Abstract
Objective:
To evaluate the diagnostic capability of signs and symptoms of sleep bruxism (SB) as per the American Academy of Sleep Medicine (AASM) criteria and a diagnostic grading system proposed by international experts for assessing SB.
Methods:
The study was conducted in three phases (interview, physical examination, and sleep studies). Subjects were asked about self-reported tooth grinding sounds occurring during sleep, muscle fatigue, temporal headaches, jaw muscle pain, and jaw locking. A visual examination was conducted to check for presence of abnormal tooth wear. A full-night polysomnography (PSG) was performed. After three phases, the subjects were divided into two groups matched by age and gender: Case Group, 45 SB subjects, and Control Group, 45 non-SB subjects. Diagnostic accuracy measurements were calculated for each sign or symptom individually and for the two diagnostic criteria analyzed.
Results:
Muscle fatigue, temporal headaches, and AASM criteria were associated with highest sensitivity (78%, 67%, 58%, respectively) and also with highest diagnostic odds ratio (OR = 9.63, 9.25, 6.33, respectively). Jaw locking, muscle pain, and the criterion of “probable SB” were associated with the worst sensitivity (16%, 18%, 22%, respectively).
Conclusions:
Presence of muscle fatigue and temporal headaches can be considered good tools to screen SB patients. None of the diagnostic criteria evaluated was able to accurately identify patients with SB. AASM criteria had the strongest diagnostic capabilities and—although they do not attain diagnostic values high enough to replace the current gold standard (PSG)—should be used as a screening tool to identify SB.
Citation:
Palinkas M, De Luca Canto G, Rodrigues LA, Bataglion C, Siéssere S, Semprini M, Regalo SC. Comparative capabilities of clinical assessment, diagnostic criteria, and polysomnography in detecting sleep bruxism. J Clin Sleep Med 2015;11(11):1319–1325.
Keywords: bruxism, diagnostic grading system, polysomnography, sleep bruxism
Although the definition and grading of bruxism has been hindered by a lack of consensus, a proposed operational term defines bruxism as the “repetitive jaw-muscle activity characterized by clenching or grinding of the teeth and/ or by bracing or thrusting of the mandible.”1 Bruxism is understood to be a sleep related movement disorder, and it has two distinct circadian manifestations: it can occur during sleep (sleep bruxism, SB) or during wakefulness (awake bruxism, AB).2
As a result of periodic mechanical grinding, SB can lead to tooth wear, tooth mobility, and other clinical findings such as tongue/cheek indentation, masticatory muscle hypertrophy, temporomandibular joint pain, headaches, and masticatory muscle pain or muscle fatigue.3
Complaints of tooth-grinding occurring during sleep decline over time, from an estimated prevalence of 14% in children to 12.8% in adults; in patients 60 years of age or older, a prevalence of 3% is reported.4,5 In a sample of individuals between 20 and 80 years of age, SB prevalence confirmed by polysomnography (PSG) was 7.4%.6
BRIEF SUMMARY
Current Knowledge/Study Rationale: Although PSG is considered the gold standard tool for SB diagnosis, the relative complexity of PSG and the inherent costs associated with this have spurred the quest for alternative diagnostic methods.
Study Impact: There is an acknowledged gap in the literature regarding diagnosis of SB, which is multifactorial in etiology and poses clinical challenges. Although a single set of simple criteria cannot capture the complexity of such a sleep movement disorder, it may be reasonable to combine the criteria evaluated in this study into a clinical algorithm that could form the basis for new diagnostic system combining intelligent methodologies and clinical insights. Future research is needed to clarify this topic and better provide tools with acceptable diagnostic test accuracy.
The diagnosis of bruxism often is challenging.7 No universally accepted criteria for AB diagnosis have been reported in the literature. One of the most widely accepted criteria for the diagnostic of SB was proposed by American Academy of Sleep Medicine (AASM).8 The 2014 update of the International Classification of Sleep Disorders (ICSD)—a clinical protocol-based manual that categorizes and describes all known sleep disorders—includes as a diagnostic criterion the report of regular or frequent tooth grinding sounds occurring during sleep and the presence of one or more of the following clinical signs and symptoms: (1) abnormal tooth wear consistent with reports of tooth grinding during sleep; (2) transient morning jaw muscle pain or fatigue; and/or temporal headaches; and/or jaw locking upon awakening consistent with reports of tooth grinding during sleep.
