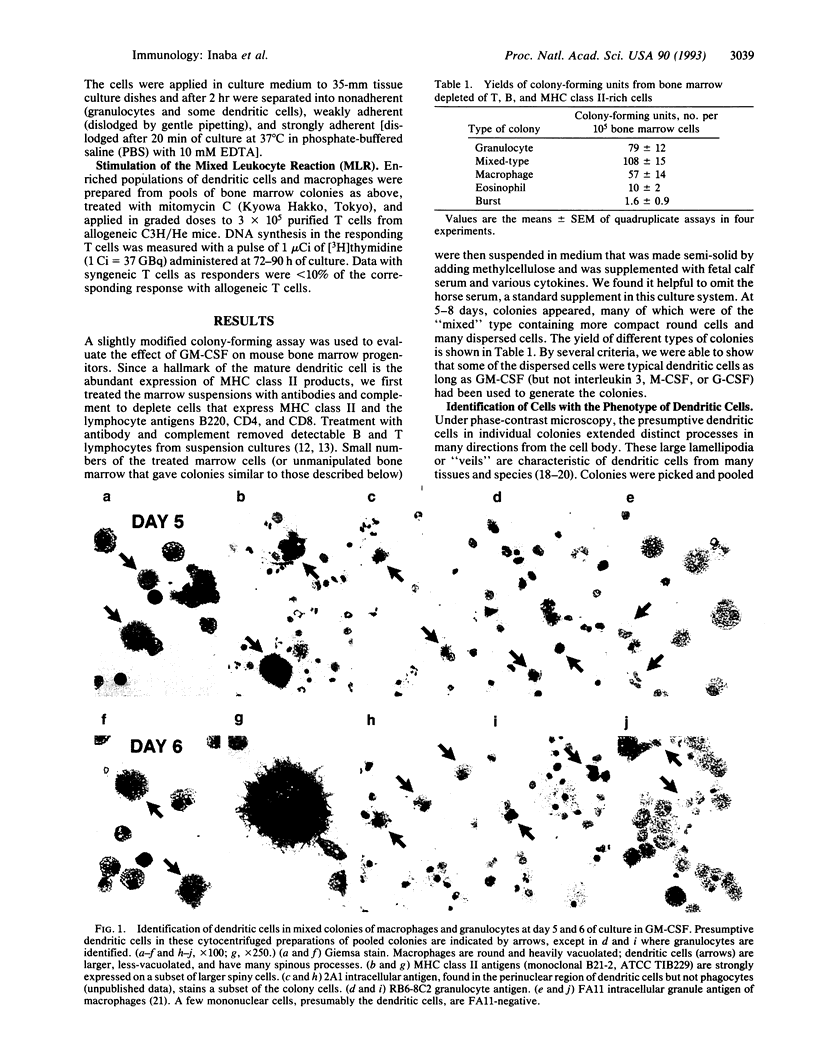
Abstract
The developmental origin of dendritic cells, a specialized system of major histocompatibility complex (MHC) class II-rich antigen-presenting cells for T-cell immunity and tolerance, is not well characterized. Granulocyte-macrophage colony-stimulating factor (GM-CSF) is known to stimulate dendritic cells, including growth and development from MHC class II-negative precursors in suspension cultures of mouse bone marrow. Here we studied colony formation in semi-solid methylcellulose cultures, a classical bioassay system in which GM-CSF induces the formation of mixed granulocyte-macrophage colonies. When colonies were induced from MHC class II-negative precursors, a small subset (1-2%) of typical dendritic cells developed alongside macrophages and granulocytes. The dendritic cells were distinguished by their cytologic features, high levels of MHC class II products, and distinct intracellular granule antigens. By using differential adherence to plastic, enriched populations of the various myeloid cell types were isolated from colonies. Only the dendritic cells stimulated a primary T-cell immune response, the mixed leukocyte reaction, and the potency was comparable to typical dendritic cells isolated from spleen. Macrophages from mixed or pure colonies were inactive as stimulator cells. Therefore, three distinct pathways of myeloid development--granulocytes, macrophages, and dendritic cells--can develop from a common MHC class II-negative progenitor under the aegis of GM-CSF.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agger R., Witmer-Pack M., Romani N., Stossel H., Swiggard W. J., Metlay J. P., Storozynsky E., Freimuth P., Steinman R. M. Two populations of splenic dendritic cells detected with M342, a new monoclonal to an intracellular antigen of interdigitating dendritic cells and some B lymphocytes. J Leukoc Biol. 1992 Jul;52(1):34–42. doi: 10.1002/jlb.52.1.34. [DOI] [PubMed] [Google Scholar]
- Bowers W. E., Berkowitz M. R. Differentiation of dendritic cells in cultures of rat bone marrow cells. J Exp Med. 1986 Apr 1;163(4):872–883. doi: 10.1084/jem.163.4.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breel M., Mebius R. E., Kraal G. Dendritic cells of the mouse recognized by two monoclonal antibodies. Eur J Immunol. 1987 Nov;17(11):1555–1559. doi: 10.1002/eji.1830171105. [DOI] [PubMed] [Google Scholar]
- Drexhage H. A., Mullink H., de Groot J., Clarke J., Balfour B. M. A study of cells present in peripheral lymph of pigs with special reference to a type of cell resembling the Langerhans cell. Cell Tissue Res. 1979 Nov;202(3):407–430. doi: 10.1007/BF00220434. [DOI] [PubMed] [Google Scholar]
- Freudenthal P. S., Steinman R. M. The distinct surface of human blood dendritic cells, as observed after an improved isolation method. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7698–7702. doi: 10.1073/pnas.87.19.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heufler C., Koch F., Schuler G. Granulocyte/macrophage colony-stimulating factor and interleukin 1 mediate the maturation of murine epidermal Langerhans cells into potent immunostimulatory dendritic cells. J Exp Med. 1988 Feb 1;167(2):700–705. doi: 10.1084/jem.167.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Inaba M., Romani N., Aya H., Deguchi M., Ikehara S., Muramatsu S., Steinman R. M. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992 Dec 1;176(6):1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Steinman R. M., Pack M. W., Aya H., Inaba M., Sudo T., Wolpe S., Schuler G. Identification of proliferating dendritic cell precursors in mouse blood. J Exp Med. 1992 May 1;175(5):1157–1167. doi: 10.1084/jem.175.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Steinman R. M. Resting and sensitized T lymphocytes exhibit distinct stimulatory (antigen-presenting cell) requirements for growth and lymphokine release. J Exp Med. 1984 Dec 1;160(6):1717–1735. doi: 10.1084/jem.160.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba M., Inaba K., Hosono M., Kumamoto T., Ishida T., Muramatsu S., Masuda T., Ikehara S. Distinct mechanisms of neonatal tolerance induced by dendritic cells and thymic B cells. J Exp Med. 1991 Mar 1;173(3):549–559. doi: 10.1084/jem.173.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson G. G. Properties of lymph-borne (veiled) dendritic cells in culture. I. Modulation of phenotype, survival and function: partial dependence on GM-CSF. Immunology. 1989 Sep;68(1):102–107. [PMC free article] [PubMed] [Google Scholar]
