Abstract
Background
p63, a member of p53 family, known to be expressed in embryonic tissues and basal regenerative layers of many epithelial tissues in the adult, is also expressed in various benign and malignant lesions of body including lesions of oral cavity. To evaluate the expression of p63 and compare the expression qualitatively and quantitatively in normal buccal mucosa, epithelial dysplasia, oral submucous fibrosis (OSMF), and oral squamous cell carcinoma (OSCC).
Methods
The study material consisted of 45 archival cases which were divided into Group I with 5 cases of normal buccal mucosa, Group II with 15 cases of epithelial dysplasia, and Group III with 10 cases of OSMF and 15 cases of OSCC. Immunohistochemical expression of p63 was assessed by using mean, standard deviation, and analysis of variance.
Results
Overexpression of p63 was seen in epithelial dysplasia, OSMF, and squamous cell carcinoma with an increased suprabasal expression in cases of epithelial dysplasia. The mean labeling index (LI) of p63 was found to be in increasing order from normal oral mucosa (33.75%), OSMF (57.37%), epithelial dysplasia (63.87%) to squamous cell carcinoma (69.76%).
Conclusion
The results suggest a possible role of p63 in oral carcinogenesis, and an increased LI as well as increased suprabasal expression of this gene in dysplastic lesions may have a potential to be utilized as a marker for premalignancy.
Keywords: IHC, p63, OSMF, OSCC, Epithelial dysplasia
1. Introduction
Accumulation of genetic alterations is the basis for the progression from a normal cell to a cancer cell. These alterations can be in the form of mutations in certain genes (tumor suppressor genes or oncogenes), gain or loss of chromosome material, or loss of heterozygosity. Even though a large proportion of oral squamous cell carcinomas (OSCCs) are thought to be preceded by visible precursor lesions and conditions like leukoplakia, erythroplakia, and oral submucous fibrosis (OSMF), it is often difficult to predict the actual malignant transformation in an individual case. Hence, it would be of interest to develop markers, which could detect early genetic changes in these lesions, and may facilitate detection of those lesions, which may have potential to progress to malignancy.
p63 gene, one of the members of p53 family, encodes a transactivation domain at N-terminal, a core DNA binding domain, and a oligomerization domain at carboxy terminal. The gene contains two transcriptional start sites that are used to generate transcripts encoding proteins with or without an N-terminal transactivation domain. Proteins with the transactivation domain are termed TAp63, and proteins lacking the transactivation domain are termed ΔNp63.1 p63 expression is absolutely essential for limb formation and epidermal morphogenesis (integument and tongue), including the formation of adnexa (teeth, hair, mammary and prostate glands, and sweat and lacrimal glands). In postnatal epidermis, p63 expression is restricted to nuclei of basal cells of normal epithelia (skin, oral mucosa, esophagus, tonsil, prostate, urothelium, ectocervix, and vagina) and to certain populations of basal cells in glandular structures of prostate, breast, and bronchi.2 The human p63 gene is located on chromosome 3q27-28 within a region that is frequently altered in epithelial dysplasia3 and OSCC.4
This study was designed to analyze and compare the expression pattern of p63 in OSCC, leukoplakia, OSMF, and normal buccal mucosa and to assess its usefulness as marker for premalignancy.
2. Materials and method
The study material comprised of biopsy samples from 15 cases of leukoplakic/erythroplakic lesions histopathologically showing epithelial dysplasia, 10 cases of clinically and histopathologically diagnosed OSMF, and 15 cases of OSCC obtained from the archives of Department of Oral Pathology, Saraswati Dental College, Lucknow. Five specimens of nonkeratinised buccal mucosa were taken from normal subjects with no oral lesions and no tobacco/alcohol/areca nut habits. Normal prostrate tissue with positive expression of p63 was taken as positive control (Fig. 1).
Fig. 1.
