Abstract
Secretoglobins are a superfamily of secreted proteins thought to participate in inflammation, tissue repair, and tumorigenesis. Secretoglobin family 2A member 1 (Scgb2a1) is a component of prostatein, a major androgen-binding protein secreted by the rat prostate. Using a rat model for developmental reprogramming of susceptibility to prostate carcinogenesis, we identified, by RNA-seq, that Scgb2a1 is significantly upregulated (>100-fold) in the prostate of adult rats neonatally exposed to bisphenol A (BPA), with increased gene expression confirmed by quantitative RT-PCR and chromatin immunoprecipitation for histone H3 lysine 9 acetylation. Bisulfite analysis of both CpG islands located within 10 kb of the Scgb2a1 promoter identified significant hypomethylation of the CpG island upstream of the transcription start site of this gene in the reprogrammed prostate. These data suggest that expression of Scgb2a1 in the adult prostate could be epigenetically reprogrammed by BPA exposure during prostate development, with potential implications for cancer risk and response to chemotherapeutics associated with prostatein binding.
Keywords: BPA, developmental reprogramming, DNA methylation, prostate cancer, prostatein, Scgb2a1
Abbreviations
- EDCs
Endocrine Disrupting Compounds
- BPA
Bisphenol A
- PIN
Prostatic Intraepithelial Neoplasia
- T
Testosterone
- E
Estrogen
- qRT-PCR
quantitative real-time PCR
- ChIP
chromatin immunoprecipitation
- RNA-seq
RNA-sequencing
Introduction
Accumulating evidence has shown that environmental exposures to endocrine disrupting chemicals (EDCs) can adversely impact human health.1,2 One EDC of concern is bisphenol A (BPA), which has been shown to induce developmental reprogramming in animal models3 and is proposed to have a myriad of effects on human health.4 BPA is a synthetic polymer that is in the top 2% of high production volume chemicals used in the manufacturing of polycarbonate plastics and epoxy resins. BPA has been shown to leach in microgram amounts from polycarbonate plastics and epoxy resins into food and water supplies,2 and exposure to BPA is nearly ubiquitous; urinary analysis reveals that BPA is detected in >93% of the population in the United States.5,6 The widespread use of BPA provides numerous sources for exposure during key periods of development.
Recent reports have linked BPA to prostate carcinogenesis, with BPA acting as a mitogen in prostate carcinoma cell lines, accelerating tumor growth after androgen ablation, and inducing cell migration.7-10 Low doses of BPA can also disturb the centrosome cycle in normal prostate cell lines by promoting centrosome amplification and enhancing microtubule aster formation.11 In vivo studies in a hormonally induced rat prostate carcinogenesis model also support a role for BPA in prostate cancer development. In this model, neonatal exposure to BPA induces dysplasia [the rodent equivalent of prostatic intraepithelial neoplasia (PIN)] in the adult prostate.12,13
Secretoglobin family 2A member 1 (Scgb2a1/prostatein C3/lipophilin C/mammaglobin B) is a major component peptide of prostatein (prostatic steroid binding protein), the principal secretory protein in rat prostatic fluid secreted by the acinar glands of the ventral prostate.14 Prostatein expression has been shown to be stimulated by androgens14 and serves as a steroid-binding protein to maintain a relatively high level of androgen within the lumen of acinar glands in the normal prostate.15 It has also been proposed to have an antioxidant and immunosuppressive function to protect sperm from immunologic damage in the female reproductive tract.16-18 Mass spectrometry analyses first hinted at a protein homologous to prostatein in humans,19,20 and humans do indeed express a homolog of this protein.21,22 In fact, the expression of prostatein is positively correlated with prostate tumor malignancy grade.23
In the present study, we found that a physiologically relevant exposure to BPA (oral) in neonatal rats increased the incidence of PIN lesions in adults, providing further validation for the link between developmental reprogramming due to early life BPA exposure and increased susceptibility to prostate cancer in a hormonally induced prostate carcinogenesis model. Importantly, Scgb2a1 gene expression was significantly upregulated in these rats, with a concomitant enrichment of histone H3 lysine 9 acetylation (H3K9Ac), a mark of active gene transcription. The CpG island nearest the promoter region of Scgb2a1 was hypomethylated in the BPA-reprogrammed prostate. Taken together, we herein present evidence for Scgb2a1, a major component of prostastein, as a novel reprogramming target of BPA.
Results
Neonatal BPA exposure promotes development of dysplasia in the adult rat prostate
Pharmacokinetic studies were performed to measure circulating BPA levels after acute oral or subcutaneous exposure to identify the oral BPA dose that would closely approximate a reference subcutaneous 10 μg/kg dose (SC10) previously shown to promote the development of prostate lesions.12,13 As shown in Figure 1A, 50 μg/kg (BP50) resulted in circulating levels of BPA most similar to that of SC10. Accordingly, we selected BP50 and 2 lower oral BPA doses (2 and 10 μg/kg) for our longitudinal developmental reprogramming studies.
Figure 1.
