Abstract
Prostaglandin E2, the major COX-2 product, acts via 4 functionally distinct prostanoid receptors, EP(1–4). PGE-2, through its receptors, feeds back to positively increase COX-2 expression augmenting its own synthesis thereby driving angiogenesis, while suppressing apoptosis and innate immunity. In addition to the well characterized PGE2/EP4/cAMP/PKA/CREB, EP4 activation increases GSK3 phosphorylation via PI3K and Akt consequently reducing β-catenin phosphorylation. EP4 induces angiogenesis by enhancing VEGF production via ERK activation. These effects of EP4 are asserted either directly or via EGFR transactivation depending on the type of cancer. In view of the safety concerns regarding long term use of COX-2 inhibitors and to find more effective alternatives, we evaluated the potential of EP4 prostanoid receptor as a target for treating cancer progression using a highly selective EP4 antagonist, 4-(4,9-diethoxy-1,3-dihydro-1-oxo-2H-benz[f]isoindol-2-yl)-N-(phenylsulfonyl)-benzeneacetamide. Oral administration of GW627368X showed significant tumor regression characterized by tumor reduction and induction of apoptosis. Reduction in prostaglandin E2 synthesis also led to reduced level of VEGF in plasma. Regulation of multiple pathways downstream of EP4 was evident by down regulation of COX-2, p-Akt, p-MAPK and p-EGFR. Considering wide distribution of the EP4 prostanoid receptor in major organs and the array of physiological processes it contributes to, the safety profile of the drug was analyzed. No major organ toxicity, immunosupression, behavioral change or change in blood parameters attributable to the drug was observed. The results assert the significance of EP4 prostanoid receptor as a therapeutic target as well as the safety of EP4 blockade by GW627368X.
Keywords: COX-2, GW627368X, prostaglandins, prostanoid receptors, VEGF
Abbreviations
- AC
Adenylyl cyclase; AIF, Apoptosis inducing factor; ALP, Serum alkaline phosphatase; BUN, Blood urea nitrogen; cAMP, Cyclic adenosine monophosphate; COX, Cyclooxygenase; CREB, cAMP response element-binding protein; EDTA, Ethylenediaminetetraacetic acid; EGFR, Epithelial growth factor receptor; ELISA, Enzyme linked immunosorbent assay; ERK, Extracellular-signal-regulated kinases; GSK3, Glycogen synthase kinase 3; H&E, Hematoxylin and Eosin; HB, Hemoglobin; HCT, Hematocrit; IgG, Immunoglobulin G; IHC, Immunohistochemistry; IL-1β, Interleukin 1 β; MAPK, Mitogen-activated protein kinases; MCH, Mean corpuscular hemoglobin; MCHC, Mean corpuscular hemoglobin concentration; MCV, Mean corpuscular volume; MTT, 3-(4,5-dimethylthiazol-2-γl)-2,5 diphenyltetrazolium bromide; PBS, Phosphate buffered saline; PGE2, Prostaglandin E2; PI, Propidium Iodide; PI3K, Phosphatidylinositol-4,5-bisphosphate 3-kinase; PKA, Protein kinase A; PLT, Platelet; RBC, Red blood cell; Rt, Reticulocytes; S180, Sarcoma 180; SGOT, Serum glutamic oxaloacetic transaminase; SGPT, Serum glutamic pyruvic transaminase; TNF-α, Tumor necrosis factor α; TP, Total protein; TUNEL, Terminal deoxynucleotidyl transferase dUTP nick end labeling; VEGF, Vascular endothelial growth factor; VEGFR, Vascular endothelial growth factor receptor; WBC, White blood cell count
Introduction
The association of inflammatory mediator, Cylooxygenase-2 with cancer progression and metastasis has been well established over the years by a series of elegant studies. Higher COX-2 expression tends to promote metastasis in breast cancer.1 COX-2 overexpression also contributes to poor prognosis, parametrial invasion and lymph node metastasis in cervical cancer. Chronic use of nonsteroidal anti-inflammatory drugs inhibiting the production of COX mediated prostaglandins lowers the incidence of a number of cancer types including colon cancer, aggressive breast cancer and cervical cancer.2 COX-2 exerts its influence by producing a group of lipid mediators called prostanoids. However, induced by inflammatory mediators like TNF-α and IL-1β, COX-2 does not lead to up-regulation of all its products but tends to drive selective overproduction of its major product PGE-2.3 PGE-2 is involved in cancer progression where as other products like prostacyclins have cardioprotective roles.4 Inhibition of COX-2 in blood vessels leads to reduced production of prostacyclins which are responsible for prevention of platelet aggregation and vasoconstriction.4 Its inhibition can therefore result in clot formation and increased blood pressure, thereby increasing the risk of cardiac failures.
PGE-2, a bioactive lipid, exerts diverse biological effects associated to inflammation and cancer ranging from cell proliferation, apoptosis, angiogenesis and immune surveillance.5 PGE-2, similar to hormones, mediates a wide array of physiological roles in autocrine or paracrine fashion.3,5 It acts via a family of 4 structurally related but functionally distinct G protein coupled prostanoid receptors, EP(1–4). Of the 4 receptors, EP2 and EP4 are known to be associated with malignant transformations.2 EP2/EP4 signaling stimulates angiogenesis by promoting VEGF production6,7 and also up-regulates COX-2 by amplifying PGE-2 production.7 PGE-2, through its receptors, feeds back to positively increase COX-2 expression further augmenting its own synthesis thereby driving angiogenesis, while suppressing apoptosis and innate immunity.8 Since direct targeting of COX-2 leads to undesirable side effects because of hindrance in synthesis of other COX-2 products, downstream effectors appear as viable targets against inflammation driven carcinogenesis. Therefore, in view of the safety concerns regarding long-term use of COX-2 inhibitors and to find more effective alternatives, inhibition of downstream targets like prostanoid receptors would be effective in limiting global prostaglandin synthesis.