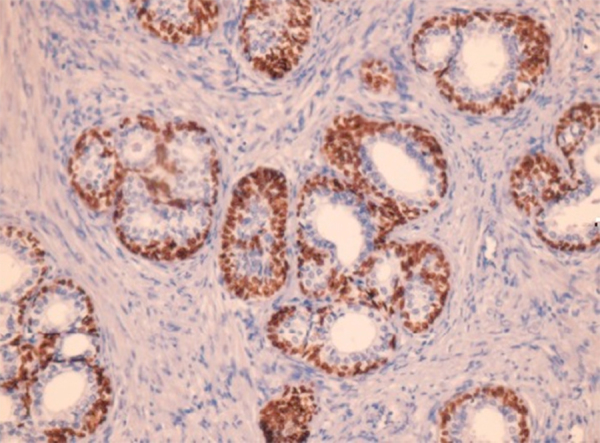
Photomicrograph showing p63 expression in normal prostrate tissue (400×).
The diagnosis and grading of dysplasia and OSCC was done according to WHO classification 2005 & Anneroth's classification, respectively, using routine Hematoxylin & Eosin-stained sections.5,6
3. Immunohistochemical procedure
The immunohistochemical staining for p63 expression was performed as per manufacturer's instructions. Briefly, tissue sections cut at approximately 4 μm were mounted on glass slides precoated with poly-l-lysine. Antigen retrieval was done using pressure cooker method in citrate buffer at pH (6.0–6.2). Sections were stained by using anti-p63 monoclonal antibody (prediluted ‘ready to use’ antibody, Clone-4A4 catalog number: AM418-5M from BioGenex Inc., San Ramon, CA, USA). Super sensitive poly-HRP (antimouse and antirabbit IgG labeled with enzyme polymer in phosphate buffered saline with stabilizers and proclin 300; catalog number: QD400-60K: Biogenex Inc., San Ramon, CA, USA) was used as secondary antibody. Color development was done with Di-amino benzidine (DAB) and sections were counterstained with hematoxylene. Positive and negative controls for staining were run along with all batches of staining of study samples. Positive control consisted of normal human prostate tissues with known antigenic reactivity to p63 in basal cells of prostatic epithelium. A negative control was performed by omitting the step of primary antibody during the staining, which resulted in lack of staining in all cases.
4. Counting criteria
Stained sections were observed and assessed on Olympus BX-51 light microscope and cases showing uniform brown coloration of DAB in nuclei of epithelial cells at low power were considered as positive. Sections showing complete lack of immunostaining of epithelial cells were considered negative. Positivity of individual cells was assessed at magnification of 200× and those cells showing uniform brown nuclear staining were considered positive, while those showing only hematoxylin staining were considered negative. Qualitative assessment of p63 expression was done based on the level of positivity of p63 in basal/parabasal, suprabasal, and superficial layers in normal oral epithelium (Fig. 2), various grades of oral epithelial dysplasia (Fig. 3), and OSMF (Fig. 4). In OSCC, the pattern of expression was assessed either as peripheral and/or central or as diffuse, based on level of staining of tumor islands (Fig. 4). For quantitative assessment, 5 non-overlapping fields selected at 400× magnification photomicrographs were taken using Olympus Live View Digital SLR Camera E-330. The photomicrographs were then analyzed using Image Pro Express 6.0 software for windows (Media Cybernetics, Inc., USA). Positive as well as total number of nuclei was counted in each field and mean scores were used to calculate the percent positive nuclei, which was considered as labeling index (LI) of p63.
Fig. 2.
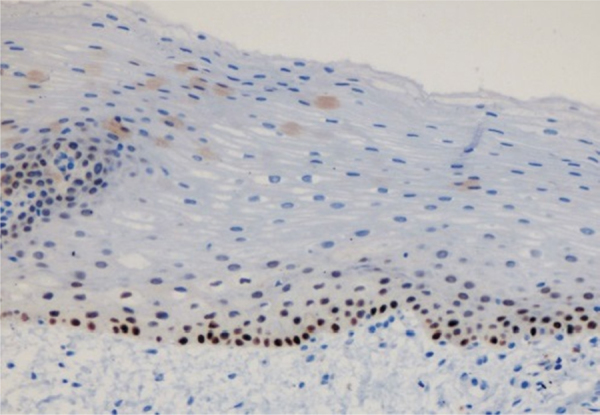
Photomicrograph showing p63 expression in normal control: basal expression was seen (400×).
Fig. 3.
