Abstract
Study Objectives:
Home sleep testing (HST) is an accepted alternative to polysomnography (PSG) for diagnosing obstructive sleep apnea (OSA) in high-risk populations. Clinical guidelines recommend PSG in cases where the HST is technically inadequate (TI) or fails to establish the diagnosis of OSA in patients with high pretest probability. This retrospective study evaluated predictors of OSA on PSG within patients who had a TI or normal HST.
Methods:
Electronic medical records were reviewed on 1,157 patients referred for HST at our sleep center. Two hundred thirty-eight patients had a TI or normal HST with subsequent PSG. Age, BMI, Epworth score, HST result, and PSG-based apnea-hypopnea index (AHI) were abstracted.
Results:
Two hundred thirty-eight consecutive patients with either a normal HST (n = 127) or TI HST (n = 111) underwent subsequent PSG. Of 127 who had a normal HST, 76% had a normal PSG and 24% had OSA (23 mild, 6 moderate, 1 severe). Of 111 who had a TI HST, 29% had a normal PSG and 71% had OSA (43 mild, 19 moderate, 17 severe). Individuals younger than 50 years old with a normal HST were more likely to have a normal PSG. Older age predicted diagnosis of OSA on PSG among individuals with a TI HST.
Conclusion:
In this retrospective analysis of a clinical sample, when the HST is interpreted as normal in a younger patient population, the subsequent PSG is likewise normal in majority of the patients, although significant OSA is sometimes discovered. When a HST is read as TI, the majority of patients have OSA.
Citation:
Zeidler MR, Santiago V, Dzierzewski JM, Mitchell MN, Santiago S, Martin JL. Predictors of obstructive sleep apnea on polysomnography after a technically inadequate or normal home sleep test. J Clin Sleep Med 2015;11(11):1313–1318.
Keywords: home sleep test, obstructive sleep apnea, polysomnography
Obstructive sleep apnea (OSA) is a common medical condition affecting 10% of men and 3% of women aged 30–49 years and 17% of men and 9% of women aged 50–70 years.1 Many cases in the population are yet undiagnosed.2 Home sleep testing (HST) is quickly becoming a primary diagnostic tool for sleep apnea in high-risk populations due to ease of access as well as third-party payer requirements. Although polysomnography (PSG) remains the “gold standard” for diagnosing OSA, HSTs are considerably less expensive to administer, usually more comfortable for the patient, and are associated with equal CPAP adherence outcomes when compared with PSG.3–5 In 2007, the American Academy of Sleep Medicine (AASM) published guidelines regarding the use of unattended portable monitors for the diagnosis of OSA.6 These guidelines indicate that HST should be performed only in individuals at high risk for OSA, after a comprehensive sleep evaluation by a practitioner who is board certified (or board eligible) in sleep medicine, for the patient to be educated by a sleep technologist or technician on how to use the HST device, and for the raw data to be reviewed and interpreted by a practitioner who is board certified (or board eligible) in sleep medicine.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Home sleep tests are becoming the first line diagnostic tool for identifying patients with OSA. As a result, understanding patient factors associated with PSG diagnosis of OSA among those not diagnosed with HST will facilitate improved triage and allocation of resources in health care systems.
Study Impact: This study suggests that older patients are more likely to require PSG if OSA is not diagnosed with HST. Cost-effectiveness research is needed to evaluate the utility of HSTs in subgroups of patients who are more likely to require a PSG.
As the use of HSTs increases, the occurrence of data loss leading to a technically inadequate (TI) study or a study in which OSA cannot be definitively diagnosed (i.e., a study resulting in an apnea-hypopnea index [AHI] below 5 events per hour) in patients with a high pretest probability of a diagnosis of OSA poses a significant challenge. Published data estimate that rates of technically inadequate studies from signal loss range from 3% to 18%, depending upon who sets up the device (technician vs. patient) and the type of device used.7 Even when HST is set up by a technician, data loss can still occur because the study is unattended, and signals can be lost overnight. In addition, a false negative rate of up to 17% has been reported.6 AASM guidelines state that “negative or technically inadequate (TI) portable monitor tests in patients with high pretest probability of moderate to severe OSA should prompt in-laboratory evaluation.” There are challenges with this approach, however, since retesting due to a TI or negative HST study can result in delays in both the diagnosis and treatment of OSA and may lead to unnecessary testing.