A recently published international consensus paper by Lobbezoo et al. on bruxism diagnosis proposed a grading system to be used for clinical as well as research purposes.1 The authors suggested the categorization of bruxism into subcategories of “possible,” “probable,” and “definite” bruxism and recommended further distinctions of “sleep” or “awake” bruxism. “Possible bruxism” was based in the Lobbezoo paper on self-report or by parent report (questionnaire or interview), “probable bruxism” was based on self-report plus inspection during clinical examination, and “definite bruxism” was based on PSG, preferably with video/audio recording.
The utility of SB diagnosis based on polysomnographic recordings is very well documented and accepted by most clinicians and researchers as the gold standard.9 Unfortunately, overnight PSG is labor-intensive and expensive, and access to this modality is significantly limited in some places. Waiting times between referral for evaluation to diagnosis commonly take 5 to 6 months in the United States and around the world.10
In this clinical context, the main objective of this research was to evaluate the relative diagnostic capabilities of SB-related signs and symptoms, AASM8 criteria and the grading system proposed by Lobbezoo et al.1 for the assessment of SB.
METHODS
The study was approved by the Research Ethics Committee of Ribeirão Preto School of Dentistry, University of São Paulo, in accordance with Resolution 466/2012 of the Brazilian Health Council under number 2012/10228-6. All participants were duly informed regarding the experiment and agreed to participate by providing their free and informed consent.
Subjects
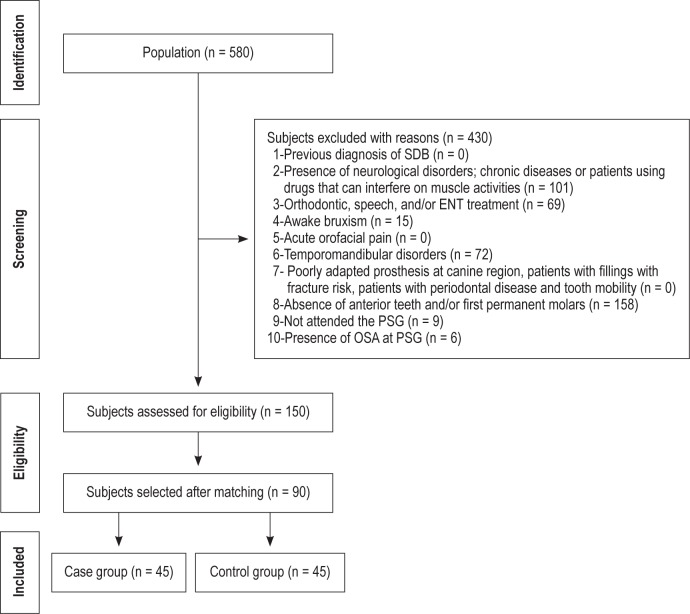
A convenience sample was selected, initially consisting of a population of 580 volunteers between 18 and 45 years of age who had been examined at Ribeirão Preto School of Dentistry, University of São Paulo, Brazil. The individuals were required to meet inclusion criteria to be included in the sample (Figure 1).
Figure 1. Inclusion and exclusion criteria.

SDB, sleep disordered breathing; ENT, ear; nose; and throat; RDC, research diagnostic criteria; TMD, temporomandibular disorder; OSA, obstructive sleep apnea; PSG, polysomnography.
Clinical Assessment
The data collection was performed in three phases: (1) Interview, (2) Physical Examination, (3) Sleep Studies. The same researcher conducted the interviews and the collection of the questionnaires. Prospective study subjects were evaluated for signs and symptoms of temporomandibular disorders, guided by the Research Diagnostic Criteria for Temporomandibular disorders (RDC/TMD)11 that was completed during the interview and the physical examination. Sleep studies were conduct by a sleep medicine physician.
In Phase 1 (interview), subjects were asked about the following symptoms: reports of regular or frequent tooth grinding sounds occurring during sleep, muscle fatigue, temporal headache, transient morning jaw muscle pain, and jaw locking upon awakening.
In Phase 2, (physical examination) researchers conducted a visual examination of each prospective subject, looking for presence or signs suggestive of abnormal tooth wear, such as the presence of teeth exhibiting flattened cusps and/or contour loss with dentin exposure. Moreover, the examiner looked for all clinical characteristics that were considered exclusion criteria as presented in Figure 1.
