Abstract
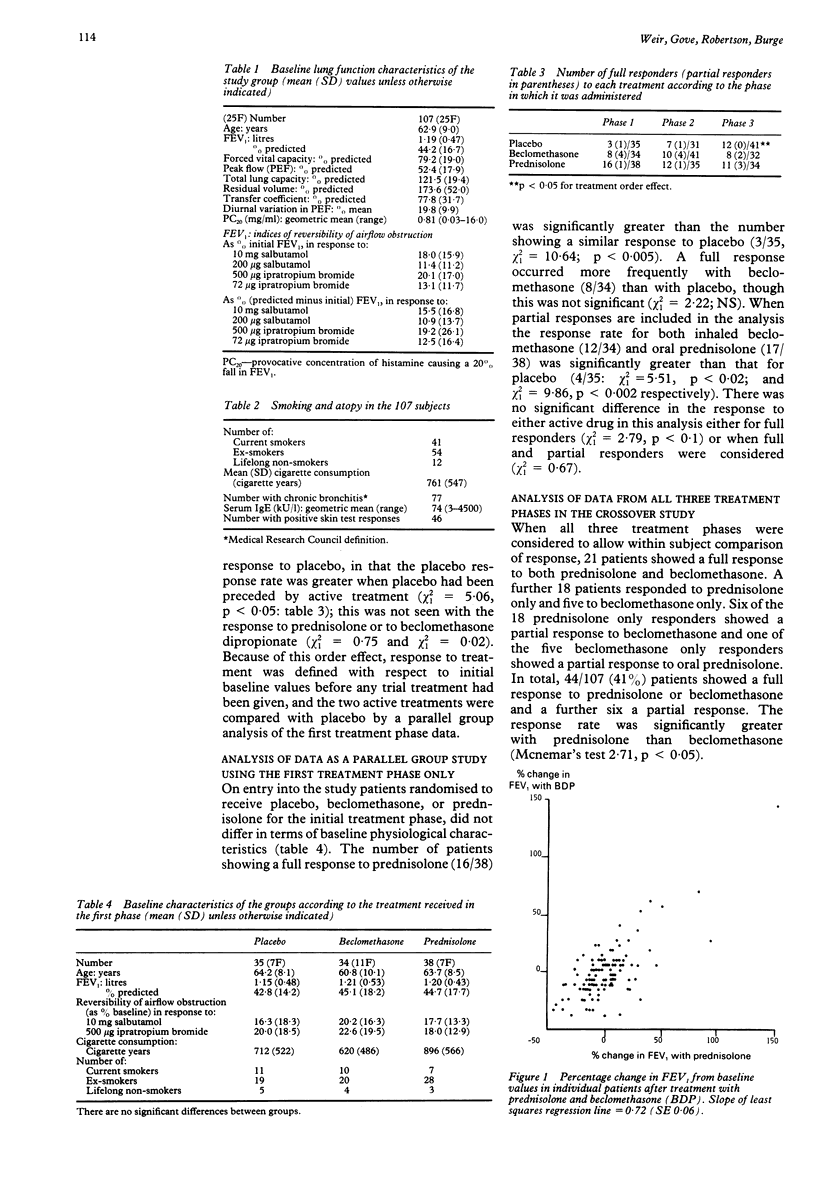
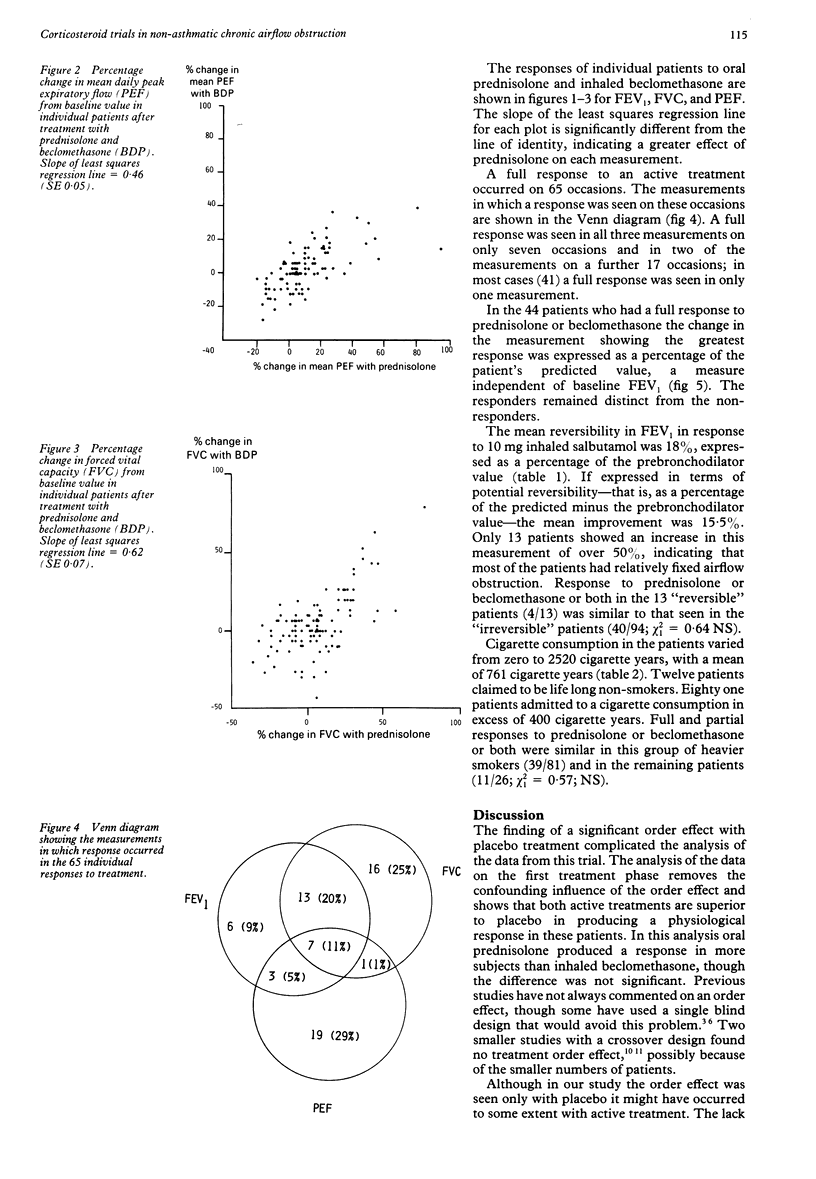
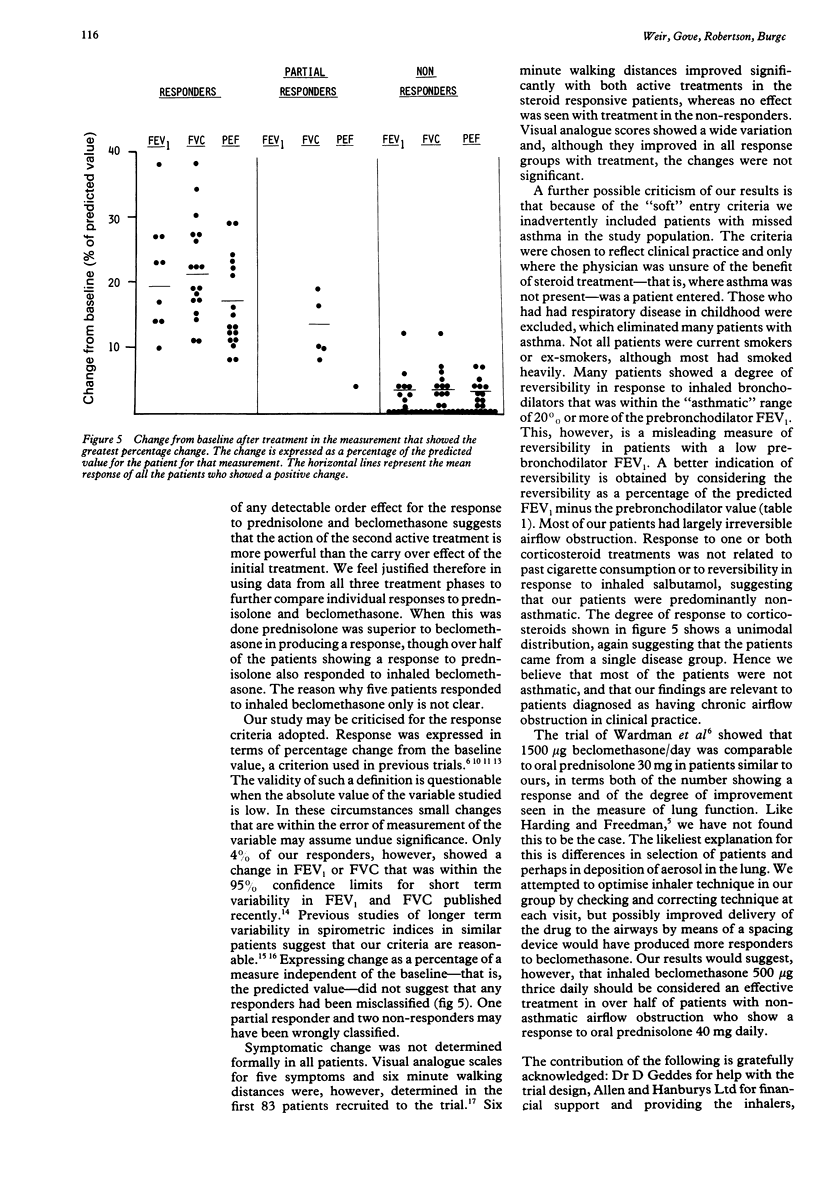
One hundred and twenty seven adults considered on clinical grounds to have non-asthmatic chronic airflow obstruction entered a randomised, double blind, placebo controlled, crossover trial comparing the physiological response to inhaled beclomethasone dipropionate 500 micrograms thrice daily with oral prednisolone 40 mg a day, both given for two weeks. One hundred and seven patients completed the study. Response was assessed as change in FEV1 and FVC measured on the last treatment day, and as change in mean peak expiratory flow (PEF) over the final seven days of treatment from home PEF recordings performed five times daily. A full response to treatment was defined as an increase in FEV or FVC, or an increase in mean daily PEF over the final seven days of treatment, of at least 20% from baseline values. An improvement in one measurement of at least 15%, or of 10% in any two measurements, was defined as a partial treatment response. Response to placebo showed a significant order effect, suggesting a carry over effect of active treatment of at least three weeks. Response to active treatment was therefore related to initial baseline values, and compared with placebo by considering responses in the first treatment phase only. A full response to oral prednisolone (16/38) was significantly more common than to placebo (3/35). The number of full responses to inhaled beclomethasone (8/34) did not differ significantly from the number responding to oral prednisolone or placebo in the first treatment phase, though full and partial responses to inhaled beclomethasone (12/34) were significantly more common than those to placebo (4/35). When all three treatment phases were considered 44/107 patients showed a full response to one or both forms of corticosteroid treatment, a response to prednisolone (39) occurring more frequently than to inhaled beclomethasone (26). Only 21 of the 44 responders showed a response to both forms of treatment. Inhaled beclomethasone dipropionate 500 micrograms thrice daily was inferior to oral prednisolone 40 mg per day, but better than placebo, in producing improvement in physiological measurements in patients thought to have nonasthmatic chronic airflow obstruction. It was, however, an effective alternative in over half of those showing a response to prednisolone.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cockcroft D. W., Killian D. N., Mellon J. J., Hargreave F. E. Bronchial reactivity to inhaled histamine: a method and clinical survey. Clin Allergy. 1977 May;7(3):235–243. doi: 10.1111/j.1365-2222.1977.tb01448.x. [DOI] [PubMed] [Google Scholar]
- FRANKLIN W., MICHELSON A. L., LOWELL F. C., SCHILLER I. W. Bronchodilators and corticosteroids in the treatment of obstructive pulmonary emphysema. N Engl J Med. 1958 Apr 17;258(16):774–778. doi: 10.1056/NEJM195804172581602. [DOI] [PubMed] [Google Scholar]
