Abstract
Plants establish highly and systemically organized stress defense mechanisms against unfavorable living conditions. To interpret these environmental stimuli, plants possess communication tools, referred as secondary messengers, such as Ca2+ signature and reactive oxygen species (ROS) wave. Maintenance of ROS is an important event for whole lifespan of plants, however, in special cases, toxic ROS molecules are largely accumulated under excess stresses and diverse enzymes played as ROS scavengers. Arabidopsis and rice contain 3 NADPH-dependent thioredoxin reductases (NTRs) which transfer reducing power to Thioredoxin/Peroxiredoxin (Trx/Prx) system for scavenging ROS. However, due to functional redundancy between cytosolic and mitochondrial NTRs (NTRA and NTRB, respectively), their functional involvements under stress conditions have not been well characterized. Recently, we reported that cytosolic NTRA confers the stress tolerance against oxidative and drought stresses via regulation of ROS amounts using NTRA-overexpressing plants. With these findings, mitochondrial NTRB needs to be further elucidated.
Keywords: NADPH-dependent thioredoxin reductase (NTR), reactive oxygen species (ROS), stress response, Thioredoxin
Plants are systemically established both in normal growth condition and in cases wherein plants need to enhance tolerance against various stress conditions caused by increase of second messengers, such as Ca2+ and ROS. Changes in intracellular Ca2+ concentration defined as a Ca2+ signature are sensed by environmental stimuli, decoded and transmitted to downstream genes by a complex of diverse Ca2+-related proteins such as calmodulins (CaMs), calcium-dependent protein kinases (CDPKs) and their interacting kinases (CIPKs).1 Like this, ROS is also maintained in plant cells with various enzymes to produce and scavenge. However, large inductions of ROS due to extreme environmental stresses work as toxic molecules, resulting in the oxidative stress in cells and ultimately trigger the cell death.2,3 These ROS are mainly produced by apoplast-localized NADPH oxidases (respiratory burst oxidase homologs, RBOHs). Moreover, detoxifying systems operate to scavenge ROS followed by several antioxidant enzymes such as superoxide dismutase (SOD), ascorbate peroxidase (APX), catalase (CAT), glutathione peroxidase (GPX) and peroxiredoxin (Prx).4-6 Prxs are a family of cysteine-dependent peroxidases, and the activity is coupled to oxidation of NADPH via thioredoxin reductase (TrxR) and thioredoxin (Trx) to reduce Prx.7,8
In Arabidopsis and rice, 3 NADPH-dependent TrxRs (NTRs) with disulfide reductase activity, namely NTRA, NTRB and NTRC have been reported existing in different subcellular localizations such as cytosol, mitochondria and chloroplast, respectively (Fig. 1).9,10 NTRs transfer electrons from NADPH to the active-site disulfide bridge (WCG/PPC) of oxidized Trx via FAD and a redox-active disulfide.8,11 Consistent with this, diverse Trxs (Trx f, h, m, o, x, and y) also exist in distinct subcellular compartments to couple thiol/disulfide exchange reactions of NTR/Trx systems.8 All NTRs possess a redox active CXXC dithiol motif.11 While Arabidopsis NTRA and NTRB share 82% sequence similarity, chloroplast localized NTRC is atypical due to additional Trx domain in C-terminus of NTRC which is absent in NTRA and NTRB.12 Compared to typical NTRA and NTRB, NTRC has been largely identified from biochemical reactions to physiological functions in Arabidopsis.13
Figure 1.
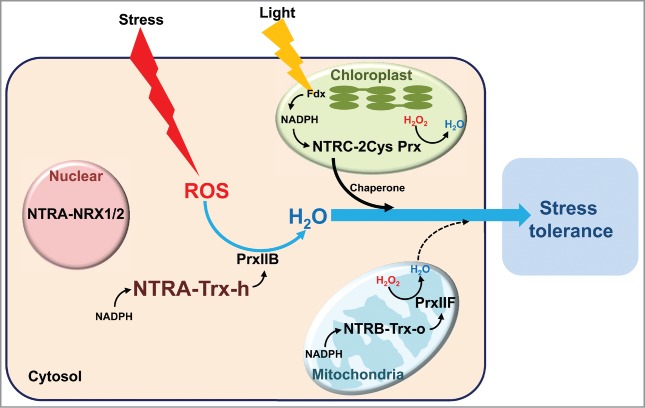
Model proposed for the function of NTRs in Arabidopsis. NTR/Trx systems exist in different subcellular compartments, such as cytosol (NTRA-Trx-h), mitochondria (NTRB-Trx-o) and chloroplast (NTRC), and reduce their distinct Prx which scavenges the H2O2. NTRA also reduces nucleus-localized thioredoxins (NRX1). Stress-induced ROS effectively reduced via the overexpression of NTRA which concomitantly confers the stress tolerance. NTR, NADPH-dependent thioredoxin reductase; Trx, thioredoxin; NRX, nucleoredoxin; ROS, reactive oxygen species; Prx, peroxiredoxin.