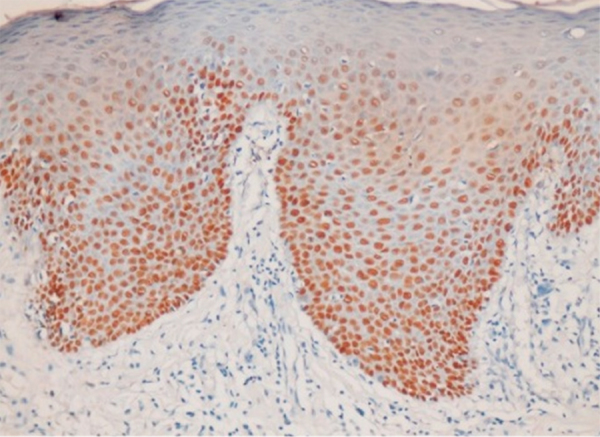
Photomicrograph showing p63 expression in dysplastic epithelium (400×).
Fig. 4.
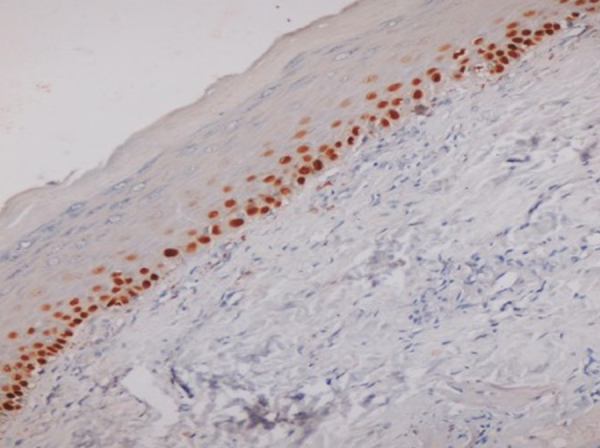
Photomicrograph showing p63 expression in basal and suprabasal layers in OSMF (400×).
5. Results
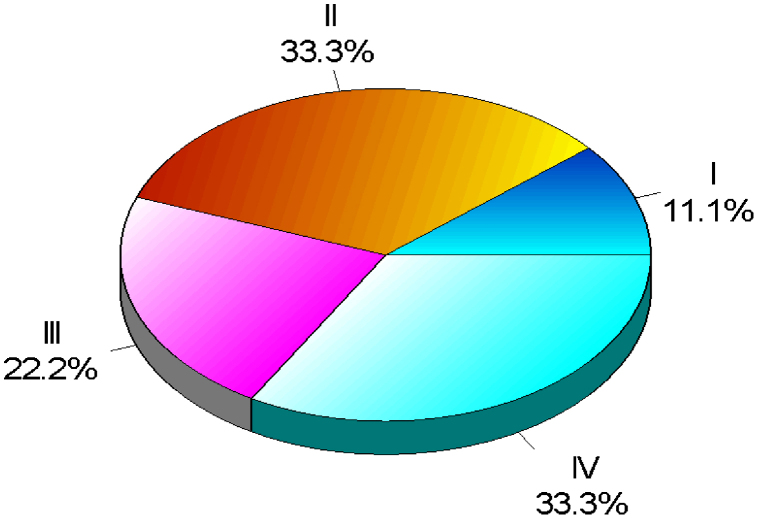
Statistical analysis was done using Chi-square test, Student t-test, and analysis of variance (ANOVA). p value of 0.05 or less was considered significant. All the cases were divided into 4 groups. Groups I, II, III, and IV comprised of normal oral mucosa, epithelial dysplasia, OSMF, and OSCC, respectively (Graph 1).
Graph 1.
Showing distribution of sample.
p63 expression was seen in all cases except 2 cases, one case each from Group I and Group IV (Table 1). p63 expression was seen in basal layer (Fig. 2) of normal epithelium except one case, which showed expression in suprabasal layer.
Table 1.
Qualitative assessment of p63 expression.
| S. No. | Group | n | No expression |
Expression |
χ2(p) | ||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | ||||
| 1. | I | 5 | 1 | 20 | 4 | 80 | 4.186 (0.242) |
| 2. | II | 15 | 0 | 0 | 15 | 100 | |
| 3. | III | 10 | 0 | 0 | 10 | 100 | |
| 4. | IV | 15 | 1 | 6.7 | 14 | 93.3 | |
χ2 = Chi-square test (degree of freedom = 3); p < 0.05 significant.
p63 expression was seen in basal and suprabasal layers of dysplastic epithelium while superficial layers did not show expression of p63 (Fig. 3).
p63 expression was seen in basal and suprabasal layers of all cases of OSMF. None of the cases showed expression extending to the superficial layers (Fig. 4).