Prostaglandin E2 or PGE-2 is widely produced in body and broadly distributed throughout the animal species.8 It is involved in a number of pathophysiological responses and whose cellular effects within tumors are exerted via a family of 4 prostanoid receptors, EP(1–4).9 Though the receptors are similar in their structure and sequence, they differ significantly in the intracellular pathways they are associated with. The role of E series of prostaglandin receptors in cancer progression is complex and has not been fully deciphered yet. All PGE receptors classically couple to Gsα, stimulate adenylyl cyclase (AC) and cAMP production.9 EP1 leads to elevation of intracellular calcium level via phospholipase C activation. EP3 has been associated with decrease in intracellular cAMP, however reverse has been observed for a splice variant of the same.9 EP2 and EP4 are coupled to protein kinase A or adenyl cyclase, thus elevating the intracellular cAMP level.9 Of all the prostanoid receptors, EP4 is the most recently discovered, identified in piglet saphenous vein.10
Interestingly, EP4 has been found to have unique pathways and biological functions. EP4 receptors have been found to be expressed in a large number of tissues and cells including immune, osteoarticular, cardiovascular, gastrointestinal and respiratory system and overexpressed in cancer cells. Intense studies with systemic null EP4 deficient mice in 1999 revealed that EP4 signaling plays multiple roles not only via cAMP pathways but via other pathways as well.11 Along with Gsα, EP4 is also associated with Giα, PI3K, β-arrestin and β-catenin. It activates extracellular signal-regulated kinase ERK1 and ERK2 by way of phosphatidylinositol 3-kinase.11 Characterization of the E series of prostaglandin receptors and their implication in carcinogenesis is relatively new and much of the information has been derived from animal models of colon carcinoma.12 These findings have made the implications of EP4 as potential therapeutic target clearer.9
In past few years, there have been number of studies exerting the importance of EP-4 receptor in carcinogenesis.9 Extensive studies have been conducted on animal models of colon cancer12-14 and various studies also support its role in breast cancer metastasis.15-18 However, very few highly potent antagonists are presently available for preclinical and clinical assessment. The present study aims at evaluating the potential of EP4 prostanoid receptor as a target for treating cancer progression using a highly selective EP4 receptor antagonist, 4-(4,9-diethoxy-1,3-dihydro-1-oxo-2H-benz[f]isoindol-2-yl)-N-(phenylsulfonyl)-benzeneacetamide (Fig. 1A), GW627368X and assessment of its safety issues on mice sarcoma model. GW627368X has been found to remarkably inhibit the proliferation and invasion of human inflammatory breast cancer cells in vitro.15 A similar selective EP-4 antagonist, RQ0015986, has been described as an orally active drug for protecting natural killer cells from PGE-2 mediated immunosupression and inhibition of breast cancer metastasis.5 In this study, we demonstrate the safety and efficiency of GW627368X as an orally administered, anti-tumor and anti-metastatic agent in a murine sarcoma model.
Figure 1.
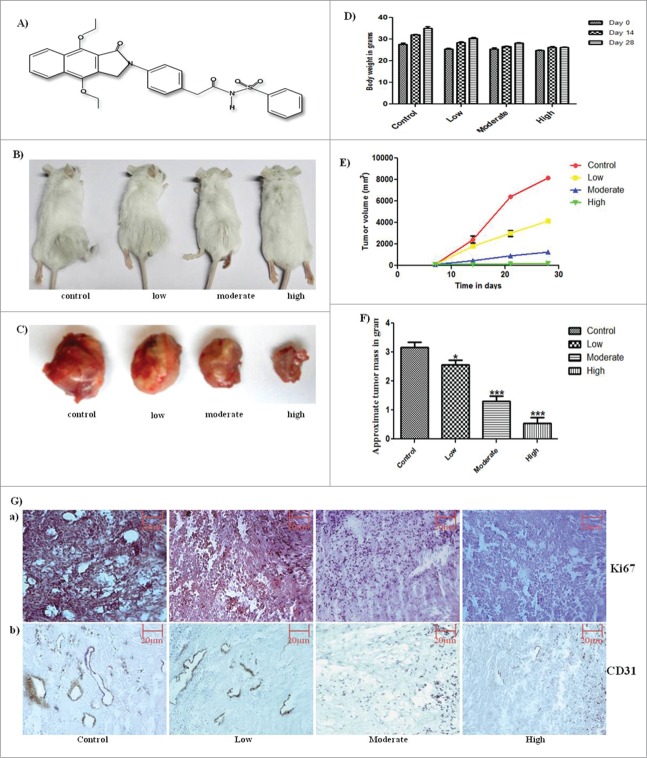
Anti-cancer potential of GW627368X. (A) Molecular structure of GW627368X. 4-(4,9-diethoxy-1,3-dihydro-1-oxo-2H-benz[f]isoindol-2-yl)-N-(phenylsulfonyl)-benzeneacetamide (C30H28N2O6S). Molecular weight 544.6; λmax: 21, 229, 295, 35 (B) Tumor bearing animals representative of each test group, (C) Excised tumor from each test group, (D) Bar graph depicting average body weight of each test group taken on day 0, 7 and 14. (E) Graph representing change in tumor volume (group average) in each test group measured every 7 days, (F) Bar graph showing average tumor mass of each test group at the end of 28 days study period. Data mean ± SD, representative of 10 animals. P < 0.05(t test), (G) Immunohistochemical analysis for expression of a) Proliferation marker, Ki67; b) Angiogenesis marker, CD31. Representative pictures taken at 20X magnification.
Results
Clinical observations
No signs of clinical illness like discolouration and change in texture of skin, fur shedding or decrease in activity or appetite were observed in any of the test groups. The animals only tended to avoid food for a brief period after treatment. All major organs were drained on filter paper and weighed. No significant change in weight or appearance of the organs was observed. Only slight enlargement of spleen in the treatment groups was suspected. To confirm, spleenocyte proliferation assay was further performed.
GW627368X displayed anti-tumor and anti-proliferative potential in sarcoma 180 bearing mice
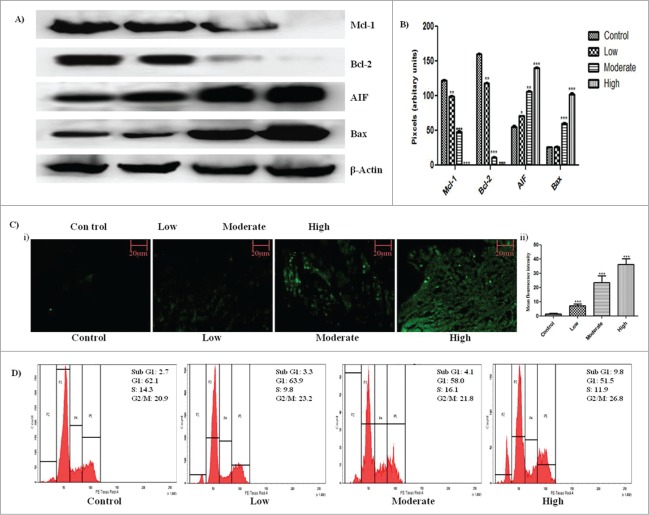
The final volume and weight of solid tumor in all treatment groups were significantly low with respect to the control group (Fig. 1B–F). The tumor volume in the highest treatment group regressed to 202.333 ± 18.800 mm3 as opposed to 8148.333 ± 76.979 mm3 in control group. Increase in DNA fragmentation, a marker of apoptosis, was observed by in the tumor sections by TUNEL assay in a dose dependent manner (Fig. 2C i.). Mean fluorescent intensity from each tumor section was calculated using Image J software and was found to increase significantly from 1.588 ± 0.4363 in control to 36.34 ± 4.047 in the highest treatment group (Fig. 2C ii). On cell cycle analysis, an increase in percentage of apoptotic cells from 2.7% in control to 9.8% in highest treatment group was observed in intraperitoneally treated animals which asserts the apoptotic potential of the drug (Fig. 2D). In antigen specific immunohistochemical analysis of the tumor sections, a significant decrease in expression of Ki67 (Fig. 1G.) and CD31(Fig. 1G.), markers for cell proliferation and angiogenesis respectively, was observed.