Loss-of function NTRC mutant in Arabidopsis showed obvious pale-green leaves and dwarf phenotypes due to defects of chloroplast biogenesis and auxin levels, respectively.14 The ntrc mutant plants were also sensitive to oxidative, salt, drought and heat stresses.9,15 NTRC protein harboring this fusion of TrxR and Trx domain directly reduces chloroplast-localized 2Cys-peroxiredoxin (2Cys Prx). Furthermore, electrons from NADPH produced in chloroplast transfer to 2Cys Prx via redox-active cysteins in TrxR and Trx domain in NTRC.13,16,17 However, cytosolic NTRA and mitochondrial NTRB are acquired to cooperate with their relevant Trx counterparts. NTRA and NTRB also share redundant function in cytosol and mitochondria both in vitro and in vivo.10,18,19 The single loss-of function mutants of NTRA and NTRB plants showed no phenotypic perturbations with wild-type Arabidopsis plant, however, ntra ntrb double mutant exhibited minor differences such as wrinkled seeds, slow plant growth and reduced fitness of pollen. It indicates that NTRA and NTRB are not essential for plant development.19 However, it has been reported that ntra ntrb double mutant plant exhibits UV-C tolerance due to high accumulation of anthocyanin.20 Recently, nuclear Trx genes (NRX1 and NRX2) have been identified in Arabidopsis, emphasizing that NRX1 was reduced by NTRA but not NRX2. Interestingly, NRX1 and NRX2 are localized in both nucleus and cytosol, while cytosolic NTRA is partially localized in the nucleus.21 Even this finding suggests that Trx system also existed in nucleus, it is still difficult to conclude whether NTRA/NRX1 system is working in the nucleus or cytosol. Thus, due to this functional redundancy between NTRA and NTRB, their physiological functions under environmental stresses are not clearly classified and remain elusive to date.
Due to difficulties of research using single loss-of function NTRA mutant (ntra-ko), NTRA-overexpressing plants (NTRA-OX) in Arabidopsis were generated and functional roles of NTRA under various stress responses were investigated. As already known, cytosolic NTRA protein reduces cytosolic Trx-h proteins.22 And Trx-h proteins are positively involved in stress responses such as pathogen and heat stress via redox regulation and chaperone function in cells.23,24 Under oxidative stress, toxic ROS are largely accumulated in plant cells and cause damage of DNA, RNA, protein and lipid.2,3 We have found that NTRA transcripts were dramatically enhanced by methyl viologen treatments which are well known to induce large accumulations of ROS and cause oxidative damage in plant cells.25 Moreover, NTRA-OX plants showed high survival rates and retarded ROS inductions under oxidative stress whereas wild-type and ntra-ko plants were almost dead with high ROS contents. The phenomenon of stress tolerance and low ROS levels were consistent when plants exposed to drought stress.25 Interestingly, transcriptional levels of drought-responsive genes (RD29A and DREB2A) were higher in NTRA-OX compared to wild-type and ntra-ko plants, and moreover, overexpression of NTRA caused induction of CuZnSOD and APX1 transcripts.25 It suggests that NTRA regulates cellular ROS levels via activated Trx systems in plant cells and protects the plants against stress.
Functional roles of mitochondrial TrxRs are well characterized under oxidative stress in yeast and human.26,27 However, mitochondrial NTRB has been known only disulfide reductase activity for Trx proteins,10 but its roles are still largely unknown in stress responses due to same reason such as NTRA. Thus, overexpression or dominant mutant analysis needs to be followed.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grants from Next-Generation BioGreen21 Program (SSAC, grant: PJ009615 and PJ009088), Rural Development Administration, Republic of Korea. J.-Y.C. were supported by a scholarship from the BK21Plus Program, the Ministry of Education, Korea.
