Abstract
Sex-possessing organisms perform sexual reproduction, in which gametes from different sexes fuse to produce offspring. In most eukaryotes, one or both sex gametes are motile, and gametes actively approach each other to fuse. However, in flowering plants, the gametes of both sexes lack motility. Two sperm cells (male gametes) that are contained in a pollen grain are recessively delivered via pollen tube elongation. After the pollen tube bursts, sperm cells are released toward the egg and central cells (female gametes) within an ovule (Fig. 1). The precise mechanism of sperm cell movement after the pollen tube bursts remains unknown. Ultimately, one sperm cell fuses with the egg cell and the other one fuses with the central cell, producing an embryo and an endosperm, respectively. Fertilization in which 2 sets of gamete fusion events occur, called double fertilization, has been known for over 100 y. The fact that each morphologically identical sperm cell precisely recognizes its fusion partner strongly suggests that an accurate gamete interaction system(s) exists in flowering plants.
Keywords: fertilization, gamete attachment, gamete fusion, GEX2, male gamete, sexual reproduction, sperm cell
Fertilization Mechanisms Reside on the Gamete Surface of Flowering Plants
Gamete fusion is a mysterious phenomenon in which 2 gametes, derived from different cell lineages, successfully recognize, dock and merge with each other. This mechanism is largely conserved in plants and animals.1 We therefore speculate that each sex gamete is programmed to express surface factors that specifically regulate fusion between different sexes. Indeed, previous studies on fertilization have identified several gamete surface factors critical for gamete recognition, attachment and fusion.1,2 Mori and colleagues identified a male-specific gamete fusion factor called GENERATIVE CELL SPECIFIC 1 (GCS1), also known as HAPLESS2 (HAP2), in lily pollen generative cells.3,4 GCS1 is a novel type I transmembrane protein, possessing an N-terminal signal sequence and a transmembrane domain. It is exclusively expressed in male gametes.3 GCS1-knockout Arabidopsis thaliana sperm cells are sterile, unable to fuse with any of the female gametes in an ovule. GCS1 therefore became the first example of a gamete surface factor that regulates double fertilization.3 At the same time, this finding predicts that additional factors surrounding GCS1 reside in both sex gametes. Molecular orchestration of these factors leads to successful double fertilization. To identify these factors, Mori and colleagues performed a new experiment using Arabidopsis gametic marker lines.5
Identification of Angiosperm Gamete Attachment Factor, GEX2
A transgenic Arabidopsis plant, expressing both central cell specific GFP and sperm nucleus specific RFP, was mutagenized by ethyl methanesulfonate (EMS) to screen for mutants with defective gamete fusion.5 A mutant candidate with an obvious gamete fusion deficiency, designated Y47, was detected. After mapping, a point mutation was found in the GAMETE EXPRESSED 2 (GEX2) gene. Therefore, the Y47 line was renamed gex2–1. Although GEX2 was previously reported to be a sperm specific protein localized to the cell membrane, its function was unknown.6 The phenotype of gex2–1 plants provided the first evidence that GEX2 is involved in gamete fusion.5 In addition, Mori and colleagues revised the previously described GEX2 gene structure and concluded that GEX2 is a type I transmembrane protein, like GCS1.5 Detailed observation of gex2–1 sperm cells revealed that incomplete double fertilization (also known as single fertilization), in which one sperm cell successfully fuses with an egg or a central cell, occasionally takes place. This finding suggests that GEX2 is involved in stabilizing gamete contact rather than functioning in a critical gamete fusion step, such as membrane fusion.5 Indeed, gex2–1 sperm cells occasionally detached from female gametes, but those of gcs1 rarely did (Fig. 2).5 To further investigate gex2–1 sperm behaviors, Mori and colleagues invented a simple method for gamete behavioral observation, in which female gametophytic cells are separated by polysaccharide-digesting enzymatic treatment.5 As a result, gex2–1 sperm cell detachment from egg and central cells was occasionally detected. This method allowed for statistical evaluation of the frequency of gamete detachment. However, most gcs1 sperm cells remained attached to female gametes. From these data, it was concluded that GEX2 protein is a gamete attachment factor expressed on the sperm cell surface, providing the first evidence of a gamete attachment process in flowering plants.5 Although the spatiotemporal relationships between GEX2-dependent attachment and GCS1-dependent fusion processes remain elusive,7 successful fertilization in flowering plants undoubtedly requires gamete contact preceding (or supporting) the fusion process, as in other organisms.8,9
Figure 1.