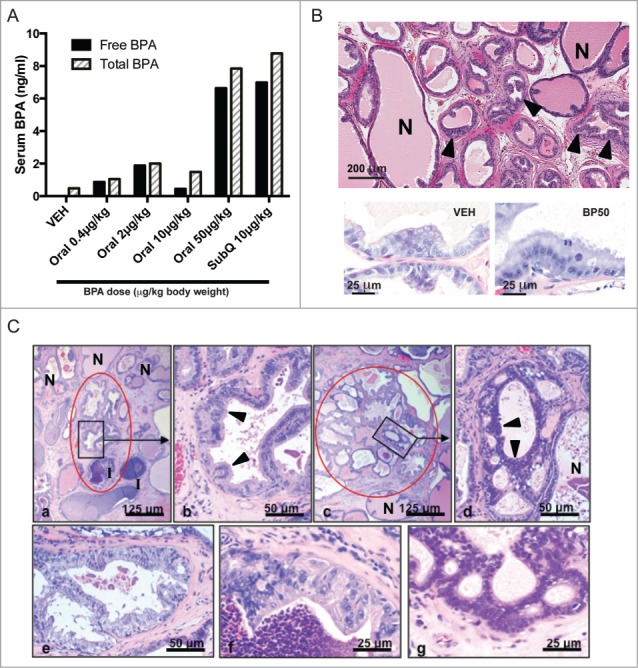
BPA pharmacokinetic studies and histopathology of adult prostates from rats exposed to BPA neonatally. (A) Circulating levels of total and free BPA were measured after acute administration of BPA via subcutaneous or oral route. Postnatal day 3 (PND3) male neonates were treated with the indicated BPA doses and serum was harvested 1 h later. Free and total BPA levels (ng/ml) are represented by black and striped bars, respectively. Each bar corresponds to one pooled sample of at least 11 male neonates. (B) Upper panel: representative image of a BPA-treated animal displaying hyperplasia (arrowheads) in the dorsolateral lobe at day 70. N: normal prostate tissue. Lower panel: example of epithelial atypia observed in the BPA-treated group (right), which was absent in the vehicle group (left). (C) Prostatic dysplasia. Representative examples of lesions scored as 1 (mild dysplasia) and 2 (moderate-marked dysplasia): Panel a – Mild dysplasia (severity score of 1): one area with dysplasia [(encircled in red; the remainder of the image contains normal glands (N)]. In the lower one third of the image there is inflammation (I), which was frequently present in T + E-treated animals, but this was not associated with dysplastic lesions. Panel b – Higher magnification of inset in Panel a; mildly atypical cells with hypochromatic nuclei (arrowheads). Panel c – Moderate-marked dysplasia (severity score of 2): one of multiple areas with dysplasia (encircled in red). Panel d – Higher magnification of inset in Panel c; crowded hyperchromatic atypical cells with gland-within-gland formation (arrowheads) and normal epithelium (N) in lower right hand of panel. Panels e and f – Moderate-markedly atypical cells with hypochromatic nuclei (severity score of 2). Panel g - Moderate-markedly atypical crowded hyperchromatic atypical cells with gland-within-gland formation (severity score of 2). The lesions were focal, leaving areas of normal tissue (N) in each prostate. The morphology of the lesions was not different between the treatment groups; there were only differences in the incidence of lesions with a degree of severity score of 1 and 2.
Pathological assessment was performed on prostates from adult animals (day 70) prior to implantation of testosterone + estrogen (T+E) pellets to induce prostate carcinogenesis. In the dorsolateral prostate, both vehicle and BPA-exposed animals exhibited hyperplasia (Fig. 1B, upper panel), with no difference in the extent of hyperplasia between both groups (data not shown). Minimal to mild epithelial atypia was observed in the BPA-treated group, but this phenotype was widespread and not localized to particular areas of hyperplasia (Fig. 1B, lower panel).
In the lateral prostate of 12 month-old animals treated with T+E at day 70, we observed dysplasia (PIN lesions) and inflammation as previously described.13 We focused on the lateral prostate because it is selectively sensitive to treatment with T+E, which induces dysplasia and inflammation in addition to carcinomas.24,25 Images representative of the different dysplasia scores are shown in Figure 1C. The incidence of more than minimal dysplasia increased significantly with increasing oral BPA dose (P < 0.05) and, in a pair-wise comparison, the incidence in the BP50 group (91%) was significantly increased over the vehicle group (63%), similar to that of the SC10 group (88%) (Table 1). Inflammation likely contributes to lesion progression26 and is associated with all major anomalies of the human prostate, including benign prostatic hyperplasia and prostate cancer.27,28 We observed inflammation in the prostate of virtually all of the 12 month-old rats, with a similar severity across the BPA and vehicle exposure groups (Table 1). Small, well-differentiated adenocarcinomas and carcinomas in situ in the periurethral prostatic ducts and lateral prostate were present in both the BPA-exposed and vehicle groups (Table 2), consistent with the susceptibility of Sprague-Dawley rats to prostate carcinogenesis induced by T+E promotion. It should be noted that overall prostate weight was not markedly increased in any BPA-exposed group compared to vehicle-treated animals (Fig. S1). These findings confirm that the neonatal oral BPA exposure developmentally reprogrammed susceptibility to prostatic carcinogenesis, as previously reported,13 and we focused further analysis on comparing the impact on the epigenome in vehicle and BP50 treated rats.
Table 1.