Sleep Studies
In phase 3, a full-night PSG was performed at the sleep laboratory, using a digital system (Sonolab polysomnograph 632 030003), and starting at 23:00. The sleep physician was blinded to the clinical diagnosis. The electrodes were soaked in electrolyte solution and placed on the scalp, face, and leg. Before starting the sleep recording, the participants performed biocalibration tests for confirmation of physiological signals (e.g., mandibular movements, coughing, swallowing, maximum voluntary contraction, rhythmic contractions), which were recognized by the operator. The PSG system consisted of 26 AC-programmable channels, 6 DC channels, multi-user software, 32-bit Windows platform to perform the analytical reports, histograms, tables, and maps. Electrodes and sensors that record electroencephalogram12 signals, electro-oculogram (bilateral) signals, noise and gnashing of teeth and tighten sounds (via microphone set in the buccinator muscle region), surface electromyographic signals (masseter and right and left temporal muscles, chin and right tibialis anterior), plethysmographic signals (respiratory effort of the chest and abdomen), body and leg movements, nasal airflow, electrocardiogram, and visual analysis through the camcorder all were utilized in the sleep recording. The diagnosis of SB was established when the PSG had ≥ 4 episodes (phasic, tonic, and mixed) per hour of sleep or ≥ 25 bursts during sleep, according to criteria published by Lavigne et al.10
After phases 1, 2, and 3, the subjects selected for study were divided into 2 groups matched by age and gender: Case Group, composed of individuals with SB; and Control Group, composed of individuals without SB.
Statistical Analysis
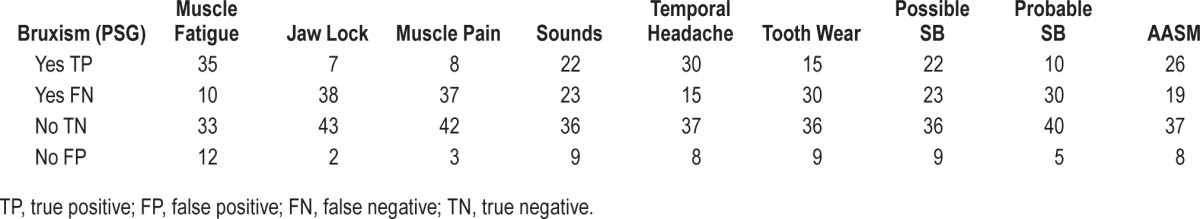
Diagnostic capability of clinical assessment was tested against the PSG (gold standard). Thus, comparing each test (sign or symptom or SB criteria) with the gold standard, 4 possible interpretations could emerge: true positive, (TP) when the test result and PSG are positive; false positive (FP), when the test result is positive and PSG is negative; false negative (FN), when the test result is negative and PSG is positive; and true negative (TN), when the test result and PSG are negative. Tables 2 × 2 were constructed with these data. From these comparative interpretations, values of sensitivity, specificity, accuracy, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (LR+), negative likelihood ratio (LR−) and the Youden's index for were calculated. Microsoft Excel was used to perform the analysis. Forest plots and receiver operating characteristic (ROC) curves were constructed in Review Manager 5.2 (RevMan 5.2, The Nordic Cochrane Centre, Copenhagen, Denmark). All statistical analyses were performed with 95% confidence intervals.
RESULTS
Subject Selection
A flowchart describing the process of identification, inclusion, and exclusion of subjects is shown in Figure 2. The population of this study was 580 subjects. After all exclusion criteria were applied, 150 subjects met our inclusion criteria. The subjects were matched by age and gender to find an ideal control group to be compared to case group. Because of this matching, only 90 subjects were finally included in this study. They were divided into two groups according to the results of PSG: 45 individuals in the Case group (SB), and 45 subjects in Control group (non-SB). Both groups were composed of 16 men and 29 women. The average age was 30.01 years (SD = 7.33).
Figure 2. Flow diagram of screening and selection criteria.
Synthesis of Results
To improve our interpretation of results, the diagnostic accuracy measurements are presented in Table 1, Figure 3 (Forest plot), and Figure 4 (ROC curve). Further, Table 2 × 2 is presented in Appendix 1. More information about diagnostic test accuracy (DTA) measurements and its interpretation can be found in Appendix 2.