To better understand patient level factors associated with the presence of OSA among patients with either a TI or normal HST, we conducted a retrospective electronic medical record review among consecutive patients in a VA Sleep Disorders Center experienced in the use of HST. The goal of this study was to compare the results of a normal or technically inadequate HST to the results of a subsequent PSG to identify predictors of OSA based on a laboratory PSG study. We hypothesized that older age, more daytime sleepiness, larger neck circumference, higher body mass index (BMI), and male gender would be associated with a greater likelihood of OSA based on PSG after a negative or technically inadequate HST.
METHODS
Participants
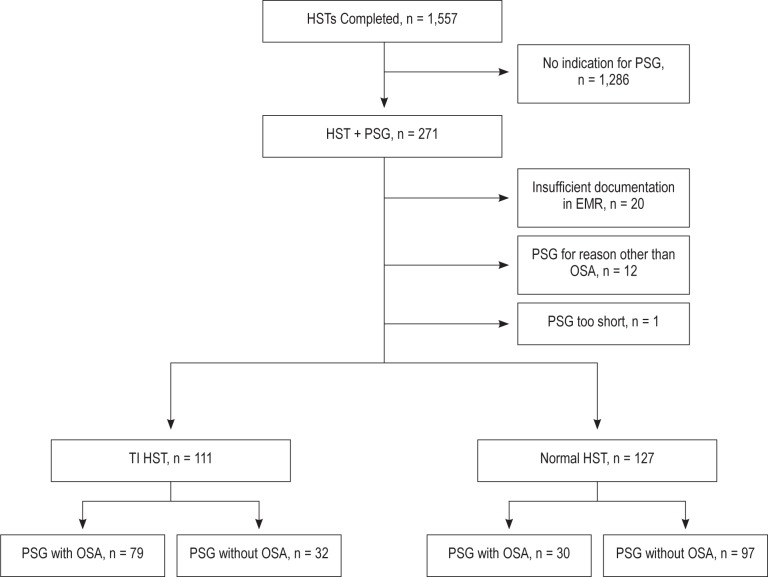
A retrospective electronic medical record review was performed via the Computerized Patient Record System (CPRS) at the VA Greater Los Angeles Healthcare System (VAGLAHS). Records were reviewed consecutively for all veterans referred to the sleep disorders center who had both HST and subsequent PSG performed from January 2010 to December 2012 (n = 271). As indicated in the AASM guidelines, all HST referrals were made by sleep specialists within the Center. A total of 1,557 patients had HSTs performed between 2010 and 2012, and 238 patients met inclusion criteria for this study (Figure 1). The inclusion criteria included (1) patient completing both home sleep testing (Stardust, Philips Respironics) and diagnostic polysomnogram (Somnostar, Viasys) from January 2010 to December 2012 and (2) patients with a high pretest probability of OSA including one or more of the following features: BMI > 25, snoring, or daytime sleepiness as described in the electronic consults to the sleep disorders center from the primary care team. Exclusion criteria were (1) home sleep testing with equipment other than the Stardust device or (2) PSG performed for purposes other than diagnostic testing for OSA (e.g., to rule REM behavior disorder or central sleep apnea).
Figure 1. Flow chart outlining individuals with home sleep tests (HSTs) who were included for final analysis and reasons for exclusion.
Patients were instructed on use of the Stardust home sleep monitor as per the clinical protocol at the VAGLAHS sleep center, which includes the patient coming in to the sleep center and having one-on-one instruction with a respiratory therapist. The patient is shown the components of the home sleep test device, how to turn the device on and off, and how to place the device on themselves. During the instructional period, the patient applies the effort belt and flow sensor on themselves and then removes them. The patient is also shown how to affix the oximetry probe. The patient is sent home with additional instructional materials regarding the device, including a pamphlet and an instructional DVD.
Data collected from the medical recorded included age, gender, race/ethnicity, body mass index (BMI), collar size, Ep-worth Sleepiness Scale (ESS),8 documented outcome of the portable HST indicating a normal HST (with AHI < 5) or a TI HST (i.e., one that could not be interpreted due to signal loss or insufficient study time), and the AHI of the subsequent polysomnogram. The HSTs were evaluated by sleep medicine physicians (board certified or board eligible) for adequacy of data and determination of the presence or absence of OSA. During this period of record review, HST studies were documented in the medical record as diagnostic of OSA, normal, or TI. However, the AHI was not routinely documented in the medical record and therefore could not be included in this study. Studies were typically considered TI due to signal loss during the night; however, the level of detail documented in the medical record about which signals were lost varied by clinician. In this clinic, one repeat attempt is made for TI HSTs prior to referral for PSG. If this occurred, the status of the second HST was included in the database. If the HST was read as TI for a second time, the patient was scheduled for an in-laboratory PSG. All patients with normal HSTs were sent for PSG without a repeat HST. All PSGs were scored per AASM guidelines and interpreted by a board-certified sleep medicine physician. This study was reviewed and approved by the VAGLAHS institutional review board, and a waiver of informed consent was obtained.