Figure 2.
Induction of apoptosis within tumor by GW627368X. (A) Western blot analysis of apoptotic (AIF and Bax) and anti-apoptotic (Mcl−1 and Bcl−2) proteins in subcutaneous tumor tissue of different test groups. (B) Densitometric analysis of Mcl−1, Bcl−2, AIF and Bax expression from protein blots. Each bar representative of 3 independent experiments, P < 0.05 (t test). (C) TUNEL staining of tumor sections for detection of apoptosis in all test groups i) Fluorescent micrographs representative of each test group ii) Bar graph representing mean fluorescence intensity of each micrograph. (D) In vivo cell cycle phase distribution study by flow cytometry. Cells were isolated from ascitic tumor bearing mice of each test group were subjected to cell cycle analysis following PI staining.
Western blot analysis revealed up regulation of apoptotic proteins and down regulation of anti-apoptotic proteins
Expression profile of apoptotic proteins was studied by western blot analysis. A significant increase in expression level of apoptotic proteins AIF and Bax and decrease in expression level of anti-apoptotic proteins Mcl-1 and Bcl-2 were observed with increase in drug dosage which further confirms the apoptotic potential of the drug (Fig. 2A, B).
GW627368X inhibited tumor proliferation via PGE-2/EP4/Cox-2, Akt and MAPK pathways
To evaluate the effect of GW627368X on signaling within tumor environment, the expression profile of various phosphorylated and non-phosphorylated proteins were studied by immunohistochemistry and western blotting (Fig. 3). A significant decrease in phosphorylation level of signaling mediators like EGFR, VEGFR, Akt, MAPK etc was observed with increase in dose of the drug while the total proteins seemed to remain equal in all the sets (Fig. 3D). In addition, highly significant down regulation of COX-2 and EP4 was observed (Fig. 3A). The key player of the cascade, Prostaglandin E2 in plasma and tissue was quantified using enzyme immune assay. The plasma and tissue levels of prostaglandin E-2 drastically reduced from 720.7 ± 11.11pg/ml in control group to 200 ± 0.7076 pg/g in high treatment group vs. 248.8 ± 16.40 pg/ml in normal mice (Fig. 3C). The effect was highly significant. To further confirm the effect, its downstream molecule, VEGF was quantified using ELISA (Fig. 3C). Lipid mediator, prostaglandin E2 is a major regulator VEGF expression which correlates with angiogenesis within tumor. A marked reduction in plasma VEGF from 482.1 ± 17.57 pg/ml to 250.5 ± 14.62 pg/ml was observed which was slightly lower compared to plasma VEGF of healthy animal (177.3 ± 14.97).
Figure 3.
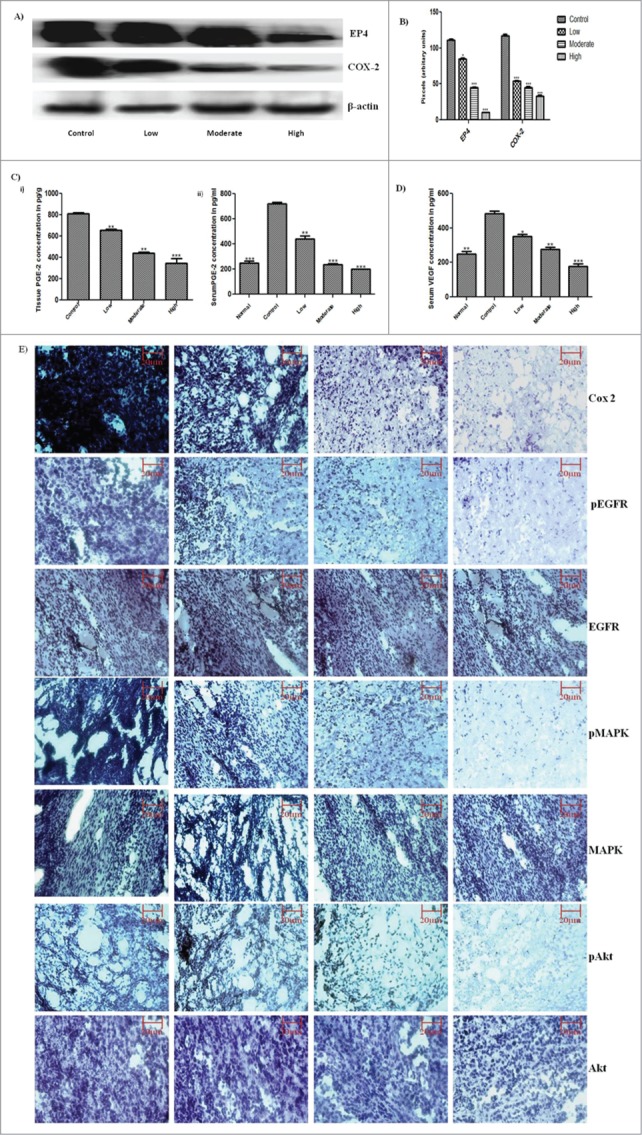
PGE-2 via EP-4 signaling, feeds back to positively increase COX-2 expression further augmenting its own synthesis, thereby driving angiogenesis via VEGF upregulation. In addition to PGE-2/cAMP/PKA pathway, EP-4 acts via PI3K/Akt pathway. It induces angiogenesis via ERK activation. These effects are asserted either directly or via EGFR transactivation. (A) Expression profile of EP4 and COX-2 protein in subcutaneous tumor tissue by western blot analysis. (B) Densitometric analysis of EP4 and COX-2 expression on protein blots. Each bar representative of 3 independent experiments, P < 0.05 (t test). (C) Prostaglandin E2 quantification by ELISA in (i) Subcutaneous tumor tissue (ii) Serum of non-cancerous, tumor bearing control and test group animals. (D) VEGF quantification by ELISA in plasma of non-cancerous, tumor bearing control and test group animals. The bars are representative of 3 independent experiments. P < 0.05 (t test). (E) Immunohistochemistry of GW627368X treated S180 mouse sacrcoma tissue. Paraffin-embedded sections of S180 tumors in swiss albino mice were processed and expression level of the key proteins, COX-2, pEGFR, EGFR, pMAPK, MAPK, pAkt and Akt was studied by immunohistochemistry. Representative pictures were taken at 20X magnification
Toxicity studies
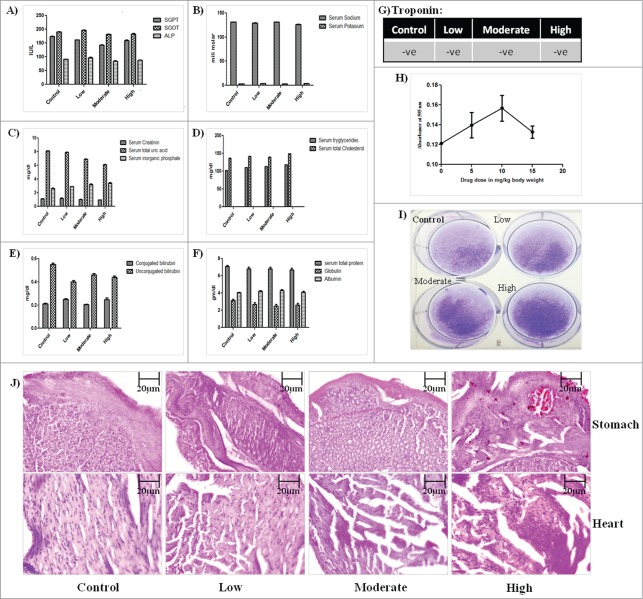