Three patterns of staining were observed in OSCC. In few cases, p63 expression was restricted only in peripheral layer of tumor islands. In some cases, p63 expression was seen in both peripheral and central cells (Fig. 5).
Fig. 5.
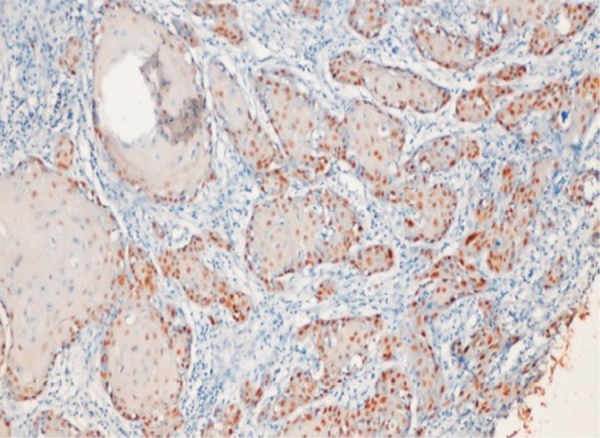
Photomicrograph showing p63 expression in peripheral cells of tumor islands (400×).
In rest of the cases, p63 expression was diffused throughout the tumor tissue (Fig. 6).
Fig. 6.
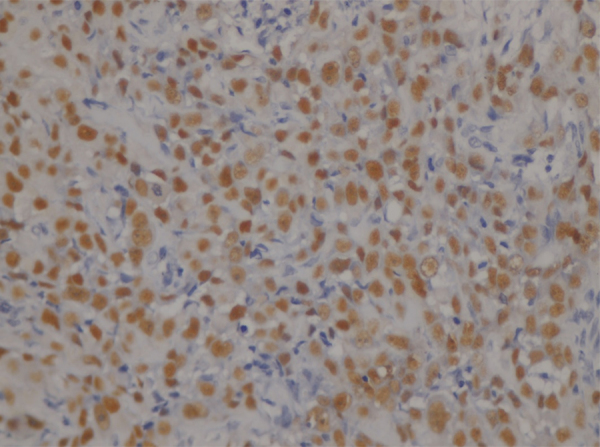
Photomicrograph showing diffuse expression of p63 in OSCC (400×).
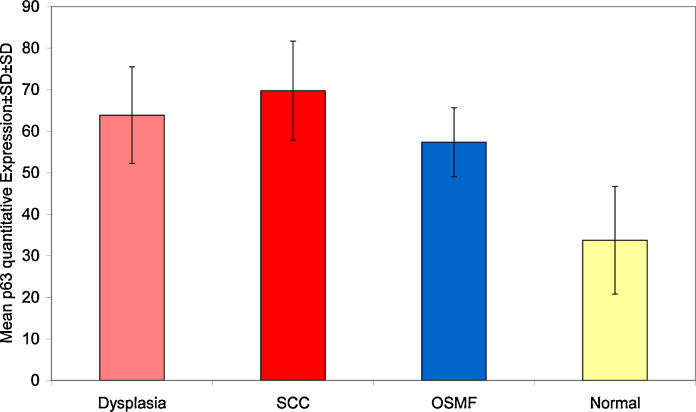
Mean LI of p63 varied from 33.75 to 69.76 with minimum value of 21.5 seen in normal to maximum of 92.6 in OSCC (Table 2, Graph 2).
Table 2.
Mean LI in different groups.
| N | Mean | Std. deviation | Minimum | Maximum | |
|---|---|---|---|---|---|
| Dysplasia | 15 | 63.87 | 11.62 | 38.2 | 76.8 |
| SCC | 14 | 69.76 | 11.92 | 51.5 | 92.6 |
| OSMF | 10 | 57.37 | 8.28 | 41.2 | 66.3 |
| Normal | 4 | 33.75 | 12.95 | 21.5 | 50.8 |
| Total | 43 | 61.47 | 14.76 | 21.5 | 92.6 |
Graph 2.
Showing mean LI in different groups.
Statistical analysis was done using ANOVA, and it was found that there was significant difference among the groups (F = 11.471; p < 0.001) (Table 3, Graph 3).
Table 3.