Histopathological assessment of dysplasia and inflammation in prostates from 12 month-old rats exposed to BPA neonatally and to T+E at day 70
| Group | All cancer (cancer + cis) | Carcinoma only | LP dysplasia score | Increase in LP dysplasia score# | Incidence of LP dysplasia score## | LP inflammation score |
|---|---|---|---|---|---|---|
| Oral Veh | 8/26 (31%) | 4/26 (15%) | 0.97 ± 0.43 (n=24 ) | — | 15/24 (63%)a | 1.63 ± 0.67 (n=26 ) |
| Oral BPA 2 μg/kg | 5/25 (20%) | 3/25 (12%) | 1.05 ± 0.56 (n=23 ) | 8% | 15/23 (65%) a | 1.75 ± 0.44 (n=24 ) |
| Oral BPA 10 μg/kg | 9/25 (36%) | 5/25 (20%) | 1.13 ± 0.52 (n=20 ) | 17% | 15/20 (75%)a | 1.86 ± 0.35 (n=22 ) |
| Oral BPA 50 μg/kg | 7/24 (29%) | 3/24 (13%) | 1.20 ± 0.49 (n=22 ) | 23% | 20/22 (91%)a,b | 1.89 ± 0.28 (n=25 ) |
| SubQ BPA 10 μg/kg | 8/28 (29%) | 4/28 (14%) | 1.23 ± 0.41* (n=27 ) | 26% | 23/26 (88%)b | 1.93 ± 0.37 (n=27 ) |
LP lateral prostate.
cis carcinoma in situ.
P < 0.05 relative to Vehicle group using a 2-sided Mann-Whitney test.
aP < 0.05 for trend with increasing dose of oral BPA using a X2 test.
bP < 0.05 in a pair-wise comparison with the Vehicle group using a Fisher exact test.
#Increase in dysplasia score over the Vehicle group.
##Number of rats with more than minimal dysplasia (score > 0.67) over the total number of rats per group (%).
BPA dose is expressed in μg/kg body weight.
Table 2.
Histopathological assessment of cancer incidence in prostates from 12 month-old rats exposed to BPA neonatally and to T+E at day 70
| Group | LP Cancer (cancer + cis) | LP Carcinoma only | PUPD Cancer (cancer + cis) | PUPD Cancer (carcinoma) |
|---|---|---|---|---|
| Oral Veh | 7/26 (27%) | 3/26 (11%) | 3/18 (17%) | 3/18 (17%) |
| Oral BPA 2 μg/kg | 2/25 (8%) | 1/25 (4%) | 4/21 (19%) | 2/21 (10%) |
| Oral BPA 10 μg/kg | 8/25 (32%) | 3/25 (12%) | 5/21 (24%) | 4/21 (19%) |
| Oral BPA 50 μg/kg | 3/24 (13%) | 2/24 (8%) | 4/21 (19%) | 1/21 (5%) |
| SubQ BPA 10 μg/kg | 4/28 (14%) | 1/28 (4%) | 5/22 (23%) | 3/22 (14%) |
PUPD Periurethral Prostate Ducts.
LP Lateral Prostate.
cis carcinoma in situ.
BPA dose is expressed in μg/kg body weight.
Scgb2a1 is a target for developmental reprogramming by BPA
RNA-seq analysis performed on day 70 rats (prior to T + E exposure) identified the Scgb2a1 gene as a target for developmental reprogramming by BPA. Prostates from rats exposed neonatally to vehicle and BPA were subjected to RNA sequencing. As shown in Figure 2A, Scgb2a1 exhibited a significant increase in expression (∼116-fold) by RNA-seq analysis, and this result was confirmed by RT-PCR. ChIP analysis of acetylated H3K9 (which is associated with transcriptionally active genes) demonstrated that H3K9Ac was enriched 500 bp upstream of the transcription start site (TSS) of Scgb2a1, with a 4-fold increase in BPA-treated prostates compared to prostates from vehicle-treated animals (Fig. 2B).
Figure 2.