The safety profile of GW627368X was illustrated by performing organ specific biochemical tests which assured the proper functioning of all major organs. Serum glutamic pyruvic transaminase (SGPT), Serum glutamic oxaloacetic transaminase(SGOT) and serum alkaline phosphatase (ALP) are enzymes commonly expressed in liver and heart. The level of SGPT, SGOT, ALP (Fig. 4A) and conjugated and unconjugated bilirubin in different test groups was found to be almost equal and close to normal indicating no hepatic toxicity (Fig. 4E). A stable level of creatinine, inorganic phosphate assured normal renal activity (Fig. 4C). However, there seems to be slight gradual decrease uric acid level with increase in drug dosage (Fig. 4C). Selective COX-2 inhibitors are usually known to be associated with cardiac toxicity and ulceration in stomach. Sections of heart and stomach were H&E stained to look for any visible damage. The heart tissue seemed normal in the low dose test group but the muscle fiber architecture in heart sections of moderate and high treatment group appeared slightly less clear (Fig. 4J). To evaluate its cardiosaftey, blood troponin level of all test groups was performed. Absence of troponin expression in blood of all the test groups confirmed its cardiosaftey (Fig. 4G). However, slight ulceration in stomach sections of moderate and high treatment groups was observed (Fig. 4J). The levels of serum sodium, potassium, triglycerides, cholesterol, albumin and globulin were found to be unaffected by drug treatment (Fig. 4B, D, F). All blood parameters like hemoglobin, platelet count, leucocyte count, reticulocyte count were found to be normal in all test groups (Table 2). To check for the activity of the spleen, spleenocytes were cultured ex vivo on a 6 well plate followed by crystal violet staining (Fig. 4I). Viability of the cells seemed to be uniform which was further confirmed by in vivo spleenocyte proliferation assay (Fig. 4H). A slight increase in spleenocyte proliferation was observed in low and moderate treatment groups, however in high treatment group it was close to the control group.
Figure 4.
28 days toxicity profile of GW627368X in mice. Bar graphs showing the levels of (A) Serum glutamic pyruvic transaminase(SGPT), Serum glutamic oxaloacetic transaminase(SGOT) and serum alkaline phosphatase(ALP) (B) Serum sodium, serum potassium (C) Serum creatinin, total uric acid, inorganic phosphate (D) Serum tryglicerides, total cholesterol (E) Conjugated and unconjugated bilirubin (F) Serum total protein, Globulin, albumin (G) Troponin in different test groups. Results represented as mean ± SD. Each bar is representative of 3 independent experiments. (H) Ex vivo spleenocyte proliferation assay. Spleenocytes isolated from animals of each test group was seeded into 96 well plate and MTT assay was performed. (I) Ex vivo culture of spleenocytes isolated from animal of each test group followed by crystal violet staining. (J) H&E stained sections of stomach and heart of animals from each test group.
Table 2.
Hematological parameters. Blood samples from each test group were collected in separate tubes containing EDTA-2K and the hematological parameters ie. red blood cell (RBC) count, hemoglobin (HB) concentration, hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelet (PLT) count, reticulocytes (Rt), white blood cell count (WBC) and WBC differential counts (lymphocyte, monocyte and granulocyte) were examined. Data mean±SE
| Blood parameters | Control | Low | Moderate | High |
|---|---|---|---|---|
| Haemoglobin | 10.5 ± 0.2gm/dl | 10.1± 0.5gm/dl | 12.6 ± 0.4gm/dl | 10.2 ± 0.1gm/dl |
| Total count | ||||
| i)Platelet ii) Leucocyte |
8,30,000 ± 1000/Cu mm 10200 ± 120/Cu mm |
6,50,000 ± 1030/Cu mm 40200 ± 300/Cu mm |
5,40,000 ± 870/Cu mm 10800 ± 100/Cu mm |
6,80,000 ± 1100/Cu mm 22800 ± 260/Cu mm |
| Diff. leucocyte count | ||||
| Neutrophil Lymphocyte Monocyte Eosinophil Basophil |
39 ± 1% 58 ± 2% 02 ± 0.03% 01 ± 0.1% 00% |
57 ± 0.5% 38 ± 1% 03 ± 0.01% 02 ± 0.06% 00% |
41± 3% 54 ± 0.8% 01± 0.005% 04 ± 0.08% 00% |
52 ± 2% 43 ± 1% 3 ± 0.007% 02 ± 0.2% 00% |
| P.C.V | 31.0 ± 0.8% | 30.0 ± 1% | 38.0 ± 0.5% | 31.0 ± 0.7% |
| M.C.V | 88.6 ± 2 fl | 96.8 ± 4 fl | 90.5 ± 10 fl | 96.9 ± 1 fl |
| M.C.H | 30.0 ± 0.5 pg | 30.6 ± 1.2 pg | 30.0 ± 0.6 pg | 31.9 ± 5 pg |
| M.C.H.C | 33.9 ± 0.7% | 33.7 ± 0.1% | 33.2± 0.5% | 32.9 ± 0.3% |
| Reticulocyte count | 0.2 ± 0.005% | 0.3 ± 0.01% | 0.2 ± 0.007% | 0.2 ± 0.02% |
Discussion
An increased level of prostaglandin E2 or PGE-2, the major COX-2 product, has often been regarded as the culprit in inflammation driven carcinogenesis. Owing to its wide array of physiological functions, it is thought that inhibition of PGE-2 rather than global prostaglandin synthesis would be useful. Intervention of downstream targets rather than COX-2 is also justified by the fact that in most of the cancers PGE-2 levels can also be contributed by COX-1, the housekeeping cyclooxygenases.19 Studies reveal that deficiency of either COX-2 or COX-1 causes similar decrease in intestinal polyps in mice.19 The total prostaglandin level in local environment is responsible for cancer progression.