ANOVA of mean p63 expression in different groups.
| Sum of squares | df | Mean square | F | Sig. | |
|---|---|---|---|---|---|
| Between groups | 4289.328 | 3 | 1429.776 | 11.471 | <0.001 |
| Within groups | 4861.019 | 39 | 124.642 | ||
| Total | 9150.347 | 42 |
Graph 3.
Showing ANOVA of mean p63 expression in different groups.
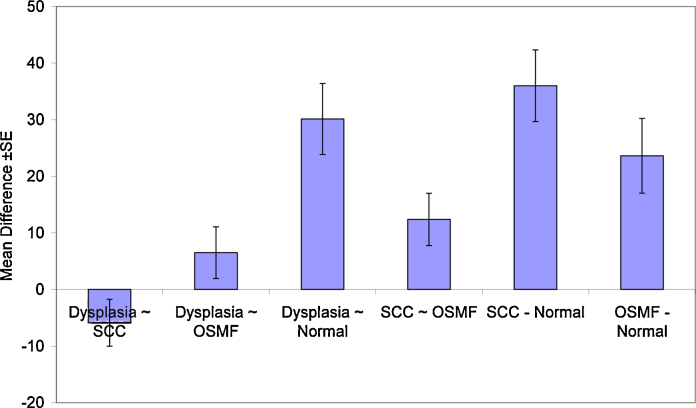
Multiple comparisons revealed a statistically significant difference between control group and all the other groups (p < 0.01), while no significant difference was observed between dysplasia, OSCC, and OSMF (p > 0.05).
A statistically significant difference was observed between OSCC and OSMF group (p = 0.05). No significant difference was observed between OSMF and dysplasia group (p = 0.492) (Table 4, Graph 4).
Table 4.
Multiple comparison between normal oral mucosa, dysplasia, OSMF, and OSCC.
| (I) Group | (J) Group | Mean difference (I − J) | Std. error | Sig. | 95% confidence interval | |
|---|---|---|---|---|---|---|
| Dysplasia | SCC | −5.89 | 4.1488 | 0.495 | −17.023 | 5.242 |
| OSMF | 6.50 | 4.5578 | 0.492 | −5.734 | 18.727 | |
| Normal | 30.12* | 6.2825 | <0.001 | 13.258 | 46.975 | |
| SCC | Dysplasia | 5.89 | 4.1488 | 0.495 | −5.242 | 17.023 |
| OSMF | 12.39 | 4.6225 | 0.050 | −0.017 | 24.791 | |
| Normal | 36.01* | 6.3296 | <0.001 | 19.023 | 52.992 | |
| OSMF | Dysplasia | −6.50 | 4.5578 | 0.492 | −18.727 | 5.734 |
| SCC | −12.39 | 4.6225 | 0.050 | −24.791 | 0.017 | |
| Normal | 23.62* | 6.6049 | 0.005 | 5.897 | 41.343 | |
| Normal | Dysplasia | −30.12* | 6.2825 | <0.001 | −46.975 | −13.258 |
| SCC | −36.01* | 6.3296 | <0.001 | −52.992 | −19.023 | |
| OSMF | −23.62* | 6.6049 | 0.005 | −41.343 | −5.897 | |
The mean difference is significant at the 0.05 level.
Graph 4.
Showing multiple comparisons between various groups.
6. Discussion
The concept of a two-step process of cancer development in the oral mucosa, i.e., the initial presence of a precursor lesion or condition subsequently developing into cancer, is well-established.7 The precancerous lesion and condition included in this study, i.e., leukoplakia, have malignant transformation rate of about 7.9% and 7.6%, respectively.8,9 The human tumor protein (TP) 63 locus lies on chromosome 3q27-28, a frequently amplified region in squamous cell carcinoma. A member of tumor suppressor p53 family, TP63 has been known to act as oncogene.10 In our study, p63 positivity was seen in all cases of leukoplakia, OSMF, and OSCC except one case of OSCC.