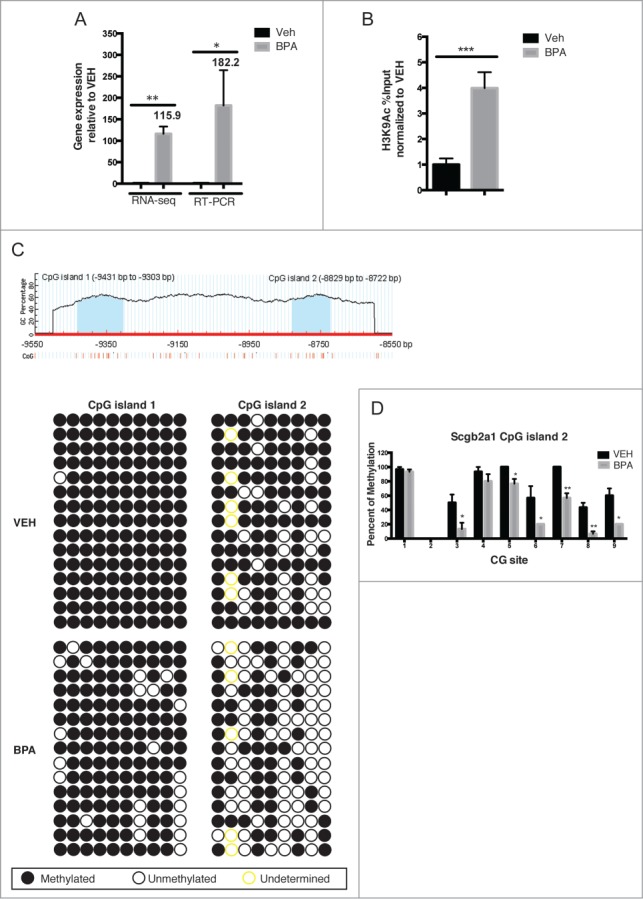
Scgb2a1 is a target for development reprogramming by neonatal BPA exposure. (A) Expression of Scgb2a1 at day 70 was analyzed with RNA-seq and RT-PCR from BP50-treated and vehicle-treated animals. Mean values ± SEM are shown, * P < 0.05, ** P < 0.005 relative to vehicle, n = 3. (B) Chromatin immunoprecipitation was performed with an anti-H3K9Ac antibody using DNA obtained from day 70 prostates from vehicle and BP50 treatment groups. Mean values ± SEM are shown, *** P < 0.001 relative to vehicle, n = 4. (C) Bisulfite genomic sequencing of Scgb2a1 gene methylation. Upper panel: schematic of CpG content (%) in the upstream region of the rat Scgb2a1 gene identifies 2 CpG islands (blue) between −9431 to −9303bp and −8829 to −8722bp, vertical lines depict individual CpG sites. Lower panel: Bisulfite genomic sequencing data from 3 independent day 70 prostate treated either with vehicle or BP50 (from 5 clones of each individual animal). (D) Quantitation of the bisulfite genomic sequencing of Scgb2a1 gene methylation. Percentage of methylation at each CpG site within CpG island 2 of Scgb2a1 (total of 9 CG sites) is shown. The percentage of methylation at each site was averaged from 3 independent prostates per treatment group (sequencing results from 10 colonies of each individual sample).
The previous data was analyzed in whole prostate samples, but the rodent prostate is a complex organ consisting of the anterior prostate (AP), ventral prostate (VP), dorsal prostate (DP), and lateral prostate (LP),29 and it has previously been shown that exposure of rats to environmental chemicals neonatally disrupts prostate development in a lobe-specific manner .30 Scgb2a1 gene expression profiles in the prostate are also known to be lobe-specific,31,32 so we checked the gene expression level in the 4 different lobes. We observed the highest expression in the VP and a near absence of expression in the AP, while expression levels in the LP and DP were intermediate between the VP and the AP (Fig. 3A). Interestingly, as shown in Figures 3B and 3C, developmental reprogramming of Scgb2a1 only occurred in the AP in BPA-treated animals (∼50-fold). In contrast to what was observed in the AP, expression of Scgb2a1 in the other lobes, which normally express higher levels of this gene, was not significantly different. Thus, the effect of this gene reprogramming was lobe-specific, which may result in increased expression of Scgb2a1, which is normally silent in the anterior lobe of the prostate.
Figure 3.
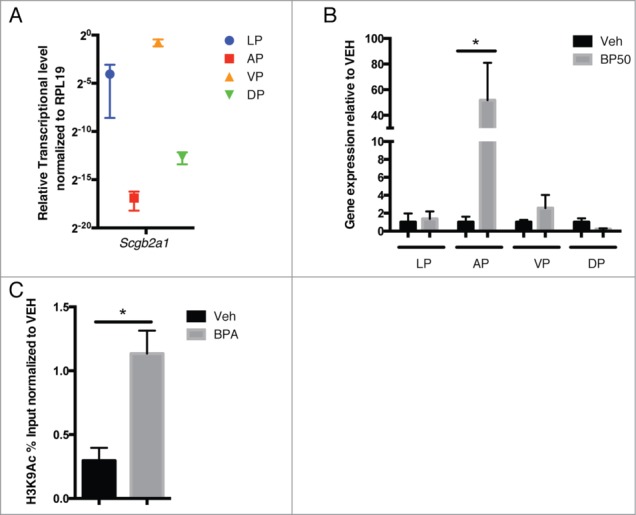
Effect of developmental reprogramming of Scgb2a1 is lobe-specific. (A) The basal expression level of Scgb2a1, in the absence of BPA treatment, was analyzed by qRT-PCR in prostates from day 70 animals and Scgb2a1 is expressed in a lobe-specific manner. Relative gene expression was normalized to the housekeeping gene RPL19. Mean values ± SEM are shown, n = 3. (B) Scgb2a1 gene expression was upregulated in the anterior lobe but not in the other 3 lobes in the day 70 BPA-treated animals. Mean values ± SEM are shown, * P < 0.05, relative to vehicle, n = 3. (C) Chromatin immunoprecipitation was performed with an anti-H3K9Ac antibody with DNA obtained from the anterior prostates of day 70 vehicle-treated and BPA-treated animals. H3K9Ac was enriched at the TSS or 500 bp downstream in BPA-treated prostates, relative to the vehicle group. Mean values ± SEM are shown, * P < 0.05 relative to vehicle, n = 3. Veh: vehicle; BP50: oral 50 μg/kg BPA.