Our study provides a preclinical evidence of the anti-tumor and anti-proliferative potential of GW627368X as an orally administered drug within effective and safe range of 5–15 mg/kg of body weight of mice bearing sarcoma 180. This highly selective prostanoid receptor antagonist holistically inhibits multiple aspects of cancer progression. Being associated with multiple pathways downstream, the prostanoid receptor EP-4 is functionally complex. Blockade of EP-4 using a highly potent antagonist, GW627368X, dramatically reduced the plasma and tissue levels of prostaglandin E2 and VEGF consistent with the down regulation of EP4 and COX-2 expression. These observations were due to the interruption of the EP-4 induced feedback loop which further augments the synthesis of inflammatory mediators. Reduction of PGE-2 directly correlates with reduced cancer proliferation since it is itself known to promote proliferation and metastasis.16 EP4 activation also leads to activation of ERK pathway further inducing VEGF production, thereby inducing angiogenesis. These effects of EP4 are asserted either directly or via EGFR transactivation depending on the type of cancer.1,5,7,9,16 Induction of angiogenesis is the main role of VEGF in tumor growth. It regulates the differentiation, migration and proliferation of endothelial cells by interacting with its receptors.20 Recently, it has been discovered that VEGF serves as a survival factor for both endothelial as well as tumor cells through induction of Bcl−2 expression.20,21 A highly significant decrease in level of Bcl−2 was also observed by western blot analysis. A strong correlation between VEGF and Bcl−2 expression exists since even Bcl−2 has been shown to induce VEGF expression in different tumor histotypes.22 EP4 signaling stimulates angiogenesis by VEGF production and upregulates COX-2 production by amplifying PGE-2 production. A decreased tumor vasculature and reduced proliferative potential is also evident from CD31 and Ki67 staining respectively. A down regulation of phosphorylated Akt levels on drug treatment by immunohistochemistry suggests a probable reduction of transcriptional activity by β-catenin via GSK3.Tumor regression by GW627368X can be attributed to inhibition of amplified transcription by nuclear factors, β-catenin and CREB via PI3K/Akt and cAMP/PKA pathways respectively and reduced tumor angiogenesis via ERK pathway.
The major limitation in usage of any anti-cancer agent is their undesirable side effects on normally proliferating cells of the body and physiological processes. Most of the COX inhibitors are associated with gastrointestinal, renal and cardiotoxicity which could be due to deregulation of protective prostaglandins like prostacyclins.4 EP4 is also known to have many physiological roles. It is widely distributed in various tissues of the circulatory system, most strongly expressed in cardiomyocytes.4 They are also reported to be expressed in the renal system and immune system.18,23 To analyze the safety profile of GW627368X as a drug, various biochemical tests were performed. In animals with solid subcutaneous tumors, normal activity and movement are restricted because of the asymmetric tumor outgrowth. Inspection of changes in respiratory, circulatory, autonomic, central nervous system, somatomotor activity, and behavior in such cases becomes complicated. To overcome this, ascitic tumor was induced in a group of 20 animals by intraperitoneal tumor injection and further subdivided into treatment groups and treated similar to the other test groups (Table 1). No behavioral, respiratory, circulatory, autonomic, central nervous system or somatomotor abnormality was observed except a slightly lowered appetite following drug treatment. SGPT, SGOT and ALP, indicators of liver and heart health did not show any variation within the test groups. Though slight change in cardiac muscle architecture was noticed, troponin expression was negative in all test groups indicating no cardiac toxicity. Other parameters of overall health like bilirubin, uric acid, serum sodium and potassium, triglycerides were normal in all test groups. Blood parameters also did not show any significant variation. No fluid retention or nephrotoxicity was observed till 15 mg/kg of drug treatment. PGE-2 is also known to effect immune functions eg. by affecting natural killer cell function.5 Macrophages are also known to produce prostaglandin E2 in large quantities.16 The released PGE-2 then acts on the macrophages themselves by exerting an inhibitory effect on early and late stage activation.16 Spleen activity is an indicator of immune response. Splenocyte proliferation assay showed a slight increase in proliferation rate in lower doses compared to the control group but seemed to normalize in the highest treatment group. However, slight ulceration of stomach in moderate and high treatment groups.
Table 1.
Experimental plan. Animals were randomized into five test groups (Control, Low, Moderate, High, Recovery; n = 10) and one intraperitoneal treatment group (N = 20; subdivided into four groups (Control,Low,Moderate,High;n = 5)). While the control group was treated with polysorbate dissolved in deionized water, the treatment groups were treated with drug dissolved in water every alternate day for 28days with an oral gavage
| Test groups | Dosage(mg/kg of body weight) | |
|---|---|---|
| Group 1 n = 10 | Control | 0 |
| Group 2 n = 10 | Low | 5 |
| Group 3 n = 10 | Moderate | 10 |
| Group 4 n = 10 | High | 15 |
| Group 5 n = 10 | Recovery | 15 |
| Group 6 N = 20 | Intraperitoneal a) Control (n = 5) b) Low (n = 5) c) Moderate (n = 5) d) High (n = 5) |
0 5 10 15 |
Conclusion
The current work elucidates the role of GW627368X, a highly selective prostanoid EP4 receptor antagonist, in inhibiting tumor progression in S180 mice sarcoma model at the same time testing its safety profile as an orally administered drug. Though detailed insight of the mechanism of action in specific cancer is required for any conclusive remark, this current study demonstrates the anti-cancer potential and preclinical safety of GW627368X in an in vivo rodent model. It affirms the potential of EP4 prostanoid receptor as a therapeutic target for cancer. Cancer being a multi factorial disease needs multiple targeting. Inflammation and angiogenesis are 2 closely related aspects which need to be exploited. Co-administration of EP4 with conventional therapies might prove useful. Multiple targeting not only enhances therapeutic potential by affecting different aspects of tumor progression but also minimizes overall toxicities by reducing dosages of individual drugs.