Statistical Analyses
Outcome Variables
The main outcome variable was the presence of any obstructive sleep apnea (OSA), defined as a PSG AHI ≥ 5. Values of 5 or larger were coded as 1 (any OSA) and values < 5 were coded as a 0 (no OSA). For descriptive purposes (see Table 1), PSG AHI was also used to categorize OSA using the following 4 categories: (1) AHI of 0 to (but excluding) 5: no OSA; (2) AHI of 5 to (but excluding) 15: mild OSA; (3) AHI of 15 to (but excluding) 30: moderate OSA; and (4) AHI of 30 and above: severe OSA.
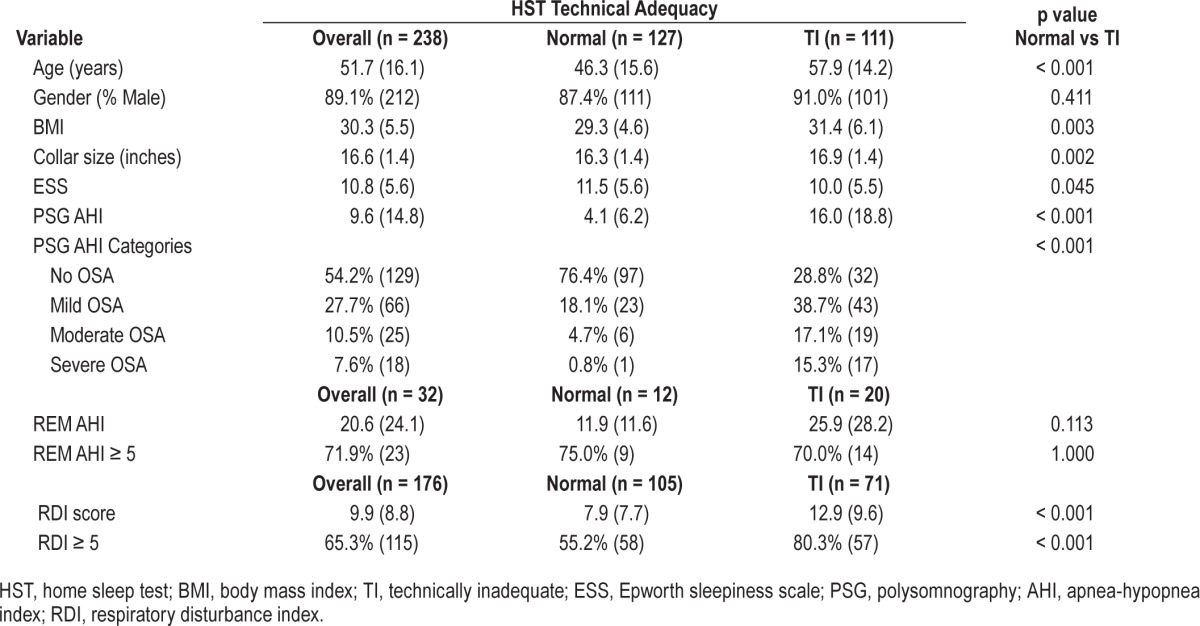
Table 1.
Patient demographics for individuals with normal (n = 127) and technically inadequate (n = 111) home sleep tests.
Predictor Variables
The predictor variables included HST technical adequacy (discussed further below), age, gender, BMI, collar size (inches), and ESS. Race/ethnicity was considered, but was ultimately omitted due to a large percentage (16.6%) of missing race/ethnicity information in the medical record.
HST technical adequacy was classified as Normal (n = 127) or Technically Inadequate (TI; n = 111). Comparisons between these 2 groups were performed using t-tests (for continuous variables) and Fisher exact tests (for binary and nominal variables).