- Harding S. M., Freedman S. A comparison of oral and inhaled steroids in patients with chronic airways obstruction: features determining response. Thorax. 1978 Apr;33(2):214–218. doi: 10.1136/thx.33.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam W. K., So S. Y., Yu D. Y. Response to oral corticosteroids in chronic airflow obstruction. Br J Dis Chest. 1983 Apr;77(2):189–198. doi: 10.1016/0007-0971(83)90027-x. [DOI] [PubMed] [Google Scholar]
- Mendella L. A., Manfreda J., Warren C. P., Anthonisen N. R. Steroid response in stable chronic obstructive pulmonary disease. Ann Intern Med. 1982 Jan;96(1):17–21. doi: 10.7326/0003-4819-96-1-17. [DOI] [PubMed] [Google Scholar]
- Mitchell D. M., Gildeh P., Dimond A. H., Collins J. V. Value of serial peak expiratory flow measurements in assessing treatment response in chronic airflow limitation. Thorax. 1986 Aug;41(8):606–610. doi: 10.1136/thx.41.8.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D. M., Gildeh P., Rehahn M., Dimond A. H., Collins J. V. Effects of prednisolone in chronic airflow limitation. Lancet. 1984 Jul 28;2(8396):193–196. doi: 10.1016/s0140-6736(84)90481-1. [DOI] [PubMed] [Google Scholar]