References
- 1. Hashimoto J, Kudla J. Calcium decoding mechanisms in plants. Biochimie 2011; 93:2054-9; PMID:21658427; http://dx.doi.org/ 10.1016/j.biochi.2011.05.019 [DOI] [PubMed] [Google Scholar]
- 2. Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Ann Rev Plant Biol 2004; 55:373-99; http://dx.doi.org/ 10.1146/annurev.arplant.55.031903.141701 [DOI] [PubMed] [Google Scholar]
- 3. Møller IM, Jensen PE, Hansson A. Oxidative modifications to cellular components in plants. Ann Rev Plant Biol 2007; 58:459-81; http://dx.doi.org/ 10.1146/annurev.arplant.58.032806.103946 [DOI] [PubMed] [Google Scholar]
- 4. Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Environ 2010; 33:453-67; PMID:19712065; http://dx.doi.org/ 10.1111/j.1365-3040.2009.02041.x [DOI] [PubMed] [Google Scholar]
- 5. Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trend Plant Sci 2004; 9:490-8; http://dx.doi.org/ 10.1016/j.tplants.2004.08.009 [DOI] [PubMed] [Google Scholar]
- 6. Torres MA, Dangl JL. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Cur Opin Plant Biol 2005; 8:397-403; http://dx.doi.org/ 10.1016/j.pbi.2005.05.014 [DOI] [PubMed] [Google Scholar]
- 7. Hall A, Nelson KJ, Poole LB, Karplus PA. Structure-based insights into the catalytic power and conformational dexterity of peroxiredoxins. Antioxid Redox Signal 2010; 402:194-209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gelhaye E, Rouhier N, Navrot N, Jacquot JP. The plant thioredoxin system. Cell Mol Life Sci 2005; 62:24-35; PMID:15619004; http://dx.doi.org/ 10.1007/s00018-004-4296-4 [DOI] [PubMed] [Google Scholar]
- 9. Serrato AJ, Pérez-Ruiz JM, Spínola MC, Cejudo FJ. A novel NADPH thioredoxin reductase, localized in the chloroplast, which deficiency causes hypersensitivity to abiotic stress in Arabidopsis thaliana. J Biol Chem 2004; 279:43821-7; PMID:15292215; http://dx.doi.org/ 10.1074/jbc.M404696200 [DOI] [PubMed] [Google Scholar]
- 10. Reichheld JP, Khafif M, Riondet C, Droux M, Bonnard G, Meyer Y. AtNTRB is the major mitochondrial thioredoxin reductase in Arabidopsis thaliana. FEBS Lett 2005; 579:337-42; PMID:15642341; http://dx.doi.org/ 10.1016/j.febslet.2004.11.094 [DOI] [PubMed] [Google Scholar]
- 11. Kirkensgaard KG, Hägglund P, Shahpiri A, Finnie C, Henriksen A, Svensson B. A novel twist on molecular interactions between thioredoxin and nicotinamide adenine dinucleotide phosphate-dependent thioredoxin reductase. Proteins 2014; 82:607-19; PMID:24123219; http://dx.doi.org/ 10.1002/prot.24437 [DOI] [PubMed] [Google Scholar]
- 12. Moon JC, Jang HH, Chae HB, Lee JR, Lee SY, Jung YJ, Shin MR, Lim HS, Chung WS, Yun DJ, et al. . The C-type Arabidopsis thioredoxin reductase ANTR-C acts as an electron donor to 2-Cys peroxiredoxins in chloroplasts. Biochem Biophys Res Commun 2006; 348:478-84; PMID:16884685 [DOI] [PubMed] [Google Scholar]
- 13. Cejudo FJ, Ferrández J, Cano B, Puerto-Galá L, Guinea M. The function of the NADPH thioredoxin reductase C-2-Cys peroxiredoxin system in plastid redox regulation and signaling. FEBS Lett 2012; 586:2974-80; PMID:22796111; http://dx.doi.org/ 10.1016/j.febslet.2012.07.003 [DOI] [PubMed] [Google Scholar]
- 14. Lepistö A, Kangasjärvi S, Luomala E-M, Brader G, Sipari N, Keränen M, Keinänen M, Rintamäki E. Chloroplast NADPH-thioredoxin reductase interacts with photoperiodic development in Arabidopsis. Plant Physiol 2009; 149:1261-76; PMID:19151130; http://dx.doi.org/ 10.1104/pp.108.133777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chae HB, Moon JC, Shin MR, Chi YH, Jung YJ, Lee SY, Nawkar GM, Jung HS, Hyun JK, Kim WY, et al. . Thioredoxin reductase type C (NTRC) orchestrates enhanced thermotolerance to Arabidopsis by its redox-dependent holdase chaperone function. Mol Plant 2013; 6:323-36; PMID:23024205; http://dx.doi.org/ 10.1093/mp/sss105 [DOI] [PubMed] [Google Scholar]