Binary logistic regression models were formed predicting the presence of OSA (AHI ≥ 5) as a function of the previously described predictors. Two model-building strategies were used. Strategy 1 began with a full model (including all predictors listed above), and the nonsignificant predictors were manually removed one at a time until only significant predictors remained. Strategy 2 began with an empty model and then manually added candidate variables, retaining variables that were significant until no more significant variables were found to enter.
All analyses were performed using Stata version 13.1.9 The logistic regression analyses were computed using the logit command. For all tests performed, p < 0.05 was considered statistically significant.
RESULTS
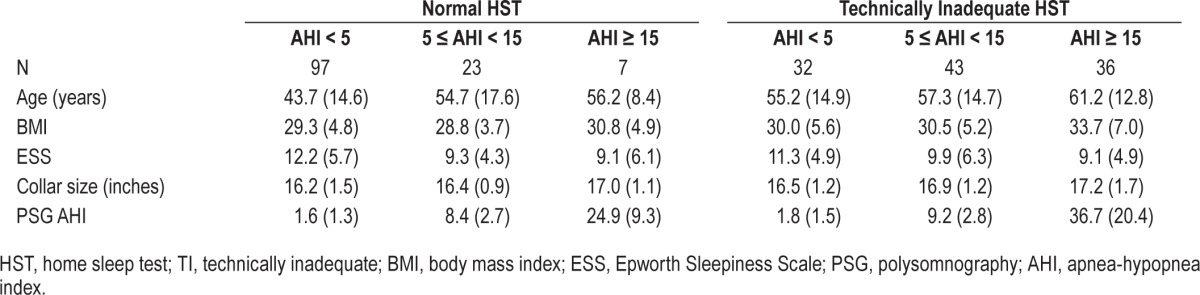
Descriptive statistics in the form of means and standard deviations (for continuous variables) and percentages and frequencies (for binary and nominal variables) are displayed in Table 1. The results are presented for the overall sample (n = 238), as well as the Normal (n = 127) and Technically Inadequate (TI; n = 111) groups. This includes overall AHI as well as REM AHI in the subset available and RDI. Comparisons of the 2 groups showed those with TI tests were older (p < 0.001), had higher BMIs (p = 0.003), larger collar sizes (p = 0.002), were less sleepy (p = 0.045), and had higher average PSG AHI scores (p < 0.001). There were also significant differences in the frequency distribution of the PSG AHI categories between the 2 groups (p = 0.001). Table 2 shows the mean and standard deviations for age, BMI, ESS, collar size, and PSG-AHI broken down by the technical adequacy of the test (Normal vs. TI) and 3 PSG-AHI categories (AHI < 5; 5 ≤ AHI < 15; AHI ≥ 15).
Table 2.
Characteristics of patients with normal and technically inadequate home sleep tests (HST) by severity of sleep disordered breathing based on subsequent laboratory polysomnography.
The logistic regression analyses predicting the presence of OSA (AHI ≥ 5) resulted in an identical model for both modeling strategies, with 3 significant predictors: HST technical adequacy (normal vs. TI), age, and ESS. The overall test of this model was significant, χ2(3) = 73.71, p < 0.001, n = 233. The odds of OSA on PSG for those with TI HSTs was 6.6 times higher than the odds of those with normal HSTs (OR = 6.6, CI [3.5, 12.2], p < 0.001). Older age was associated with a higher odds of OSA (OR = 1.03, CI [1.01, 1.05], p = 0.005), and lower ESS scores were associated with a higher odds of OSA (OR = 0.94, CI [0.88, 0.99], p = 0.022).
To seek a more parsimonious model and one that is simpler to visualize, ESS was omitted based on being the weakest of the 3 predictors. In this model, the odds of OSA for those with technically inadequate HSTs was 6.3 times that of those with normal HSTs (CI [3.4, 11.5], p < 0.001) and older age was associated with a higher odds of OSA as well (OR = 1.03, CI [1.01, 1.06], p = 0.001). To aid in the interpretation of these results, Figure 2 shows the probability of any OSA (AHI ≥ 5) with a 95% CI as a function of age with separate lines for tests that were TI and normal. The figure shows how the probability of having any OSA (AHI ≥ 5) differs between those with TI and normal HST at any specified age. For example, at age 40 the modeled probability (as a percentage) of having any OSA (AHI ≥ 5) for those with a TI HST was 58% (95% CI [46%, 71%]) compared to 18% (95% CI [11%, 25%]) for those with a normal HST.