Hypomethylation of the Scgb2a1 promoter by BPA
We next asked if increased expression of Scgb2a1 was due to reprogramming of promoter-associated DNA CpG islands as a result of neonatal BPA exposure. Using MethPrimer33 to predict CpG islands within 10 kb upstream and downstream of the Scgb2a1 TSS, 2 regions were identified located −8829 to −8722, and −9431 to -9303, from the TSS (Fig. 2C, top panel). Bisulfite sequencing was performed to determine the DNA methylation status of both CpG islands. Of the two, the CpG island located nearest to the promoter at −8829 to −8722 showed significant hypomethylation (20–40%) in day 70 BPA-treated prostates compared to prostates from vehicle-treated animals (Fig. 2C, bottom panel and Fig. 2D).
Discussion
Previous studies of BPA in animal models were criticized for using subcutaneous injections, as this is a non-physiological route of exposure. Thus, one of the goals of this study was to investigate the reprogramming of BPA on the developing prostate using the more relevant, oral route of exposure. In our longitudinal studies, BPA exposure resulted in a dose-related increase in the percentage of animals with more than minimal dysplasia at 12 months of age (P < 0.05 for trend). It is possible that a greater difference would be observed in animals older than 12 months. Importantly, our data generated in Sprague-Dawley rats that were bred, treated, and maintained in a different facility with independent histological analysis of prostate lesions provide independent validation of previous studies13 and confirm the ability of BPA to promote dysplasia in the rat prostate in a model of hormonally-induced lesion formation.
We have identified the secretoglobin polypeptide family member Scgb2a1 as a novel reprogramming target of BPA. Upon BPA treatment, Scgb2a1 expression was robustly upregulated, with a parallel increase in H3K9Ac. A previous report has shown that early estrogenic exposure could epigenetically modify the prostate through alterations in DNA methylation and cause permanent changes in prostate development and carcinogenesis susceptibility.12 We observed altered DNA methylation for a CpG island located −8829 to −8722 upstream of the TSS of Scgb2a1. Detailed analysis of this region revealed several potential cis-elements (harboring CpG dinucleotides), which could be bound by the transcription factors IK-2, CREB binding protein, GATA-1/2, CREB, and STAT. Although there is a correlation between hypomethylation of CpG dinuceleotides and increased Scgb2a1 gene expression, we did not directly test whether this epigenetic change is the driver for increased gene expression in this study. It is possible that hypomethylation of CpG dinucleotides may lead to increased transcription factor accessibility and increased Scgb2a1 expression. Alternatively, it is possible that CpG island hypomethylation may enhance AR binding at the ARE motif in the first intron through looping. It is known that Scgb2a1 is an AR-regulated gene that exhibits androgenic responsiveness in in vitro reporter assays.34
The functional consequence of BPA-mediated developmental reprogramming of Scgb2a1 in the prostate is not clearly defined, although there is some evidence that the gene may be a marker of carcinogenesis in a variety of tissues. For example, studies have shown that Scgb2a1 may serve as a biomarker for ovarian cancer,35,36 is associated with disease recurrence in ovarian cancer patients,37 and may be an attractive candidate for immunotherapy in ovarian cancer patients exhibiting chemotherapeutic resistance.38 In addition, Scgb2a1 is also overexpressed in endometrial cancer39 and lung cancer.40 Recently, a role for Scgb2a1 in chemoresistance and radioresistance in colorectal tumors was reported.41 Finally, Scgb2a1 has also been proposed to be a marker for lymph node micrometastasis in patients with abdominal cancers,42 bilary tract carcinoma,43 and breast cancer.44 Scgb2a1 could be a “permissive” gene that can be reprogrammed and, thus, serves as a biomarker of reprogramming, but not necessarily as a driver for increased risk for prostate lesions. In fact, Scgb2a1 expression is increased in the anterior prostate and we did not observe an increase in lesions in this lobe (data not shown). However, increased Scgb2a1 could also be having a paracrine/endocrine effect and increasing the potency of T+E regime, both possibilities that require further exploration.
As mentioned previously, Scgb2a1 is a major component of prostatein. Prostatein, also known as estramustine binding protein (EMBP), can bind to the anticancer drug estramustine phosphate and its major metabolites with a relatively high affinity and lead to high uptake in the ventral prostate and in prostatic tumor tissue45,46. Estramustine phosphate, a carbamate ester combining 17β-estradiol and nor-nitrogen mustard, is a cytotoxic drug used in the treatment of advanced prostatic carcinoma in conjunction with other chemotherapeutic agents such as docetaxel (reviewed in).47 As such, prostatein has been proposed as a marker to predict response to therapy in prostate cancer patients23. Alteration of Scgb2a1 gene expression and methylation by BPA indicates that Scgb2a1 could potentially serve as a sensitive marker for early life BPA exposure, prostate cancer risk, and response to estramustine chemotherapy. Future studies of the functional significance of the reprogramming of Scgb2a1 in prostate have the potential to yield new insights into the role of environmental exposures in development and therapy for this disease.