In normal buccal mucosa, the expression pattern of p63 was restricted to the basal/parabasal layers as reported previously.4 p63 expression has been seen in embryonic ectoderm as early as seventh to eleventh day,11 and it is suggested that it is the first gene product that distinguishes stem cells from their transient amplified progeny in stratified squamous epithelia.12 The finding that p63 is specifically expressed by stem cells of human epidermis, limbal and not by transient cells strongly suggests that p63 can be recognized as a stem cell marker.13
In our study, out of 15 cases of OSCC, 14 cases were positive and 1 well-differentiated case was negative for p63. The number of p63 positive cells was outstandingly increased when compared to normal buccal epithelium. These findings are consistent with the studies done by other authors on OSCC.3 Potential role for p63 in tumorigenesis is supported by the finding that p63 is a target of genomic amplification and/or overexpression in >80% of primary head and neck squamous cell carcinomas (HNSCC) as well as other squamous epithelial malignancies, thus suggesting the role of p63 as an oncogene.14 The 3q26 region that contains the p63 locus, and is frequently amplified in many tumors, also contains genes for phosphoinositide-3-kinase (PI3K), a positive regulator of pathway. Barbieri et al. showed that ΔNp63α levels are regulated by phosphoinositide-3-kinase pathway downstream of the epidermal growth factor receptor and hence amplification of PIK3CA would possibly explain p63 overexpression in select squamous cell carcinomas.15 In our study, well and moderately differentiated SCCs showed p63 positive cells present mainly at the periphery of tumor islands while few central cells showed immunoreactivity. Tumor islands are composed of peripheral less-differentiated cells close to their embryogenic state, while central cells of tumor islands are the differentiated cells and hence did not show any expression. In poorly differentiated SCCs, p63 expression was seen in almost all the cells.16 The overexpression of p63 reflects the immaturity of the tumor cell lineage, which in turn may cause disruption of terminal differentiation and consequently preserve their ability to multiply.
Dominant negative ΔNp63α isoforms have been considered to be the preferential isoforms to be expressed in squamous cell carcinoma.17 Even though the monoclonal antibody (4A4) used in our study stains all isoforms of p63, it is probable that the increased expression of p63 in malignant and premalignant lesions is due to overexpression of the ΔNp63α isoforms, which acts as an oncogenic protein. Preferential overexpression of ΔNp63α mRNA has previously been shown in cases of head and neck squamous cell carcinoma and epithelial dysplasia.18,19 The role of ΔNp63α form in tumorigenesis is based on the hypothesis that this form can act in a dominant negative manner toward both p53 and transactivating version of p63 such as TAp63, both of which have tumor suppressive functions. Hence, overexpression of ΔNp63α may cause inhibition of tumor suppressor function of p53, leading to propagation of mutated clones.
Insulin growth factor binding protein-3 (IGFBP-3) is a growth inhibitory pro-apoptotic protein, and is a direct transcriptional target of negative regulation by endogenous ΔNp63α in squamous epithelial cells and tissues. Tumors overexpressing p63 have been reported to show loss of IGFBP-3, suggesting that ΔNp63α and IGFBP-3 expression patterns are inversely correlated in normal squamous epithelium and squamous cell carcinomas, and that this repression represents a mechanism by which tumors that overexpress p63 may be protected from apoptosis.20 Another target of p63 in its oncogenic action may be HSP70, one of the most abundant heat shock protein. Its overexpression is associated with metastasis, whereas repression results in inhibition of tumor cells proliferation and induction of apoptosis. Strong association between HSP70 and ΔNp63α has been seen in head and neck squamous cell carcinoma, and it was suggested that ΔNp63 actively upregulates HSP70 similarly to mutant.21
One case each of normal mucosa and OSCC did not show p63 expression even on repeat staining. The most probable explanation may be because of lack of unmasking of the antigen possibly related to fixation of the tissues. Immunohistochemistry is a sensitive technique and fixation is one of its most critical aspects, and hence needs to be kept in consideration before interpreting the results.22 Negative expression of p63 in some cases of OSCC has been reported previously.23
All 15 cases of epithelial dysplasia included in our study showed positive p63 expression in dysplastic epithelium with increase in LI as compared to normal buccal epithelium. p63 positive cells were found in substantial numbers in suprabasal layers along with strong staining in basal/parabasal region as compared to normal epithelium, which is in concordance with some of previous studies.19. In stratified squamous epithelia, basal cells express ΔNp63α, which maintains the proliferative undifferentiated state of basal keratinocytes cells, while TAp63 is required for the stratification program.19 Upon dysplastic change (i.e. transition from normal oral mucosa to epithelial dysplasia), dysplastic keratinocytes above the basal layers may shift to a status similar to the embryogenesis condition, and are still able to express p63 protein producing an antidifferentiation effect as well as an increased proliferative capacity of dysplastic cells.19 Increase in the level of expression and p63 LI in dysplastic epithelium as compared to normal may thus establish the pre-assumption that p63 could be considered as marker of premalignancy.