Figure 2. The probability of a diagnosis of sleep apnea (based on PSG AHI > 5) as a function of age for individuals with a normal and technically inadequate home sleep test.
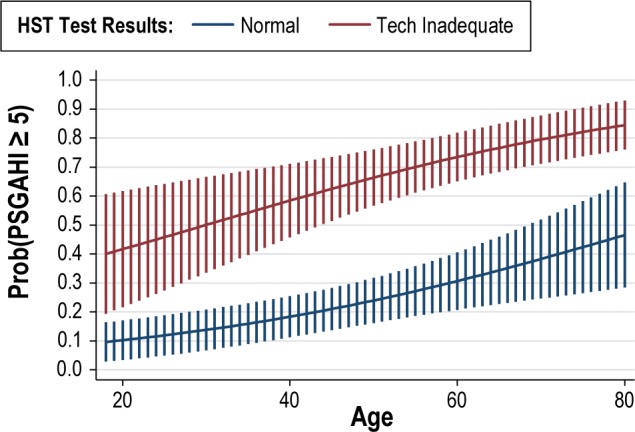
The probability of AHI > 5 on PSG is higher among patients with a technically inadequate tests, and increases as a function of age in both groups.
DISCUSSION
This study describes a clinical implementation of the AASM guidelines regarding use of PSG after normal or TI HSTs in patients with a high pretest suspicion for OSA. To date, the available data supporting the use of PSG after a negative home sleep test comes mainly from validation studies of portable monitoring devices, performed in prospective studies, often of nonclinical samples, which show a false negative rate of 3% to 18%.7 This study attempted to identify predictors of “true negative” results in the HST as determined by laboratory PSG. We found that within our VA population, when the HST was interpreted as normal by a sleep medicine physician, the subsequent PSG was likewise normal or of mild severity in the majority of patients. However, 5.5% of patients with a normal home sleep were diagnosed with moderate to severe OSA. All individuals with a normal HST and an AHI > 15 were 56 years of age or older. This is in contrast to a previous study by Bixler and colleagues,10 who found a higher prevalence of OSA in a younger male patient population but that the most severe OSA occurred in young subjects and milder OSA was seen in older patients. A possible explanation for this difference is the difference in the populations sampled. The Bixler study encompassed a large unscreened epidemiologic sample rather than a prescreened sleep disorders clinic population.
Also, in our study, lower BMI and smaller collar size were not significant predictors of a true negative HST, although multiple other factors such as hypertension, additional measures of body habitus, and more detailed questionnaires regarding witnessed apneas or snoring were not collected and therefore could not be included as possible predictors. Prior studies have typically shown BMI as a strong predictor for the presence of OSA11; however, the average BMI in our study was below the cutoff BMI of 32.7 kg/m2 which was predictive of OSA in a study of young commercial truck drivers, so this may account for the differences in our findings for the younger patients.12 Interestingly, the ESS was higher in the group of patients with a normal HST and a PSG with AHI < 5 when compared to individuals with a normal HST and OSA diagnosed on the PSG. This may be unique to the VA population studied due to the multiple comorbid conditions in the younger veteran population who have a high prevalence of traumatic brain injury, depression and posttraumatic stress disorder possibly causing daytime sleepiness. Some comorbid conditions may also lead to poor sleep in the sleep laboratory (e.g., PTSD), which could increase the rate of negative studies if sufficient sleep is not achieved for a definitive diagnosis. Further research would be necessary to evaluate this hypothesis. In addition, some studies show that multiple nights can increase the accuracy of HST in the diagnosis of OSA and we recorded only one night if the study was normal (although a second attempt was made if the HST was TI). One study found that 10% of patients were misclassified as having a normal AHI when an abnormal AHI was detected on a subsequent night of study.13 Other limitations of this study include the inclusion of only a veteran population which consists predominantly of male patients and the absence of AHI documentation from the HST, which precluded any analyses using this variable. It is unclear if the findings would be consistent in female patients, who have a lower prevalence of OSA and may have different challenges with the use of HST equipment.
When the HST was interpreted as TI, the majority of patients had OSA, with 32.4% of the group having moderate-to-severe disease. Older age also predicted having OSA. Older age may account for more difficulty with using the home sleep monitor due to poorer dexterity or cognitive limitations, although prior data has shown that HSTs are suitable in an elderly population.14 The elderly veteran population in the Greater Los Angeles area may be different. For example, there may be less spousal and family support as well as high rates of cognitive impairment and comorbid diseases. The use of a simpler device or having a technician hook-up the device at the patient's home might mitigate this problem and lead to fewer technically inadequate studies in older patients.