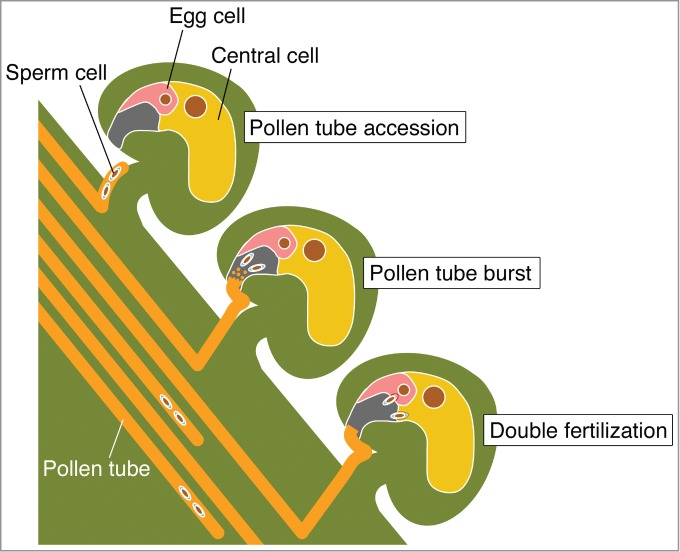
Illustration of the fertilization process in flowering plants. First, each pollen tube accesses an ovule containing egg and central cells. Next, the 2 sperm cells face the female gametes in the ovule after the pollen tube bursts. Finally, each sperm cell simultaneously fuses with either egg or central cell.
Figure 2.
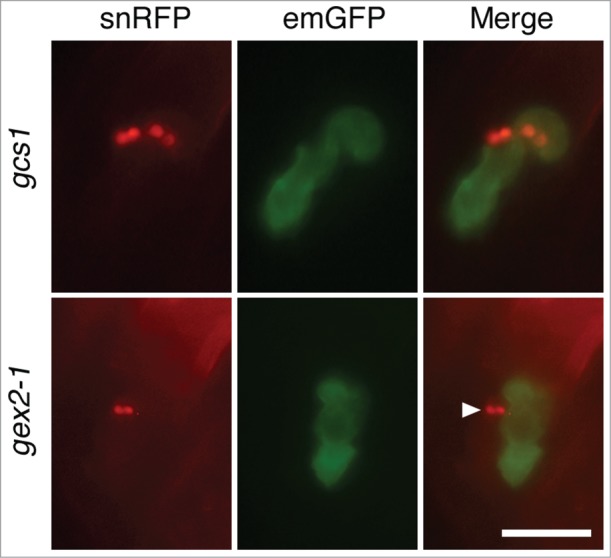
Gamete attachment step, revealed by analyses of Arabidopsis fertilization mutants. GCS1- or GEX2-knockout sperm cells expressing sperm nucleus RFP (snRFP) were observed in ovules expressing egg membrane GFP (emGFP). The top panels depict 2 pairs of GCS1-knockout (gcs1) sperm cells released from 2 pollen tubes, most likely because the first pollen tube failed to fertilize and the second pollen tube, which was guided by the fertilization recovery system,16 similarly failed. All the unfused gcs1 sperm cells are attached to the egg cell. In contrast, GEX2-knockout (gex2–1) sperm cells occasionally detached from the egg cell (bottom panels, arrowhead). The scale bar represents 20 μm.
GEX2 is Vascular Plant-Specific and Possesses a Filamin-Like Domain(s)
According to the revised GEX2 gene structure, GEX2 is a type I transmembrane protein composed of an N-terminal signal sequence, filamin-like domains and a C-terminal transmembrane domain.5 The filamin domain was originally defined in filamin proteins that function in the crosslinking of actin filaments. They are known to form an immunoglobulin-like fold in humans and Dictyostelium discoideum (slime mold).10 The filamin domains are repetitive and form an arm structure in a filamin molecule.10 GEX2 proteins are present in multiple flowering plants, and all possess at least one filamin-like domain (Fig. 3).5 In addition to flowering plants, the lycophyte Selaginella moellendorffii also possesses putative GEX2 and GCS1 orthologs (Fig. 3 and not shown, respectively). The Selaginella GEX2 structure contains at least 5 copies of the filamin-like domain (Fig. 3). Although it is unknown whether Selaginella GEX2 and GCS1 function similarly in fertilization, the presence of GEX2 and GCS1 orthologs in the Selaginella genome suggests that gamete attachment based on GEX2 has been established since the emergence of primitive vascular plants and that angiosperm GEX2 has been inherited from that of motile sperm. Furthermore, the differences in copy number and primary structure of detectable filamin-like domains among plant species may have triggered reproductive isolation and speciation.5
Figure 3.