- Matzinger P., Guerder S. Does T-cell tolerance require a dedicated antigen-presenting cell? Nature. 1989 Mar 2;338(6210):74–76. doi: 10.1038/338074a0. [DOI] [PubMed] [Google Scholar]
- Mazda O., Watanabe Y., Gyotoku J., Katsura Y. Requirement of dendritic cells and B cells in the clonal deletion of Mls-reactive T cells in the thymus. J Exp Med. 1991 Mar 1;173(3):539–547. doi: 10.1084/jem.173.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metlay J. P., Witmer-Pack M. D., Agger R., Crowley M. T., Lawless D., Steinman R. M. The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. J Exp Med. 1990 May 1;171(5):1753–1771. doi: 10.1084/jem.171.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito K., Inaba K., Hirayama Y., Inaba-Miyama M., Sudo T., Muramatsu S. Macrophage factors which enhance the mixed leukocyte reaction initiated by dendritic cells. J Immunol. 1989 Mar 15;142(6):1834–1839. [PubMed] [Google Scholar]
- Rabinowitz S. S., Gordon S. Macrosialin, a macrophage-restricted membrane sialoprotein differentially glycosylated in response to inflammatory stimuli. J Exp Med. 1991 Oct 1;174(4):827–836. doi: 10.1084/jem.174.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid C. D., Fryer P. R., Clifford C., Kirk A., Tikerpae J., Knight S. C. Identification of hematopoietic progenitors of macrophages and dendritic Langerhans cells (DL-CFU) in human bone marrow and peripheral blood. Blood. 1990 Sep 15;76(6):1139–1149. [PubMed] [Google Scholar]
- Reid C. D., Stackpoole A., Meager A., Tikerpae J. Interactions of tumor necrosis factor with granulocyte-macrophage colony-stimulating factor and other cytokines in the regulation of dendritic cell growth in vitro from early bipotent CD34+ progenitors in human bone marrow. J Immunol. 1992 Oct 15;149(8):2681–2688. [PubMed] [Google Scholar]
- Scheicher C., Mehlig M., Zecher R., Reske K. Dendritic cells from mouse bone marrow: in vitro differentiation using low doses of recombinant granulocyte-macrophage colony-stimulating factor. J Immunol Methods. 1992 Oct 2;154(2):253–264. doi: 10.1016/0022-1759(92)90199-4. [DOI] [PubMed] [Google Scholar]
- Schuler G., Steinman R. M. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J Exp Med. 1985 Mar 1;161(3):526–546. doi: 10.1084/jem.161.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. J., Koch G. L. Differential expression of murine macrophage surface glycoprotein antigens in intracellular membranes. J Cell Sci. 1987 Feb;87(Pt 1):113–119. doi: 10.1242/jcs.87.1.113. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Kaplan G., Witmer M. D., Cohn Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. V. Purification of spleen dendritic cells, new surface markers, and maintenance in vitro. J Exp Med. 1979 Jan 1;149(1):1–16. doi: 10.1084/jem.149.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Witmer M. D. Lymphoid dendritic cells are potent stimulators of the primary mixed leukocyte reaction in mice. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5132–5136. doi: 10.1073/pnas.75.10.5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witmer-Pack M. D., Olivier W., Valinsky J., Schuler G., Steinman R. M. Granulocyte/macrophage colony-stimulating factor is essential for the viability and function of cultured murine epidermal Langerhans cells. J Exp Med. 1987 Nov 1;166(5):1484–1498. doi: 10.1084/jem.166.5.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. W., Koulova L., Soergel S. A., Clark E. A., Steinman R. M., Dupont B. The B7/BB1 antigen provides one of several costimulatory signals for the activation of CD4+ T lymphocytes by human blood dendritic cells in vitro. J Clin Invest. 1992 Jul;90(1):229–237. doi: 10.1172/JCI115840. [DOI] [PMC free article] [PubMed] [Google Scholar]