Materials and Methods
GW627368X (Cayman Chemical Item Number 10009162, CAS 439288-66-1) was procured from Cayman chemicals, Ann Arbor, Michigan. For Western blot analysis and immunohistochemistry, the following antibodies were used: rabbit monoclonal anti-COX-2, anti-AIF, anti-Bax, anti-Bcl−2,anti- VEGFR, anti-p-VEGFR, anti-EGFR, anti-p-EGFR, anti-Akt, anti-p-Akt, anti-MAPK, anti-p-MAPK,anti-Ki67, anti-CD31(Cell Signaling Technology, Beverly, MA, USA), mouse monoclonal anti-β-actin, anti-MCL−1 (Cell Signaling Technology, Beverly, MA, USA) and goat monoclonal anti-EP4 (Imgenex, India), horseradish peroxidase conjugated goat anti-rabbit IgG and goat anti-mouse IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Chemiluminescent peroxidase substrate, Propidium Iodide (PI), CelLytic™ cell lysis reagent for mammalian tissue and 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT) (Sigma Aldrich, St. Louis, MO, USA) ApopTag in situ Apoptosis detection kit (Promega, Madison, WI, USA), Fetal bovine serum (FBS) (Gibco-BRL, Invitrogen Corporation, CA, USA) were purchased from the corresponding company. Stock solutions of PtdIns and MTT were prepared by dissolving 1 mg of each compound in 1 ml PBS. The solution was protected from light, stored at 4°C, and used within 1 month. Stock concentrations of 10 mg/ml RNase A (Sigma Aldrich, St. Louis, MO, USA) was prepared and kept at −20°C. PGE2 immunoassay kit (Imgenex, India), mouse VEGF quantikine ELISA kit (R&D systems, Minneapolis, MN, USA) and IHC detection system (Biogenex, Fremont, CA, USA) were procured from respective companies.
Animal maintenance
6 to 8 weeks old male and female Swiss albino mice were housed in the institutional animal facility, Indian Institute of Technology, Kharagpur, India. Mice were acclimatized in pathogen free environment at institute animal facility for 1 week prior to injection with S180 mouse sarcoma cells. The animals were grouped and housed in wire cages with not more than 5 animals per cage, under good laboratory conditions (temperature 25 ± 2°C; relative humidity 50 ± 20%) with dark and light cycle (12/12 hr). After the acclimatization period only healthy animals were assigned for the study. The animals had access to standard balanced diet and water ad libitum. The study was approved by the Department of Biotechnology (DBT), INDIA under the project number: E-1/MM-SMST/14, at Indian Institute of Technology Kharagpur, INDIA and the mice were maintained in accordance with the institute animal ethical committee (IAEC) guidelines approved by Indian Council of Medical Research (ICMR), New Delhi.
Induction of tumor and treatment regimen
Exponentially growing S180 cells were harvested and a tumorigenic dose of 3 × 106 cells was injected subcutaneously in the right flank of mice. Solid tumors were allowed to grow in the mice for 7 days and the animals were randomized into 5 treatment groups (10 mice per group), 1.Control (Vehicle), 2.Low (5 mg/kg), 3.Moderate (10 mg/kg), 4.High (15 mg/kg) and 5.Recovery (15 mg/kg) doses (Table 1). In another group of 20 animals ascetic tumor was developed by injecting tumorigenic dose of 3 × 106 S180 cells intraperitoneally (Table 1). This group was introduced to confirm the anticancer potential of the drug in solid as well as ascetic model and to study the physical and behavioral changes subsequent to drug treatment. The drug was suspended in deionized water. While the control group was treated with polysorbate dissolved in deionized water, the treatment groups were treated with drug dissolved in water, orally by an oral gavage, every alternate day for 28 d. The tumor volume was measured regularly using a side caliper and the animals were weighed on day 0, 14 and 28. Body weight and size were monitored regularly. After 28 days of treatment, the animals were sacrificed. The tumors were excised, weighed and preserved in 10% formalin for immunohistochemical analysis. Blood was collected immediately in separate vials with and without EDTA for analysis of various biochemical and hematological parameters. The major organs, liver, heart, kidney and stomach were dissected, weighed and preserved for H&E staining. The spleen was excised and immediately processed for spleenocyte proliferation assay. From the animals bearing ascetic tumor, cells were harvested and processed for flow cytometric analysis. For western blot analysis, tumor tissues were excised from each test group and stored in −80°C. For quantification of tissue and plasma levels prostaglandin E2, tissues were snap-frozen in liquid nitrogen and stored in −80°C. To check the reversibility of any possible toxicity and development of any post treatment toxic symptom, the recovery group was observed for 8 days after withdrawal of treatment.
Anti-carcinogenic potential of GW627368X
TUNEL assay
Tumor sections of each test group were dewaxed and rehydrated. The tissue sections were permeabilized with 20 µg/ml of proteinase K in 10 mM Tris (pH 7.5) and 5 mM EDTA for 15–20 mins. The slides were rinsed with PBS and stained with TUNEL cocktail as per manufacturer's protocol. The slides were incubated in humidified chamber in dark at 37°C for 60 mins. The slides were gently washed with PBS dried and mounted.24 Images were obtained at appropriate wavelength with Confocal Laser scanning microscope(Olympus Fluoview FV100, Version 1.7.1.0, Shiyunku, Tokyo, Japan and images captured using FLUOVIEW 1000(version 1.2.4.0) imaging software(Olympus, Tokyo, Japan).
Flowcytometry
Cells isolated from animals bearing intraperitoneal tumor were fixed in 70% ethanol and stored in −20°C. Later they were stained with propidium iodide and cell cycle analysis was performed using BD FACS Aria III and the data analyzed using BD FACS DIVA software.25
Western blot and Immunohistochemistry
Tissue samples stored at −80°C were homogenized in liquid nitrogen using mortar and pestle and lysed in CelLytic™ MT mammalian tissue lysis/extraction reagent for 4–5 h at 4°C with regular vortexing. The lysate was centrifuged at 10,000 rpm for 10 mins at 4°C. The supernatant was collected and protein concentration was estimated by Bradford's method. Tissue extracts containing 50 μg of protein were separated on a sodium dodecyl sulfate-polyacrylamide electrophoretic gel and transferred to nitrocellulose membranes, which were blocked in 3% bovine serum albumin for 2 h. After blocking, the membranes were incubated with primary antibodies overnight at 4°C and then with horseradish peroxidase-conjugated secondary antibody for 2 h at room temperature. Proteins were visualized by exposing the chemiluminescence substrate (Sigma) to X-OMAT AR autoradiography film (Eastman Kodak, Rochester, NY, USA). For immunohistochemical analysis, tissue sections were deparafinized followed by antigen retrieval. IHC for specific proteins were carried out on tumor tissue sections using Biogenex IHC detection system according to manufacturer's guidelines.24,25
Prostaglandin E2 and VEGF quantification by ELISA
Snap frozen tissue samples preserved in −80°C were pulverized in presence of ethanol and level of prostaglandin E2 in tissue and serum were quantified by ELISA using IMGENEX PGE2 EIA kit according to manufacturer's protocol.15 For serum prostaglandin levels, serum separated from blood and stored in −80°C was purified and PGE-2 was quantified using IMGENEX PGE2 EIA kit. Sample preparation, purification and recovery were done in accordance with kit guidelines. From the stored sample, level of VEGF in serum of treated and untreated groups were quantified using VEGF quantikine ELISA kit, R&D systems according to kit guidelines.25
Toxicological study
Gross behavioral examination
All animals were inspected twice a day during the course of the study and monitored carefully during weighing/feeding period. Animals were observed for reaction to treatment such as changes in skin, fur, eyes, and mucous membranes. Respiratory, circulatory, autonomic, central nervous system, somatomotor activity, and behavior patterns were also monitored along with any other signs of ill health. These evaluations required examination for general appearance, respiration, abnormalities for behavior and movement, and included external organs, skin, and any lesion.26-29 The unfasted body weight of each animal was recorded prior to its assignment to the relevant group. Each animal was again weighed on day 7, 14 and 28 prior necropsy. The food intake of each animal was monitored daily during the study period.