Although OSMF is a known pre-malignant condition, the molecular basis of progression of OSMF to squamous cell carcinoma is still not established. In our study, we found a significantly higher p63 expression in the epithelium of OSMF as compared to normal oral epithelium with mean LI close to the group of oral epithelial dysplasia. These findings are consistent with some previous reports.24,25 The relationship between malignant transformation of OSMF and presence of epithelial dysplasia is still debatable. The finding that even those cases of OSMF, which lacked epithelial dysplasia, also showed a high p63 LI indicating that molecular changes predisposing toward malignant transformation do take place in the OSMF epithelium, even in the absence of morphologically apparent dysplastic changes. Also, grading of dysplasia in OSMF is highly subjective and variable, especially due to the varying degree of epithelial atrophy, which may lead to over or under grading of dysplastic changes. Studies have reported aberrant expression of p53 in OSMF as well as that of ΔNp63α isoform, which has action similar to mutant p53 and may be associated with the carcinogenesis involving OSMF.24
7. Conclusion
To conclude, the results of our study demonstrate a significantly increased p63 expression in oral premalignant and malignant lesions as compared to the normal oral mucosa. p63 LI was found to be highest for OSCC followed by epithelial dysplasia and OSMF. The finding that p63 expression was stronger in regions known to show lack of differentiation suggests that increased p63 expression in oral epithelium is suggestive of increased proliferative potential and aberrant maturation, which in turn would predispose to malignant transformation, and hence may have a role as a marker for premalignancy.
Conflicts of interest
The authors have none to declare.
References
- 1.Westfall M.D., Pietenpol J.A. p63: molecular complexity in development and cancer. Carcinogenesis. 2004;25(6):857–864. doi: 10.1093/carcin/bgh148. [DOI] [PubMed] [Google Scholar]
- 2.Moll M.U., Neda S. p63 and p73: roles in development and tumor formation. Mol Cancer Res. 2004;2(7):371–386. [PubMed] [Google Scholar]
- 3.Bortoluzzi M.C., Yurgel L.S., Dekker P.N., Jordan R.C.K., Regezi J.A. Assessment of p63 expression in oral squamous cell carcinomas and dysplasias. Oral Surg Oral Med Oral Pathol. 2004;98:698–704. doi: 10.1016/j.tripleo.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Takeda T., Sugihara K., Hirayama Y., Hirano M., Tanuma J.-I., Semba I. Immunohistological evaluation of Ki-67, p63, CK19 and p53 expression in oral epithelial dysplasias. J Oral Pathol Med. 2006;35:369–375. doi: 10.1111/j.1600-0714.2006.00444.x. [DOI] [PubMed] [Google Scholar]
- 5.Siriwardena B.S.M.S., Tilakaratne A., Amaratunga E.A.P.D. Analysis of histopathological and immunohistochemical differences of oral squamous cell carcinoma in young and old patients in Sri Lanka. J Oral Pathol Med. 2007;36:357–362. doi: 10.1111/j.1600-0714.2007.00548.x. [DOI] [PubMed] [Google Scholar]
- 6.Warnakulasuriya S., Reibel J., Bouquot J., Dabelsteen E. Oral epithelial dysplasia classification systems: predictive value, utility, weaknesses and scope for improvement. J Oral Pathol Med. 2008;37:127–133. doi: 10.1111/j.1600-0714.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- 7.Reibel J. Prognosis of oral premalignant lesions: significance of clinical, histopathological, and molecular biological characteristics. Crit Rev Oral Biol Med. 2003;14(1):47–62. doi: 10.1177/154411130301400105. [DOI] [PubMed] [Google Scholar]
- 8.Amagasa T., Yamashiro M., Ishikawa H. Oral leukoplakia related to malignant transformation. Oral Sci Int. 2006;3(2):45–53. [Google Scholar]