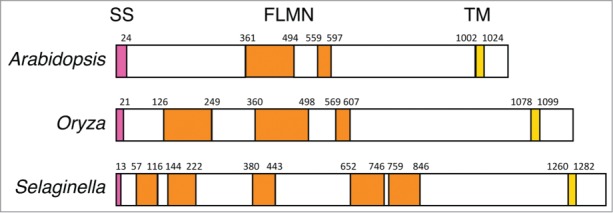
GEX2 structures of vascular plants. All the GEX2 proteins identified in vascular plants are type I transmembrane proteins composed of an N-terminal signal sequence (SS), filamin-like domain(s) (FLMN) and a C-terminal transmembrane domain (TM). Amino acid positions of those domains are indicated. Accession numbers are as follows: AB743888 (Arabidopsis), BAD33437 (Oryza) and XP_002994042 (Selaginella). SS and TM domains were predicted by SOSUI (http://harrier.nagahama-i-bio.ac.jp/sosui/) and FLMN domains were predicted by PROSITE (http://prosite.expasy.org/).
Gamete Attachment Mechanisms are Both Alternative and Conservative
The green alga Chlamydomonas reinhardtii possesses 2 mating types (i.e., sexes) designated mt+ and mt-. When mating, mt+ gametes extrude a tube-shaped protrusion (tubular mating structure; TMS) to dock with mt- gametes (Fig. 4).11 A type I transmembrane protein, FUS1, has been identified as an mt+ specific gamete attachment factor. It is expressed in TMSs.9,12 Wild type TMSs are capable of adhering to the mating mt- gametes, but FUS1-knockout TMSs are not.9 In the mt- gametes, GCS1 is specifically expressed and regulates the post-attachment step leading to gamete membrane fusion.13 GCS1-knockout mt- gametes retain the ability to adhere to wild type mt+ gametes.13 These data suggest that FUS1 and GCS1 do not bind to each other. Recently, it was found that a mammalian sperm specific type I transmembrane protein, IZUMO1, binds JUNO, a glycophosphatidylinositol (GPI)-anchored protein expressed in eggs, for successful fertilization (Fig. 4).14,15 Because neither IZUMO1-knockout sperm nor JUNO-knockout eggs are fertile, this provided the first example of a direct interaction between male and female gamete fusion factors that is essential for fertilization.15 In addition, it is likely that the IZUMO1-JUNO interaction functions only in gamete attachment.15 Cultured somatic cells expressing JUNO were able to adhere to those expressing IZUMO1 but not able to fuse.15 GEX2, FUS1 and IZUMO1 are specific to vascular plants, green algae and mammals, respectively, and their primary structures are quite different, suggesting that they arose independently in eukaryotic evolution. Nevertheless, it is still noteworthy that these proteins are all type I transmembrane proteins containing immunoglobulin-like domain(s) and most likely serve as gamete attachment factors (Fig. 4). The nature of gamete attachment systems might unexpectedly be conserved among phylogenetically distant eukaryotes.
Figure 4.
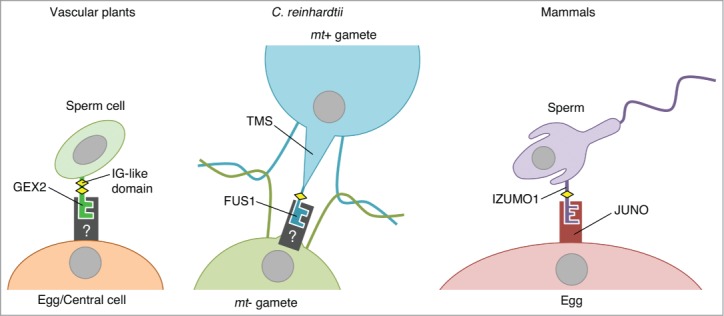
Gamete attachment factors containing immunoglobulin (IG)-like domain(s). Filamin-like domains are known to form an IG-like fold. Both GEX2 and FUS1 possess filamin-like domain(s). IZUMO1 is a member of the IG superfamily and possesses an IG-like domain.