- Mungall I. P., Hainsworth R. Assessment of respiratory function in patients with chronic obstructive airways disease. Thorax. 1979 Apr;34(2):254–258. doi: 10.1136/thx.34.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozas C. J., Goldman A. L. Daily spirometric variability: normal subjects and subjects with chronic bronchitis with and without airflow obstruction. Arch Intern Med. 1982 Jul;142(7):1287–1291. doi: 10.1001/archinte.142.7.1287. [DOI] [PubMed] [Google Scholar]
- Rudd R. Corticosteroids in chronic bronchitis. Br Med J (Clin Res Ed) 1984 May 26;288(6430):1553–1554. doi: 10.1136/bmj.288.6430.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro S. G., Souhami R. L. Duration of chemotherapy in small cell lung cancer. Thorax. 1990 Jan;45(1):1–2. doi: 10.1136/thx.45.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes T. C., Shaylor J. M., O'Reilly J. F., Harrison B. D. Assessment of steroid responsiveness in patients with chronic airflow obstruction. Lancet. 1982 Aug 14;2(8294):345–348. doi: 10.1016/s0140-6736(82)90545-1. [DOI] [PubMed] [Google Scholar]
- Tweeddale P. M., Alexander F., McHardy G. J. Short term variability in FEV1 and bronchodilator responsiveness in patients with obstructive ventilatory defects. Thorax. 1987 Jul;42(7):487–490. doi: 10.1136/thx.42.7.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardman A. G., Simpson F. G., Knox A. J., Page R. L., Cooke N. J. The use of high dose inhaled beclomethasone dipropionate as a means of assessing steroid responsiveness in obstructive airways disease. Br J Dis Chest. 1988 Apr;82(2):168–171. doi: 10.1016/0007-0971(88)90038-1. [DOI] [PubMed] [Google Scholar]