Table 1.
Diagnostic test accuracy.
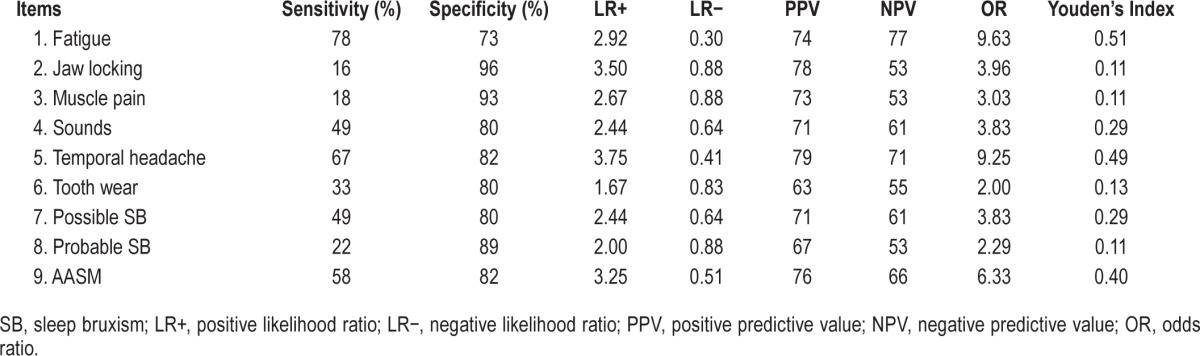
Figure 3. Forest plot with diagnostic test accuracy (sensitivity, specificity, and 95% confidence interval).
TP, true positive; FP, false positive; FN, false negative; TN, true negative.
Figure 4. Receiver operating characteristic (ROC) curve.
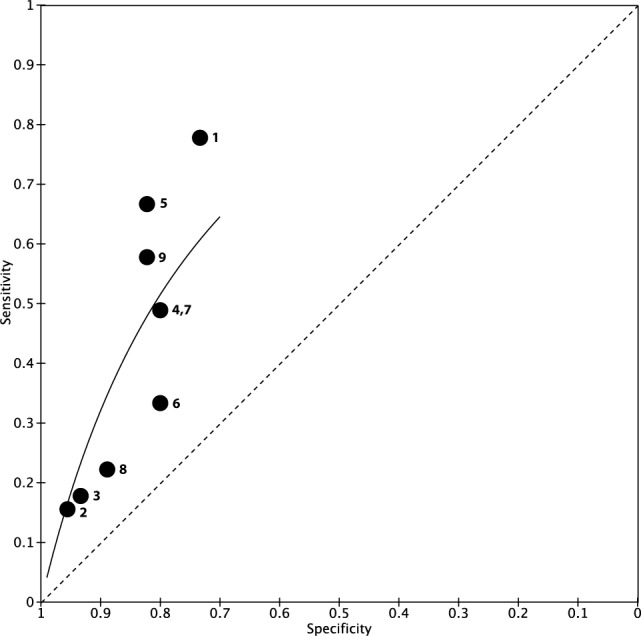
1 = Fatigue, 2 = Jaw locking, 3 = Muscle pain, 4 = Sounds. 5 = Temporal Headache, 6 = Tooth wear, 7 = Possible SB, 8 = Probable SB, 9 = AASM criteria.
In our analysis of signs and symptoms, greater diagnostic sensitivity was associated with 2 symptoms: muscle fatigue (78%) and temporal headache (67%). The greatest specificity was associated with the symptoms of jaw locking (96%), followed by jaw muscle pain (93%), report of regular or frequent tooth grinding sounds occurring during sleep (80%), abnormal tooth wear (80%), and temporal headache (82%; Table 1).
Regarding the diagnostic criteria analyzed, the AASM criteria showed the greatest sensitivity (58%). All diagnostic criteria reported good specificity (Table 1).
Additional Analysis
No single item or combined diagnostic criteria reported LR values were considered excellent or acceptable in terms of DTA.
Temporal headaches had the highest PPV values (79%); muscle fatigue had the highest NPV (77%). Temporal headaches and muscle fatigue were associated with the greatest predictive value. Muscle fatigue, temporal headaches, and AASM criteria were associated with the highest diagnostic odds ratio (OR = 9.63, 9.25, 6.33, respectively).