Serum biochemistry
For clinical biochemistry, blood was collected in tubes without anticoagulant. Blood was allowed to coagulate at room temperature for 30 minutes, centrifuged at 3,000 rpm for 10 minutes and serum was separated. Biochemical tests for total protein (TP), blood urea nitrogen(BUN), serum glutamate pyruvate transaminase (SGPT), serum glutamate oxaloacetate transaminase (SGOT), and alkaline phosphatase (ALP), troponin, cholesterol, creatinin, uric acid, bilirubin, serum sodium, serum potassium, serum inorganic phosphate and triglycerides were performed at Medilab, Clinical pathology laboratory, Kolkata, India
Spleenocyte proliferation assay
The spleen was dissected and immediately suspended in sterile PBS. The cell strainer was placed on a petridish and the spleen along with 1 ml of PBS was placed directly on to the strainer. The plunger from a 2 ml syringe was removed and with the rubber end, the spleen was gently mashed with periodic perfusions with PBS till only white connective tissue of the spleen was left on the strainer. The cell suspension was transferred into a universal tube and centrifuged at 400 g for 10 mins at room temperature. The cell pallet was resuspended in 2 ml of PBS and appropriate amount of ammonium chloride lysing reagent was added and left in dark at room temperature for 15 mins. The suspension was centrifuged at 400 g for 5mins and supernatant discarded. Following another wash with PBS the spleenocytes were seeded in RPMI medium in 96 well (3,000cells/well) and 6 well (5 × 105 cell/well) plates for MTT assay25 and crystal violet staining respectively.
Histopathology
After sacrificing the animals by cervical decapitation, samples of major organs, liver, kidney, lung, stomach and heart were collected for histological examination. Organs were blotted on filter paper and weights noted. Organs were immediately fixed in neutral, phosphate-buffered 10% formalin. They were then paraffin embedded after routine processing and sectioned (2 µm). Hematoxylin-eosin staining of heart and stomach sections was performed, dried, mounted and visualized under compound microscope (1:10 scale).
Hematology
Blood of overnight fasted animals was collected by orbital sinus venipuncture from retro orbital sinus of mice by capillary tube. Blood samples from each test group were collected in separate tubes containing EDTA-2K and analyzed. Hematological parameters ie. red blood cell (RBC) count, hemoglobin (HB) concentration, hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelet (PLT) count, reticulocytes (Rt), white blood cell count (WBC) and WBC differential counts (lymphocyte, monocyte and granulocyte) were examined (Table 2).
Statistical analysis
Statistical analyses were performed by comparing the values obtained in the control group and the treatment groups. All data were subjected to paired t test using graph pad, prism 5 software. Data was presented as mean ± standard deviation (SD). Level of significance in all tests were taken as P < 0.05
Funding Statement
University Grants Commission (UGC), India; Council of Scientific and Industrial Research (CSIR), India; Department of Biotechnology (DBT), India.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors are thankful to all members of Cancer Biology lab, School of Medical Science and Technology, IIT Kharagpur, Colleagues from Dept. of Biotechnology, The Institutional Animal Ethical Committee, IIT Kharagpur, University grants commission (UGC), India, Council of Scientific and Industrial Research (CSIR), India and Department of Biotechnology DBT, India.
References
- 1.Xin X, Majumder M, Girish GV, Mohindra V, Maruyama T, Lala PK. Targeting COX-2 and EP4 to control tumor growth, angiogenesis, lymphangiogenesis and metastasis to the lungs and lymph nodes in a breast cancer model. Laboratory Investigation 2012; 92:1115-28; PMID:22641101; http://dx.doi.org/ 10.1038/labinvest.2012.90 [DOI] [PubMed] [Google Scholar]
- 2.Fulton AM, Ma X, Kundu N. Targeting prostaglandin E EP receptors to inhibit metastasis. Cancer Res 2006; 66:9794-7; PMID:17047037; http://dx.doi.org/ 10.1158/0008-5472.CAN-06-2067 [DOI] [PubMed] [Google Scholar]
- 3.Ricciotti E, FitzGerald GA. Prostaglandin and inflammation. Arterioscler Thromb Vasc Biol 2011; 31:986-1000; PMID:21508345; http://dx.doi.org/ 10.1161/ATVBAHA.110.207449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fries S, Grosser T. The cardiovascular pharmacology of COX-2 inhibition. Hematology 2005; 2005:445-51; PMID:16304418; http://dx.doi.org/ 10.1182/asheducation-2005.1.445 [DOI] [PubMed] [Google Scholar]
- 5.Ma X, Holt D, Kundu N, Reader J, Goloubeva O, Take Y, Fulton AM. A prostaglandin E (PGE) receptor EP4 antagonist protects natural killer cells from PGE2-mediated immunosuppression and inhibits breast cancer metastasis. OncoImmunology 2013; 2:e22647; PMID:23482441; http://dx.doi.org/ 10.4161/onci.22647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao L, Wu Y, Xu Z, Wang H, Zhao Z, Li Y, Yang P, Wei X. Involvement of COX-2/PGE2 signalling in hypoxia-induced angiogenic response in endothelial cells. J Cell Mol Med 2012; 16:1840-55; PMID:22050691; http://dx.doi.org/ 10.1111/j.1582-4934.2011.01479.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hull MA, Ko SC, Hawcroft G. Prostaglandin EP receptors: Targets for treatment and prevention of colorectal cancer? Mol Cancer Ther 2004; 3:1031-9; PMID:15299086; http://dx.doi.org/ 10.4161/cbt.3.10.1227 [DOI] [PubMed] [Google Scholar]
- 8.Leduc M, Breton B, Gale´s C, Gouill CL, Bouvier M, Chemtob S, Heveker N. Functional selectivity of natural and synthetic prostaglandin EP4 receptor ligands. J Pharmacol Exp Thera 2009; 331:297-307; PMID:19584306; http://dx.doi.org/ 10.1124/jpet.109.156398 [DOI] [PubMed] [Google Scholar]
- 9.Yokoyama U, Iwastubo K, Umemura M, Fujita T, Ishikawa Y. The prostanoid EP4 receptor and its signaling pathway. Pharmacol Rev 2013; 65:1010-52; PMID:23776144; http://dx.doi.org/ 10.1124/pr.112.007195 [DOI] [PubMed] [Google Scholar]