Materials and Methods
Animals and BPA treatments
Sprague-Dawley rats aged 6–8 weeks were purchased from Harlan (Madison, WI) and maintained according to the guidelines of the MDACC Animal Care and Use Committee. The animals were treated humanely and with regard for alleviation of suffering. This strain was chosen because microsatellite DNA results revealed its high resemblance to the strain used in previous BPA prostate carcinogenesis studies.12 The animals were allowed to acclimate for 2 weeks prior to mating. Afterwards, at least 12 breeding pairs were set up to produce neonates, which were treated as described below. The rats were given phytoestrogen-reduced diet (Zeigler Bros. Inc.., Gardners, PA) ad libitium and maintained on a 14:10 light/dark cycle. BPA (from NIEHS) was dissolved in sesame oil and given to neonates on postnatal days (PND) 1, 3, and 5 subcutaneously or orally. Subcutaneous injection was performed using a 1 ml syringe with attached 27 g needle inserted into the nape of the neck while oral BPA was delivered via a pipette tip. To minimize litter effects, neonates of the same litter (total of >12 litters) were randomized to receive either BPA or vehicle. A total of 280 male neonates were treated with either vehicle, BPA subcutaneously at 10 μg/kg body weight or BPA orally at 2, 10, and 50 μg/kg body weight. These animals were subsequently used for the following analyses: serum BPA levels (n = 60), 12 month longitudinal studies (n = 130) and prostate molecular studies (including RNA-seq and DNA methylation) at day 70 prior to T + E (n = 90). For the longitudinal studies, starting from day 70, the rats were implanted with an estradiol (E)- and 2 testosterone (T)-containing capsules (Dow Corning, Midland, MI) to drive prostate carcinogenesis and the capsules were replaced every 2 months. At 12 months of age, the animals were sacrificed for histopathological assessment.
Serum BPA analysis
Pharmacokinetic studies were performed to define oral BPA doses for the longitudinal studies, as previously reported.48 Post-natal day (PND) 3 neonates were treated either with vehicle, or BPA subcutaneously (also referred to as SC10 reference dose, as it was previously shown to drive prostatic intraepithelial neoplasia in adult rats12), or orally with increasing doses (0.4–50 μg/kg body weight). Serum was pooled from the same treatment group 30–60 minutes after treatment and analyzed for free (active) and total (active plus BPA-glucuronide) BPA as previously described.49
RNA isolation, RNA sequencing and data analysis
Whole genome transcriptome analysis was performed on day 70 prostates (before treatment with T + E implants) from rats that were exposed neonatally to either vehicle, oral BPA at a dose of 50 μg/kg (BP50) or SC10. Total RNA was isolated (n = 4 per treatment) and 100 ng of total RNA was used for cDNA synthesis. RNA samples were treated with DNAseI and the RNA purity and quality was checked by Bioanalyzer prior to cDNA synthesis. cDNA libraries were constructed using SPRI-works Fragment Library System I (Beckman Coulter, Brea, CA), which were then PCR-enriched and purified. Ten pM DNA was loaded into the paired end flow cell on cBOT for cluster generation and thereafter loaded on the HiSeq 2000 sequencer (Illumina Inc.., San Diego, CA) to generate single 36 bp sequence reads. Sequence reads were aligned to the rat reference genome (rn4; version 3.4 by BCM HGSC) using TopHat aligner and aligned read counts were summarized using ShortRead and associated Bioconductor packages. The R program was used to generate heatmaps, and differential gene expression analysis was performed. Statistical analysis to identify differentially expressed genes was performed using the negative-binomial model. False discovery rate (FDR) adjusted P-values were used to control for multiple testing. Genes with FDR <0.1 and P-values <0.01 were considered significantly differentially expressed.
BPA longitudinal studies and pathological assessment
Thirty male neonates, 6 per group, were treated on PND1, 3 and 5 with vehicle, SC10 or BPA oral groups of 2, 10, and 50 μg/kg (BP2/10/50). Starting from day 70, the rats were implanted with an estradiol (E)-containing capsule (1 cm tube; internal diameter 1.5 mm and external diameter 2 mm) and 2 testosterone (T)-containing capsules (2 cm tubes) (Dow Corning, Midland, MI). The T+E is necessary to drive prostate carcinogenesis and the capsules were replaced every 2 months. At 12 months of age, the animals were sacrificed. Whole prostates were weighed and processed for histological staining using standard procedures. The prostate sections were analyzed in a blinded fashion and scored for the presence of lesions, as well as the degree of severity, and the extent of lesions, which were classified as hyperplasia, dysplasia (PIN in rats), and inflammation. Dysplasia was defined as atypical hyperplasia, showing gland-within-gland formation with variable frequency, and characterized by focal crowding of small epithelial cells with hyperchromatic nuclei, with occasional prominent appearing nucleoli. All sections were randomly examined 3 times to semi-quantitatively score dysplastic and inflammatory lesions in the lateral prostate lobe as 0 (absent), 0.5 (minimal), 1 (slight), and 2 (moderate-marked for inflammation and moderate for dysplasia), taking into account both the extent and severity of inflammation, and the severity, size, and number of dysplastic lesions which were (multi) focal (see Fig. 1C). The mean of the 3 reads was calculated for each animal. The absence or presence of adenocarcinoma and carcinoma in situ was determined using criteria previously described24,50,51. The data were analyzed using non-parametric methods (Mann-Whitney to compare 2 groups; Kruskal-Wallis with Dunn's post-hoc for multiple comparisons), and for linear trend analysis, a parametric one-way ANOVA was used. In a separate experiment, histopathological defects were assessed on day 70 prostates retrieved from vehicle, SC10 and BP50 groups, prior to T+E treatment.