The Forest plot (Figure 3) shows wide variation in sensitivity values. In this graph, we can see that muscle fatigue, temporal headache, and AASM criteria have better position than the others. Regarding specificity, the graph showed more homogeneous results. In the same sense, the ROC curve showed 3 items close to the left corner of the graph: items 1 (muscle fatigue), 5 (temporal headache), and 9 (AASM criteria).
In summary, only two symptoms (muscle fatigue and temporal headaches) were able to screen patients with SB. In contrast, the absence of most of the signs and symptoms, especially jaw muscle pain and abnormal tooth wear, was helpful in defining who does not have SB.
No diagnostic criteria evaluated in our study were able to correctly identify 100% of patients with SB. Perhaps not surprisingly in our study, the diagnostic criteria issued by AASM showed the greatest sensitivity (58%).8 All diagnostic criteria reported good specificity (Table 1).
DISCUSSION
This clinical study tested clinical assessment for SB against gold standard (PSG). To our knowledge, this is the first study that used a sample with SB subjects confirmed by PSG as a gold standard to test clinical assessments. The first controlled sleep laboratory study of bruxism was completed by Reding et al.13 in 1968. Almost 30 years later, Lavigne et al.9 validated the PSG as an SB research criterion. However, the Lavigne study had a different design from ours. Lavigne considered the clinical assessment as a gold standard and tested the PSG against it, reporting 72% sensitivity and 94% specificity. In contrast, our study was designed to classify bruxers and non-bruxers initially using PSG, and subsequently to test the clinical assessment to determine its predictive value in diagnosing SB.
Owing to the scarcity of reliable and valid tools for SB diagnosis, this topic is important for clinicians and researchers. The results of our study can help clinicians to re-evaluate the current methods used to SB diagnosis, and also perhaps to reappraise the appropriate role of tooth wear and sounds in diagnosing and grading SB. Indeed, our study demonstrated that tooth wear and sounds had poor specificity; in other words, they were not able to identify patients with SB. Conversely, only two symptoms evaluated in our study—muscle fatigue and temporal headache—can be considered good tools in the screening of patients to look for SB.
Two signs and symptoms—jaw locking and jaw muscle pain—had excellent diagnostic specificity (96% and 93%, respectively). In clinical practice, this would suggest that more than 93% of patients without complaints of jaw locking and jaw muscle pain do not have SB. In the same sense, approximately 80% of patients without reports of regular or frequent tooth grinding sounds occurring during sleep, abnormal tooth wear, or temporal headache probably do not have SB. The use of specificity can help clinicians to correctly classify healthy individuals according to the absence of some signs and symptoms.
Regarding the two sources of diagnostic criteria analyzed,1,8 neither was able to diagnose SB. However, AASM criteria8 reported better sensitivity and can be used as an effective screening tool. The criteria for “possible SB” and “probable SB,” which incorporate the most common approach used by dentists—sounds and wear facets—failed in this diagnostic study, demonstrating that neither reports of grinding sounds nor tooth wear can help diagnose SB accurately. According to Carra et al.,14 grinding sounds caused by tooth contacts are the pathognomonic signs of SB; however, not all rhythmic masticatory muscle activity (RMMA) episodes are accompanied by tooth grinding, and many patients or family members may not be aware of this. Clinical examination of the oral cavity allows for the identification of signs and symptoms. However, none of them constitutes direct proof of current SB activity. In the same sense, although tooth wear is widely acknowledged in the literature as the classic dental sign of bruxism,14 arguments against the use of tooth wear alone as an absolute pathognomonic criterion to assess SB severity have been suggested.15 Tooth wear may be related to many other factors that can induce attrition and erosion on dental surfaces, such as diet,16 gastroesophageal reflux,17 ingestion of alcoholic and/or acidic drinks, or medications.18 Furthermore, tooth wear is permanent and, even if caused by bruxism, tooth wear does not provide information regarding timing. In contrast, it is very relevant that both diagnostic criteria analyzed in this study can be used to identify individuals who do not have SB, because these criteria had specificity of around 80%. Thus, in the absence of tooth wear and/or complaints of sounds during sleep, a patient probably does not have SB.