To our knowledge, this is the first study to report the clinical implementation of the AASM Guidelines regarding PSG subsequent to nondiagnostic home sleep testing for OSA. Due to the large volume of patients studied at our institution as well as the 5- to 8-month waiting period for a full PSG, it is important to delineate factors which would identify a subset of patients for whom a subsequent PSG may not be necessary after a negative HST. Likewise, our results suggest that the subset of patients with TI HST who are older should be considered highest priority for PSG as they are most likely to have severe OSA. Further prospective studies utilizing additional predictive criteria including aspects of care that may reduce rates of TI HST are needed. In addition, future studies on the cost/benefit ratio for strategies of triaging patients initially to HST or PSG are necessary to confirm and build upon our findings.
DISCLOSURE STATEMENT
This was not an industry supported study. Supported by: VA Greater Los Angeles Healthcare System Sleep Medicine Fellowship Program, VA Rehabilitation Research and Development 1RX000135-01 (Martin) the VA Greater Los Angeles Healthcare System Geriatric Research, Education and Clinical Center, UCLA Claude Pepper Older Americans Independence Center 5P30AG028748 (Dzierzewski) and NIH/NCATS UCLA CTSI UL1TR000124 (Dzierzewski), and Research Service of the VA Greater Los Angeles Healthcare System. Dr. Martin has received research support from Equinox Fitness. The other author have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Katia Chavez, RRT and Joyce Convis, RRT who greatly helped with this study.
REFERENCES
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–14. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kapur V, Strohl KP, Redline S, Iber C, O'Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath. 2002;6:49–54. doi: 10.1007/s11325-002-0049-5. [DOI] [PubMed] [Google Scholar]
- 3.Kuna ST, Gurubhagavatula I, Maislin G, et al. Noninferiority of functional outcome in ambulatory management of obstructive sleep apnea. Am J Respir Crit Care Med. 2011;183:1238–44. doi: 10.1164/rccm.201011-1770OC. [DOI] [PubMed] [Google Scholar]
- 4.Rosen CR, Auckley D, Benca R, et al. A multisite randomized trial of portable sleep studies and positive airway pressure autotitration versus laboratory-based polysomnography for the diagnosis and treatment of obstructive sleep apnea: the HomePAP study. Sleep. 2012;35:757–67. doi: 10.5665/sleep.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masa JF, Corral J, Sanchez de Cos J, et al. Effectiveness of three sleep apnea management alternatives. Sleep. 2013;36:1799–807. doi: 10.5665/sleep.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3:737–47. [PMC free article] [PubMed] [Google Scholar]
- 7.Flemons WW, Littner MR, Rowley JA, et al. Home diagnosis of sleep apnea: a systematic review of the literature. An evidence review cosponsored by the American Academy of Sleep Medicine, the American College of Chest Physicians, and the American Thoracic Society. Chest. 2003;124:1543–79. doi: 10.1378/chest.124.4.1543. [DOI] [PubMed] [Google Scholar]
- 8.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 9.College Station, TX: StataCorp, LP; 2013. Stata Statistical Software: Release 13.1. [Google Scholar]
- 10.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men. Am J Respir Crit Care Med. 1998;157:144–8. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 11.Gurubhagavatula I, Maislin G, Pack AI. An algorithm to stratify sleep apnea risk in a sleep disorders clinic program. Am J Respir Crit Care Med. 1993;164:1904–9. doi: 10.1164/ajrccm.164.10.2103039. [DOI] [PubMed] [Google Scholar]
- 12.Gurubhagavatula I, Maislin G, Nkwuo JE, Pack AI. Occupational screening for obstructive sleep apnea in commercial drivers. Am J Respir Crit Care Med. 2004;170:371–6. doi: 10.1164/rccm.200307-968OC. [DOI] [PubMed] [Google Scholar]
- 13.Stepnowsky CJ, Orr WC, Davidson TM. Nightly variability of sleep-disordered breathing measured over 3 nights. Otolaryngol Head Neck Surg. 2004;131:837–43. doi: 10.1016/j.otohns.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Morales CR, Hurley S, Wick LC, et al. In-home, self-assembled sleep studies are useful in diagnosing sleep apnea in the elderly. Sleep. 2012;35:1491–501. doi: 10.5665/sleep.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]