- 16. Kirchsteiger K, Pulido P, González M, Cejudo FJ. NADPH-thioredoxin reductase C controls the redox status of chloroplast 2-Cys peroxiredoxins in Arabidopsis thaliana. Mol Plant 2009; 2:298-307; PMID:19825615; http://dx.doi.org/ 10.1093/mp/ssn082 [DOI] [PubMed] [Google Scholar]
- 17. Pascual MB, Mata-Cabana A, Florencio FJ, Lindahl M, Cejudo FJ. A comparative analysis of the NADPH oxidase C-2-Cys peroxiredoxin system from plants and cyanobacteria. Plant Physiol 2011; 155:1806-16; PMID:21335525; http://dx.doi.org/ 10.1104/pp.110.171082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Trotter EW, Grant CM. Non-reciprocal regulation of the redox state of the glutathione/glutaredoxin and thioredoxin systems. EMBO Rep 2003; 4:184-9; PMID:12612609; http://dx.doi.org/ 10.1038/sj.embor.embor729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reichheld JP, Khafif M, Riondet C, Droux M, Bonnard G, Meyer Y. Inactivation of thioredoxin reductases reveals a complex interplay between thioredoxin and glutathione pathways in Arabidopsis development. Plant Cell 2007; 19:1851-65; PMID:17586656; http://dx.doi.org/ 10.1105/tpc.107.050849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bashandy T, Taconnat L, Renou J-P, Meyer Y, Reichheld JP. Accumulation of flavonoids in an ntra ntrb mutant leads to tolerance to UV-C. Mol Plant 2009; 2:249-58; PMID:19825611; http://dx.doi.org/ 10.1093/mp/ssn065 [DOI] [PubMed] [Google Scholar]
- 21. Marchal C, Delorme-Hinoux V, Bariat L, Siala W, Belin C, Saez-Vasquez J, Riondet C, Reichheld J-P. NTR/NRX define a new thioredoxin system in the nucleus of Arabidopsis thaliana cells. Mol Plant 2014; 7:30-44; PMID:24253198; http://dx.doi.org/ 10.1093/mp/sst162 [DOI] [PubMed] [Google Scholar]
- 22. Jacquot J-P, Rivera-Madrid R, Marinho P, Kollarova M, Le Maréchal P, Miginiac-Maslow M, Meyer Y. Arabidopsis thaliana NAPHP thioredoxin reductase. cDNA characterization and expression of the recombinant protein in Escherichia coli. J Mol Biol 1994; 235:1357-63; PMID:8308900; http://dx.doi.org/ 10.1006/jmbi.1994.1091 [DOI] [PubMed] [Google Scholar]
- 23. Tada Y, Spoel SH, Pajerowska-Mukhtar K, Mou Z, Song J, Dong X. Plant immunity requires conformational changes of NPR1 via S-nitrosylation and thioredoxins. Science 2008; 321:952-6; PMID:18635760; http://dx.doi.org/ 10.1126/science.1156970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Park SK, Jung YJ, Lee JR, Lee YM, Jang HH, Lee SS, Park JH, Kim SY, Moon JC, Lee SY, et al. . Heat-shock and redox-dependent functional switching of an h-type Arabidopsis thioredoxin from a disulfide reductase to a molecular chaperone. Plant Physiol 2009; 150:552-61; PMID:19339505; http://dx.doi.org/ 10.1104/pp.109.135426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cha J-Y, Kim JY, Jung IJ, Kim MR, Melencion A, Alam SS, Yun D-J, Lee SY, Kim MG, Kim W-Y. NADPH-dependent thioredoxin reductase A (NTRA) confers elevated tolerance to oxidative stress and drought. Plant Physiol Biochem 2014; 80:184-191; PMID:24792388; http://dx.doi.org/ 10.1016/j.plaphy.2014.04.008 [DOI] [PubMed] [Google Scholar]
- 26. Lopert P, Day BJ, Patel M. Thioredoxin reductase deficiency potentiates oxidative stress, mitochondrial dysfunction and cell death in dopaminergic cells. PLOS One 2012; 7:e50683; PMID:23226354; http://dx.doi.org/ 10.1371/journal.pone.0050683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Greetham D, Kritsiligkou P, Watkins RH, Carter Z, Parkin J, Grant CM. Oxidation of the yeast mitochondrial thioredoxin promotes cell death. Antioxid Redox Sign 2013; 18:376-85; http://dx.doi.org/ 10.1089/ars.2012.4597 [DOI] [PMC free article] [PubMed] [Google Scholar]