Additional analysis performed to provide more power to our results reported similar values. No single item or combined diagnostic criteria reported LR values considered excellent or acceptable DTA. Regarding PPV values, 79% of patients with temporal headache had SB. The odds ratio (OR) for headaches showed a similar result (OR = 9.25), indicating that individuals with temporal headaches had nine times odds of having SB than did individuals without temporal headaches. These results are similar to findings from a recent systematic review19 that reported that the presence of SB significantly increased the odds for headaches. Muscle fatigue also was associated with a high diagnostic OR (9.63), meaning that the presence of muscle fatigue significantly increased the odds for SB.
AASM criteria also reported high diagnostic OR (6.33), indicating that the patients with SB diagnosed by AASM criteria had six times greater odds to have SB than non-SB patients.
The Forest plot (Figure 3) and ROC curve (Figure 4) highlight the results. The Forest plot showed that the best way to determine who has SB is for a clinician to ask about muscle fatigue or temporal headache, or to try to question the patient about criteria specified by the AASM. In the same way, the ROC curve showed that only 3 items are close to the left corner of the graph, which means better accuracy: these are items 1 (muscle fatigue), 5 (temporal headache), and 9 (AASM criteria).
In summary, only two symptoms—muscle fatigue and temporal headache—were able to screen patients with SB. In contrast, most of the signs and symptoms, especially jaw muscle pain and abnormal tooth wear were extremely useful in helping to define who does not SB. No diagnostic criteria tested were able to identify patients with SB.
Although this study found that two items, muscle fatigue and temporal headache, have sensitivity and specificity of an acceptable value to be of use in the clinic, we must manage this information warily. The exact etiological factors of SB are still unknown. The etiologic mechanisms for SB genesis can include sleep arousal, autonomic sympathetic-cardiac activation, genetic predisposition, neurochemicals, psychosocial components, exogenous factors, and comorbidities.7 A single set of simple criteria cannot capture the complexity of such a sleep movement disorder. However, these items may be combined in a new clinical algorithm that could play an important role in a new diagnostic system combining research methodology with acceptable diagnostic capability and ease of use.
Future research is needed to clarify this topic and better provide diagnostic tools with acceptable DTA. The future direction for SB assessment would be to develop a handy tool that can directly, reliably, and rapidly measure ongoing bruxism activity and that can be used in both clinical and research settings.14
Considering our results that demonstrated the poor diagnosis capability of tooth wear in the diagnostic of SB, dentists and physicians should focus more on insightful clinical interviewing of patients. Also, to the extent possible, screening of patients for SB using AASM criteria8 would be effective and could help to ensure a more appropriate selection of patients for referral to PSG.
Limitations
There were some limitations in this study. First, the subjects stayed only one night at the sleep laboratory; they perhaps needed more time to adapt. Although there is an expected effect of the first night in a sleep laboratory possibly resulting in an increase in the variability of the parameters of sleep,6 the cost of PSG made the protocol to stay two nights at the sleep laboratory unfeasible. Moreover, it cannot be ruled out that those individuals who have not been diagnosed with SB at PSG are in fact free of this condition, since there could be SB episodes on subsequent days.6 Hasegawa et al.20 assessed the first-night effect on SB by performing a retrospective polysomnographic analysis of data from a sample of SB patients recorded in a sleep laboratory over two consecutive nights. They concluded that in clinical practice, one-night sleep recording could be sufficient for moderate-high frequency SB patients. A small sample size was a second limitation of our study. Future research with a larger sample size needs to be conducted.
CONCLUSIONS
Presence of muscle fatigue and temporal headaches can be considered a good indicator of diagnosis of sleep bruxism. Absence of jaw muscle pain, sounds during sleep, muscle fatigue, and abnormal tooth wear could be a good screening tool to diagnose patients without SB.
None of the diagnostic criteria studied was able to correctly identify those with SB. However, the AASM criteria8 had the best diagnostic accuracy of the evaluated tests. As it does not attain diagnostic values high enough to replace the current gold standard (PSG), it should be used as a screening tool to identify SB.
DISCLOSURE STATEMENT
This was not an industry supported study. This research was supported by FAPESP/SP/Brazil, process number 2012/10228-6. The authors have indicated no financial conflicts of interest. This work was conducted at the Ribeirão Preto School of Dentistry, University of São Paulo, São Paulo, Brazil.