Funding Statement
This study was supported by a Grant-in-Aid for Scientific Research on Innovative Areas to T.M. (21112008) and a Grant-in-Aid for Young Scientists (B) to T.M. (24770062).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Márton ML, Dresselhaus T. A comparison of early molecular fertilization mechanisms in animals and flowering plants. Sex Plant Reprod 2008; 21:37-52; http://dx.doi.org/ 10.1007/s00497-007-0062-8 [DOI] [Google Scholar]
- 2. Swanson WJ, Vacquier VD. The rapid evolution of reproductive proteins. Nat Rev Genet 2002; 3:137-44; PMID:11836507; http://dx.doi.org/ 10.1038/nrg733 [DOI] [PubMed] [Google Scholar]
- 3. Mori T, Kuroiwa H, Higashiyama T, Kuroiwa T. GENERATIVE CELL SPECIFIC 1 is essential for angiosperm fertilization. Nat Cell Biol 2006; 8:64-71; PMID:16378100; http://dx.doi.org/ 10.1038/ncb1345 [DOI] [PubMed] [Google Scholar]
- 4. von Besser K, Frank AC, Johnson MA, Preuss D. Arabidopsis HAP2 (GCS1) is a sperm-specific gene required for pollen tube guidance and fertilization. Development 2006; 133:4761-9; PMID:17079265; http://dx.doi.org/ 10.1242/dev.02683 [DOI] [PubMed] [Google Scholar]
- 5. Mori T, Igawa T, Tamiya G, Miyagishima SY, Berger F. Gamete attachment requires GEX2 for successful fertilization in Arabidopsis. Curr Biol 2014; 24:170-5; PMID:24388850; http://dx.doi.org/ 10.1016/j.cub.2013.11.030 [DOI] [PubMed] [Google Scholar]
- 6. Engel ML, Holmes-Davis R, McCormick S. Green sperm. Identification of male gamete promoters in Arabidopsis. Plant Physiol 2005; 138:2124-33; PMID:16055690; http://dx.doi.org/ 10.1104/pp.104.054213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dresselhaus T, Snell WJ. Fertilization: a sticky sperm protein in plants. Curr Biol 2014; 24:R164-6; PMID:24556441; http://dx.doi.org/ 10.1016/j.cub.2013.12.044 [DOI] [PubMed] [Google Scholar]
- 8. Vacquier VD, Moy GW. Isolation of bindin: the protein responsible for adhesion of sperm to sea urchin eggs. Proc Natl Acad Sci U S A 1977; 74:2456-60; PMID:267939; http://dx.doi.org/ 10.1073/pnas.74.6.2456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Misamore MJ, Gupta S, Snell WJ. The Chlamydomonas Fus1 protein is present on the mating type plus fusion organelle and required for a critical membrane adhesion event during fusion with minus gametes. Mol Biol Cell 2003; 14:2530-42; PMID:12808049; http://dx.doi.org/ 10.1091/mbc.E02-12-0790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Popowicz GM, Schleicher M, Noegel AA, Holak TA. Filamins: promiscuous organizers of the cytoskeleton. Trends Biochem Sci 2006; 31:411-9; PMID:16781869; http://dx.doi.org/ 10.1016/j.tibs.2006.05.006 [DOI] [PubMed] [Google Scholar]
- 11. Wilson NF, Foglesong MJ, Snell WJ. The Chlamydomonas mating type plus fertilization tubule, a prototypic cell fusion organelle: isolation, characterization, and in vitro adhesion to mating type minus gametes. J Cell Biol 1997; 137:1537-53; PMID:9199169; http://dx.doi.org/ 10.1083/jcb.137.7.1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferris PJ, Woessner JP, Goodenough UW. A sex recognition glycoprotein is encoded by the plus mating-type gene fus1 of Chlamydomonas reinhardtii. Mol Biol Cell 1996; 7:1235-48; PMID:8856667; http://dx.doi.org/ 10.1091/mbc.7.8.1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu Y, Tewari R, Ning J, Blagborough AM, Garbom S, Pei J, Grishin NV, Steele RE, Sinden RE, Snell WJ, Billker O. The conserved plant sterility gene HAP2 functions after attachment of fusogenic membranes in Chlamydomonas and Plasmodium gametes. Genes Dev 2008; 22:1051-68; PMID:18367645; http://dx.doi.org/ 10.1101/gad.1656508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Inoue N, Ikawa M, Isotani A, Okabe M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature 2005; 434:234-8; PMID:15759005; http://dx.doi.org/ 10.1038/nature03362 [DOI] [PubMed] [Google Scholar]
- 15. Bianchi E, Doe B, Goulding D, Wright GJ. Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature 2014; 508:483-7; PMID:24739963; http://dx.doi.org/ 10.1038/nature13203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kasahara RD, Maruyama D, Hamamura Y, Sakakibara T, Twell D, Higashiyama T. Fertilization recovery after defective sperm cell release in Arabidopsis. Curr Biol 2012; 22:1084-9; PMID:22608509; http://dx.doi.org/ 10.1016/j.cub.2012.03.069 [DOI] [PubMed] [Google Scholar]