- 9.Murti P.R., Bhonsle R.B., Pindborg J.J., Daftary D.K., Gupta P.C., Mehta F.S. Malignant transformation rate in oral submucous fibrosis over a 17-year period. Commun Dent Oral Epidemiol. 1985;13(6):340–341. doi: 10.1111/j.1600-0528.1985.tb00468.x. [DOI] [PubMed] [Google Scholar]
- 10.Yang A., Kaghad M., Wang Y. p63, a p53 homologue at 3q27-29 encodes multiple products with transactivating, death inducing, and dominant-negative activities. Mol Cell. 1998;2:305–316. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- 11.Cernochova D., Pospišilova E., Kylarova D. Expression of p53, p63 and p73 in the orofacial region of human embryos. Biomed Papers. 2004;148(2):203–204. [PubMed] [Google Scholar]
- 12.Pellegrini G., Dellambra E., Golisano O. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci U S A. 2001;98:3156–3161. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang A., Schweitzer R., Sun D. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- 14.Hibi K., Trink B., Patturajan M. AIS is an oncogene amplified in squamous cell carcinoma. PNAS. 2000;97(10):5462–5467. doi: 10.1073/pnas.97.10.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbieri C.E., Barton C.E., Pietenpol J.A. ΔNp63α expression is regulated by the phosphoinositide 3-kinase pathway. J Biol Chem. 2003;278:51408–51414. doi: 10.1074/jbc.M309943200. [DOI] [PubMed] [Google Scholar]
- 16.Parsa R., Yang A., Mckeon F., Green H. Association of p63 with proliferative potential in normal and neoplastic human keratinocytes. J Invest Dermatol. 1999;113:1099–1105. doi: 10.1046/j.1523-1747.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- 17.Joseph C., Sniezek, Matheny K.E., Westfall M.D., Pietenpol J.A. Dominant negative p63 isoform expression in head and neck squamous cell carcinoma. Laryngoscope. 2004;114:2063–2072. doi: 10.1097/01.mlg.0000149437.35855.4b. [DOI] [PubMed] [Google Scholar]
- 18.Lo Muzio L., Santarelli A., Caltabiano R., Rubini C., Pieramici T., Trevisiol L. p63 overexpression associates with poor prognosis in head and neck squamous cell carcinoma. Human Pathol. 2005;36:187–194. doi: 10.1016/j.humpath.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y.K., Hsue S.S., Lin L.M. Expression of p63 protein and mRNA in oral epithelial dysplasia. J Oral Pathol Med. 2005;34:232–239. doi: 10.1111/j.1600-0714.2004.00277.x. [DOI] [PubMed] [Google Scholar]
- 20.Barbieri C.E., Perez C.A., Johnson K.N., Ely K.A., Billheimer D., Jennifer A. Pietenpol, IGFBP-3 is a direct target of transcriptional regulation by ΔNp63α in squamous epithelium. Cancer Res. 2005;65(6):2314–2320. doi: 10.1158/0008-5472.CAN-04-3449. [DOI] [PubMed] [Google Scholar]
- 21.Wu G., Osada M., Guo Z., Fomenkov A., Begum S. Np63 up-regulates the HSP70 gene in human cancer. Cancer Res. 2005;65(3):758–766. [PubMed] [Google Scholar]
- 22.Norman P.M. Techniques in immunocytochemistry-application to diagnostic pathology. Arch Pathol Lab Med. 1989;113:641–644. [PubMed] [Google Scholar]
- 23.Cao L.Y., Yin Y., Li H., Jiang Y., Hong-Fu, Zhang H.F. Expression and clinical significance of S100A2 and p63 in esophageal carcinoma. World J Gastroenterol. 2009;15(33):4183–4188. doi: 10.3748/wjg.15.4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haniffa A.M., Saitoh M., Abiko Y., Takeshima M., Nishimura M., Yamazaki M. Expression pattern of p63 in oral epithelial lesions and submucous fibrosis associated with betel-quid chewing in Sri Lanka. Med Mol Morphol. 2007;40:203–207. doi: 10.1007/s00795-007-0383-6. [DOI] [PubMed] [Google Scholar]
- 25.Das R.K., Pal M., Barui A. Assessment of malignant potential of oral submucous fibrosis through evaluation of p63 E-cadherin and CD105 expression. J Clin Pathol. 2010;63:894–899. doi: 10.1136/jcp.2010.078964. [DOI] [PubMed] [Google Scholar]