ACKNOWLEDGMENTS
The authors thank Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for financial support. We also thank Dr. João Spir Filho for his work in the realization of sleep studies.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- DTA
diagnostic test accuracy
- EDS
excessive daytime sleepiness
- FN
false negative
- FP
false positive
- ICSD
International Classification of Sleep Disorders
- LR+
positive likelihood ratio
- LR−
negative likelihood ratio
- NPV
negative predictive value
- OR
odds ratio
- PPV
positive predictive value
- PSG
polysomnography
- RevMan 5.2
Review Manager 5.2
- RDC/TMD
Research Diagnostic Criteria for Temporomandibular disorders
- ROC
receiver operating characteristic curves
- SB
sleep bruxism
- TN
true negative
- TP
true positive
APPENDICIES
Appendix 1.
Table 2 × 2 with all variables.
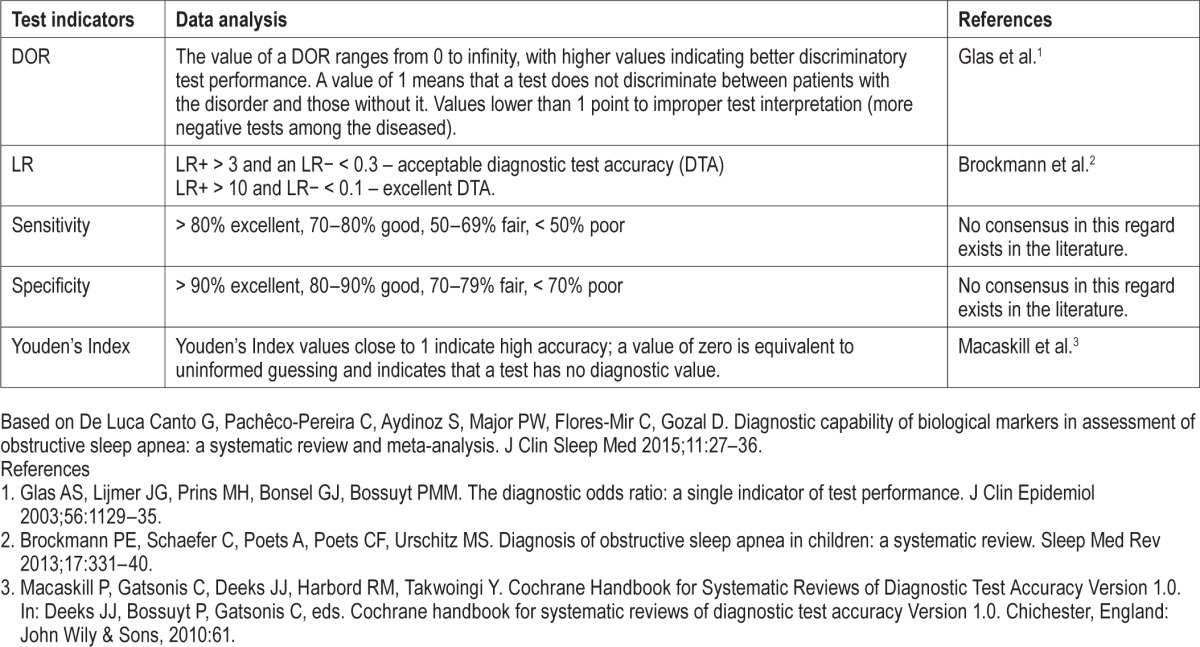
Appendix 2.
Additional analysis complementary data.