- 10.Coleman RA, Grix SP, Head SA, Louttit JB, Mallett A, Sheldrick RL. A novel inhibitory prostanoid receptor in piglet saphenous vein. Prostaglandins 1994; 47:151-68; PMID:8016385; http://dx.doi.org/ 10.1016/0090-6980(94)90084-1 [DOI] [PubMed] [Google Scholar]
- 11.Coleman RA, Smith WL, Narumiya S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev 1994; 46:205-29; PMID:7938166 [PubMed] [Google Scholar]
- 12.Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C, Kaidi A. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 2009; 30:377-86; PMID:19136477; http://dx.doi.org/ 10.1093/carcin/bgp014 [DOI] [PubMed] [Google Scholar]
- 13.Mosa AS, Hansen MB, Tilotta CM, Bindslev N. EP4 and EP2 receptor subtypes involved in colonic secretion in rat. Basic Clin Pharmacol Toxicol 2008; 103:214-21; PMID:18684231; http://dx.doi.org/ 10.1111/j.1742-7843.2008.00257.x [DOI] [PubMed] [Google Scholar]
- 14.Wilson RJ, Giblin GM, Roomans S, Rhodes SA, Cartwright KA, Shield VJ, Brown J, Wise A, Chowdhury J, Pritchard S, et al.. GW627368X ((N-{2-[4-(4,9-diethoxy-1-oxo-1,3-dihydro-2Hbenzo[f]isoindol-2-yl)phenyl]acetyl} benzene sulphonamide): a novel, potent and selective prostanoid EP4 receptor antagonist. Br J Pharmacol 2006; 148:326-39; PMID:16604093; http://dx.doi.org/ 10.1038/sj.bjp.0706726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robertson FM, Simeone AM, Mazumdar A, Shah AH, McMurray JS, Ghosh S, Cristofanilll M. Molecular and pharmacological blockade of the EP4 receptor selectively inhibits both proliferation and invasion of human inflammatory breast cancer cells. J Exp Thera Oncol 2008; 7:299-312; PMID:19227010 [PubMed] [Google Scholar]
- 16.Reader J, Holt D, Fulton A. Prostaglandin E2 EP receptors as therapeutic targets in breast cancer. Cancer Metastasis Rev 2011; 30:449-63; PMID:22002714; http://dx.doi.org/ 10.1007/s10555-011-9303-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parida S, Mandal M. Inflammation induced by human papilloma virus in cervical cancer and its implication in prevention. Eur J Cancer Prev 2014; 23:432-48; PMID:24787377 [DOI] [PubMed] [Google Scholar]
- 18.Fairbrother SE, Smith JE, Borman RA, Helen M. Cox EP4 receptors mediate prostaglandin E2, tumour necrosis factor alpha and interleukin 1beta-induced ion secretion in human and mouse colon mucosa. Eur J Pharmacol 2012; 694:89-97; PMID:22732652; http://dx.doi.org/ 10.1016/j.ejphar.2012.06.020 [DOI] [PubMed] [Google Scholar]
- 19.Dannenberg AJ, Lippman SM, Mann JR, Subbaramaiah K, DuBois RN. Cyclooxygenase-2 and eidermal growth factor peceptor: pharmacologic targets for chemoprevention. J Clin Oncol 2005; 23:254-66; PMID:15637389; http://dx.doi.org/ 10.1200/JCO.2005.09.112 [DOI] [PubMed] [Google Scholar]
- 20.Pidgeon GP, Barr MP, Harmey JH, Foley DA, Bouchier-Hayes DJ. Vascular endothelial growth factor (VEGF) upregulates BCL-2 and inhibits apoptosis in human and murine mammary adenocarcinoma cells. Br J Cancer 2001; 85:273-8; PMID:11461089; http://dx.doi.org/ 10.1054/bjoc.2001.1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nor JE, Christenesen J, Mooney DJ, Polvereni PJ. Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am J Pathol 1999; 154:375-84; PMID:10027396; http://dx.doi.org/ 10.1016/S0002-9440(10)65284-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biroccio A, Candiloro A, Mottolese M, Sapora O, Albini A, Zupi G, Bufalo DD. Bcl-2 overexpression and hypoxia synergistically act to modulate vascular endothelial growth factor expression and in vivo angiogenesis in a breast carcinoma line. FASEB 2000; 14:652-60; PMID:10744622 [DOI] [PubMed] [Google Scholar]
- 23.De Keijzer S, Meddens MB, Torensma R, Cambi A. The multiple faces of prostaglandin E2 G-Protein coupled receptor signaling during the dendritic cell life cycle. Int J Mol Sci 2013; 14:6542-55; PMID:23528886; http://dx.doi.org/ 10.3390/ijms14046542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajput S, Kumar BN, Sarkar S, Das S, Azab B, Santhekadur PK, Das SK, Emdad L, Sarkar D, Fisher PB, et al.. Targeted apoptotic effects of thymoquinone and tamoxifen on XIAP mediated Akt regulation in breast cancer. PLoS One 2013; 8:e61342; PMID:23613836; http://dx.doi.org/ 10.1371/journal.pone.0061342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar BN, Rajput S, Dey KK, Parekh A, Das S, Mazumdar A, Mandal M. Celecoxib alleviates tamoxifen-instigated angiogenic effects by ROS-dependent VEGF/VEGFR2 autocrine signaling. BMC Cancer 2013; 13:273; PMID:23731702; http://dx.doi.org/ 10.1186/1471-2407-13-273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Messinger H, Bar A. Oral 4-week and 13-week toxicity studies of polyvinyl acetate vinyl laurate copolymer in rats. Regul Toxicol Pharmacol 2014; 70:1-6; PMID:24932800; http://dx.doi.org/ 10.1016/j.yrtph.2014.06.008 [DOI] [PubMed] [Google Scholar]
- 27.Clarke K, Tchabanenko K, Pawlosky R, Carter E, Knight NS, Murray AJ, Cochlin LE, Todd King M, Wong AW, Roberts A, et al.. Oral 28-day and developmental toxicity studies of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate. Regul Toxicol Pharmacol 2012; 63:196-208; PMID:22504461; http://dx.doi.org/ 10.1016/j.yrtph.2012.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nandi U, Karmakar S, Das AK, Ghosh B, Padman A, Chatterjee N, Pal TK. Pharmacokinetics, pharmacodynamics and toxicity of a combination of metoprolol succinate and telmisartan in Wistar albino rats: Safety profiling. Regul Toxicol Pharmacol 2013; 65:68-78; PMID:23201407; http://dx.doi.org/ 10.1016/j.yrtph.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 29.Li Z, Qiao Y, Li J, An C, Hud K, Tang M. Acute and sub-chronic toxicity studies of the extract of Thunberg Fritillary Bulb. Regul Toxicol Pharmacol 2014; 68:370-7; PMID:24486111; http://dx.doi.org/ 10.1016/j.yrtph.2014.01.007 [DOI] [PubMed] [Google Scholar]