Validation of RNA-sequencing genes by quantitative real-time PCR (qPCR)
Total RNA was isolated as described above and cDNA synthesis was performed using SuperScript III First Strand Synthesis System (Life Technologies). qPCR was carried out using the Viia7 RT-PCR system (Life Technologies). RT-PCR primers were designed to span an exon-exon boundary and sequences are listed in Table S1. Ribosomal protein L19 was used as the housekeeping gene as its expression is not altered throughout life when animals are exposed to BPA neonatally.12 Fold change of gene expression was calculated based on cycle differences between vehicle and BP50 samples using the ΔΔCT method. Student t-test was used to assess statistical significance.
Chromatin immunoprecipitation (ChIP) and qPCR
Adult rats were neonatally treated with vehicle, SC10 or BP50 (n ≥ 3 per treatment) and sacrificed at day 70. Whole prostate was finely minced in ice cold PBS with added protease inhibitors and the tissue was then crosslinked with 1.5% formaldehyde, followed by incubation with 125 mM glycine, washed with PBS, and homogenized. After centrifugation, the pellet was resuspended in cell lysis buffer (5 mM PIPES, pH 8; 85 mM KCl; 0.5% NP40), and dounced. The samples were spun and the pellet was resuspended in nuclear lysis buffer (50 mM Tris-HCl, pH 8.1; 10 mM EDTA; 1% SDS) and then sonicated using the Bioruptor (Diagenode, Denville, NJ) to obtain sheared sizes of 100–500 bp. ChIP was performed according to the instructions for Magna ChIP kit (Millipore, Billerica, MA) using 5 μg of anti-H3K9Ac antibody (Cat. no. 39137 Active Motif, Carlsbad, CA). The ChIP'ed DNA was then analyzed by qPCR using SYBR green master mix and the primers listed in Table S1 to measure the percentage of co-precipitating DNA relative to input (% input). There were 2 mapped CpG sites, which are located at −8829 to −8722 and −9431 to −9303 (approximately 9.3 kb and 9.8 kb upstream of the transcription start site). The ChIP region is located 500 bp downstream of the transcription start site.
Bisulfite sequencing
Genomic DNA (1 μg) from day 70 whole prostates from vehicle-treated and BPA-treated animals was isolated, and bisulfite conversion was performed in agarose beads as described previously.52 Nested PCR or common PCR was performed using the Kapa 2G Robust HS Readymix (Kapa Biosystems, Woburn, MA) to amplify the CpG island regions after bisulfite conversion, and the cycling conditions were 94°C for 3 minutes, 35 cycles of denaturing (94°C for 15 seconds), annealing (60°C for 15 seconds), and extension (72°C for 15 seconds) followed by a 10 minute final extension. The PCR products were then purified and cloned into pMD19-T vectors (Clontech, Mountain View, CA). Ten randomly selected clones from each sample were sequenced and analyzed. The PCR primers are listed in Table S1.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank Tia Berry and Amanda Martin for technical assistance with this work and the Histology Core (MDACC, Smithville, TX) for assistance with histological evaluation.
Funding
The support for this work was funded by the National Institute of Environmental Health Sciences (RC2ES018789, P30ES023512 and ES023206), the Cancer Prevention Research Institute of Texas (RP120855) and the Welch Foundation (BE-0023, Houston, TX) to CLW. Additional support for this work was funded by the National Institute of Environmental Health Sciences (P30ES006096) to SMH.
References
- 1. Henley DV, Korach KS. Endocrine-disrupting chemicals use distinct mechanisms of action to modulate endocrine system function. Endocrinology 2006; 147:S25-32; PMID:16690802; http://dx.doi.org/ 10.1210/en.2005-1117 [DOI] [PubMed] [Google Scholar]
- 2. Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ Health Perspect 2003; 111:994-1006; PMID:12826473; http://dx.doi.org/ 10.1289/ehp.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mileva G, Baker SL, Konkle AT, Bielajew C. Bisphenol-A: epigenetic reprogramming and effects on reproduction and behavior. Int J Environ Res Public Health 2014; 11:7537-61; PMID:25054232; http://dx.doi.org/ 10.3390/ijerph110707537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rochester JR. Bisphenol A and Human Health: A review of the literature. Reprod Toxicol 2013; 42:132-55; PMID:23994667 [DOI] [PubMed] [Google Scholar]
- 5. Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect 2005; 113:391-5; PMID:15811827; http://dx.doi.org/ 10.1289/ehp.7534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the US population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ Health Perspect 2008; 116:39-44; PMID:18197297; http://dx.doi.org/ 10.1289/ehp.10753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wetherill YB, Petre CE, Monk KR, Puga A, Knudsen KE. The xenoestrogen bisphenol A induces inappropriate androgen receptor activation and mitogenesis in prostatic adenocarcinoma cells. Mol Cancer Ther 2002; 1:515-24; PMID:12479269 [PubMed] [Google Scholar]