REFERENCES
- 1.Lobbezoo F, Ahlberg J, Glaros AG, et al. Bruxism defined and graded: an international consensus. J Oral Rehabil. 2013;40:2–4. doi: 10.1111/joor.12011. [DOI] [PubMed] [Google Scholar]
- 2.Manfredini D, Lobbezoo F. Role of psychosocial factors in the etiology of bruxism. J Orof Pain. 2009;23:153–66. [PubMed] [Google Scholar]
- 3.Bader G, Lavigne G. Sleep bruxism; an overview of an oromandibular sleep movement disorder. Sleep Med Rev. 2000;4:27–43. doi: 10.1053/smrv.1999.0070. [DOI] [PubMed] [Google Scholar]
- 4.Lavigne GJ, Montplaisir JY. Restless legs syndrome and sleep bruxism: prevalence and association among Canadians. Sleep. 1994;17:739–43. [PubMed] [Google Scholar]
- 5.Manfredini D, Restrepo C, Diaz-Serrano K, Winocur E, Lobbezoo F. Prevalence of sleep bruxism in children: a systematic review of the literature. J Oral Rehabil. 2013;40:631–42. doi: 10.1111/joor.12069. [DOI] [PubMed] [Google Scholar]
- 6.Maluly M, Andersen ML, Dal-Fabbro C, et al. Polysomnographic study of the prevalence of sleep bruxism in a population sample. J Dental Res. 2013;92:97S–103S. doi: 10.1177/0022034513484328. [DOI] [PubMed] [Google Scholar]
- 7.Carra MC, Huynh N, Lavigne G. Sleep bruxism: a comprehensive overview for the dental clinician interested in sleep medicine. Dent Clin North Am. 2012;56:387–413. doi: 10.1016/j.cden.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 8.American Academy of Sleep Medicine. International classification of sleep disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 9.Lavigne GJ, Rompre PH, Montplaisir JY. Sleep bruxism: validity of clinical research diagnostic criteria in a controlled polysomnographic study. J Dental Res. 1996;75:546–52. doi: 10.1177/00220345960750010601. [DOI] [PubMed] [Google Scholar]
- 10.Gozal D. Serum, urine, and breath-related biomarkers in the diagnosis of obstructive sleep apnea in children: is it for real? Curr Opin Pulm Med. 2012;18:561–7. doi: 10.1097/MCP.0b013e328358be2d. [DOI] [PubMed] [Google Scholar]
- 11.Schiffman E, Ohrbach R, Truelove E, et al. Diagnostic criteria for temporomandibular disorders for clinical and research applications: recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J Oral Facial Pain Headache. 2014;28:6–27. doi: 10.11607/jop.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulz R, Hummel C, Heinemann S, Seeger W, Grimminger F. Serum levels of vascular endothelial growth factor are elevated in patients with obstructive sleep apnea and severe nighttime hypoxia. Am J Res Crit Care Med. 2002;165:67–70. doi: 10.1164/ajrccm.165.1.2101062. [DOI] [PubMed] [Google Scholar]
- 13.Reding GR, Zepelin H, Robinson JE, Zimmerman SO, Smith VH. Nocturnal Teeth-grinding: all-night psychophysiologic studies. J Dent Res. 1968;47:786–97. doi: 10.1177/00220345680470052001. [DOI] [PubMed] [Google Scholar]
- 14.Carra MC, Huynh N, Lavigne G. Sleep bruxism: a comprehensive overview for the dental clinician interested in sleep medicine. Dent Clin North Am. 2012;56:387–413. doi: 10.1016/j.cden.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Abe S, Yamaguchi T, Rompre PH, De Grandmont P, Chen YJ, Lavigne GJ. Tooth wear in young subjects: a discriminator between sleep bruxers and controls? Int J Prosthodont. 2009;22:342–50. [PubMed] [Google Scholar]
- 16.Li H, Zou Y, Ding G. Dietary factors associated with dental erosion: a meta-analysis. PloS one. 2012;7:e42626. doi: 10.1371/journal.pone.0042626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marsicano JA, de Moura-Grec PG, Bonato RC, Sales-Peres Mde C, Sales-Peres A, Sales-Peres SH. Gastroesophageal reflux, dental erosion, and halitosis in epidemiological surveys: a systematic review. Eur J Gastroenterol Hepatol. 2013;25:135–41. doi: 10.1097/MEG.0b013e32835ae8f7. [DOI] [PubMed] [Google Scholar]
- 18.Lussi A, Megert B, Shellis RP, Wang X. Analysis of the erosive effect of different dietary substances and medications. Brit J Nutr. 2012;107:252–62. doi: 10.1017/S0007114511002820. [DOI] [PubMed] [Google Scholar]
- 19.De Luca Canto G, Singh V, Bigal ME, Major PW, Flores-Mir C. Association between tension-type headache and migraine with sleep bruxism: a systematic review. Headache. 2014;54:1460–9. doi: 10.1111/head.12446. [DOI] [PubMed] [Google Scholar]
- 20.Hasegawa Y, Lavigne G, Rompre P, Kato T, Urade M, Huynh N. Is there a first night effect on sleep bruxism? A sleep laboratory study. J Clin Sleep Med. 2013;9:1139–45. doi: 10.5664/jcsm.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]