate their child's health-related quality of life? Results of a systematic review. Qual Life Res. 2001;10:347–57. doi: 10.1023/a:1012253723272. [DOI] [PubMed] [Google Scholar]
- 59.Meltzer LJ, Mindell JA. Sleep and sleep disorders in children and adolescents. Psychiatr Clin N Am. 2006;29:1059–76. doi: 10.1016/j.psc.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 60.Guilleminault C, Froherz S. Narcolepsy: diagnosis and management. In: Kryger MH, Roth T, Dement DC, editors. Principles and practice of sleep medicine. Philadelphia: Elsevier; 2005. [Google Scholar]
- 61.Morgantaler T, Kapur V, Brown T, et al. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin: an American Academy of Sleep Medicine report. Sleep. 2007;12:1705–11. doi: 10.1093/sleep/30.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vignatelli L, Plazzi G, Peschechera F, et al. A 5-year cohort study on health-related quality of life in patients with narcolepsy. Sleep Med. 2010;12:19–23. doi: 10.1016/j.sleep.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 63.Vignatelli L, D'Alessandro, Mosconi P, et al. Health related quality of life in Italian patients with narcolepsy: the SF-36 health survey. Sleep Med. 2004;5:467–75. doi: 10.1016/j.sleep.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 64.Murray DW, Arnold LE, Swanson J, et al. A clinical review of outcomes of the multimodal treatment study of children with ADHD (MTA) Curr Psychiatry Rep. 2008;10:24–31. doi: 10.1007/s11920-008-0068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jensen PS, Hinshaw SP, Swanson JM, et al. Findings from the NIMH multimodal treatment study of ADHD (MTA): Implications and applications for primary care providers. J Dev Behav Pediatr. 2001;22:60–73. doi: 10.1097/00004703-200102000-00008. [DOI] [PubMed] [Google Scholar]