- 8. Wetherill YB, Fisher NL, Staubach A, Danielsen M, de Vere White RW, Knudsen KE. Xenoestrogen action in prostate cancer: pleiotropic effects dependent on androgen receptor status. Cancer Res 2005; 65:54-65; PMID:15665279 [PubMed] [Google Scholar]
- 9. Wetherill YB, Hess-Wilson JK, Comstock CE, Shah SA, Buncher CR, Sallans L, Limbach PA, Schwemberger S, Babcock GF, Knudsen KE. Bisphenol A facilitates bypass of androgen ablation therapy in prostate cancer. Mol Cancer Ther 2006; 5:3181-90; PMID:17172422; http://dx.doi.org/ 10.1158/1535-7163.MCT-06-0272 [DOI] [PubMed] [Google Scholar]
- 10. Derouiche S, Warnier M, Mariot P, Gosset P, Mauroy B, Bonnal JL, Slomianny C, Delcourt P, Prevarskaya N, Roudbaraki M. Bisphenol A stimulates human prostate cancer cell migration remodelling of calcium signalling. SpringerPlus 2013; 2:54; PMID:23450760; http://dx.doi.org/ 10.1186/2193-1801-2-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tarapore P, Ying J, Ouyang B, Burke B, Bracken B, Ho SM. Exposure to bisphenol A correlates with early-onset prostate cancer and promotes centrosome amplification and anchorage-independent growth in vitro. PloS one 2014; 9:e90332; PMID:24594937; http://dx.doi.org/ 10.1371/journal.pone.0090332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ho SM, Tang WY, Belmonte de Frausto J, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res 2006; 66:5624-32; PMID:16740699; http://dx.doi.org/ 10.1158/0008-5472.CAN-06-0516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Prins GS, Ye SH, Birch L, Ho SM, Kannan K. Serum bisphenol A pharmacokinetics and prostate neoplastic responses following oral and subcutaneous exposures in neonatal Sprague-Dawley rats. Reprod Toxicol 2011; 31:1-9; PMID:20887781; http://dx.doi.org/ 10.1016/j.reprotox.2010.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Parker M, Needham M, White R. Prostatic steroid binding protein: gene duplication and steroid binding. Nature 1982; 298:92-4; PMID:6896362; http://dx.doi.org/ 10.1038/298092a0 [DOI] [PubMed] [Google Scholar]
- 15. Lea OA, Petrusz P, French FS. Prostatein. A major secretory protein of the rat ventral prostate. J Biol Chem 1979; 254:6196-202; PMID:447706 [PubMed] [Google Scholar]
- 16. Mukherjee AB, Ulane RE, Agrawal AK. Role of uteroglobin and transglutaminase in masking the antigenicity of implanting rabbit embryos. Am J Reprod Immunol 1982; 2:135-41; PMID:6126131; http://dx.doi.org/ 10.1111/j.1600-0897.1982.tb00154.x [DOI] [PubMed] [Google Scholar]
- 17. Mukherjee A, Chakrabarti J, Chakrabarti A, Banerjee T, Sarma A. Effect of 'Pan Masala' on the germ cells of male mice. Cancer Lett 1991; 58:161-5; PMID:1855192; http://dx.doi.org/ 10.1016/0304-3835(91)90095-Y [DOI] [PubMed] [Google Scholar]
- 18. Maccioni M, Riera CM, Rivero VE. Identification of rat prostatic steroid binding protein (PSBP) as an immunosuppressive factor. J Reprod Immunol 2001; 50:133-49; PMID:11334995; http://dx.doi.org/ 10.1016/S0165-0378(01)00060-2 [DOI] [PubMed] [Google Scholar]
- 19. Carter DB, Resnick MI. High resolution analysis of human prostatic fluid by two-dimensional electrophoresis. Prostate 1982; 3:27-33; PMID:6176986; http://dx.doi.org/ 10.1002/pros.2990030106 [DOI] [PubMed] [Google Scholar]
- 20. Lehrer RI, Xu G, Abduragimov A, Dinh NN, Qu XD, Martin D, Glasgow BJ. Lipophilin, a novel heterodimeric protein of human tears. FEBS Letters 1998; 432:163-7; PMID:9720917; http://dx.doi.org/ 10.1016/S0014-5793(98)00852-7 [DOI] [PubMed] [Google Scholar]
- 21. Becker RM, Darrow C, Zimonjic DB, Popescu NC, Watson MA, Fleming TP. Identification of mammaglobin B, a novel member of the uteroglobin gene family. Genomics 1998; 54:70-8; PMID:9806831; http://dx.doi.org/ 10.1006/geno.1998.5539 [DOI] [PubMed] [Google Scholar]
- 22. Zhao C, Nguyen T, Yusifov T, Glasgow BJ, Lehrer RI. Lipophilins: human peptides homologous to rat prostatein. Biochem Biophys Res Commun 1999; 256:147-55; PMID:10066439; http://dx.doi.org/ 10.1006/bbrc.1999.0274 [DOI] [PubMed] [Google Scholar]
- 23. Fluchter SH, Nelde HJ, Bjork P, Muntzing J, Bichler KH. Effect of treatment on the expression of estramustine-binding protein (EMBP) in prostatic cancer patients: an immunohistochemical study. Prostate 1989; 14:27-43; PMID:2648345; http://dx.doi.org/ 10.1002/pros.2990140105 [DOI] [PubMed] [Google Scholar]
- 24. Bosland MC, Ford H, Horton L. Induction at high incidence of ductal prostate adenocarcinomas in NBL/Cr and Sprague-Dawley Hsd:SD rats treated with a combination of testosterone and estradiol-17 β or diethylstilbestrol. Carcinogenesis 1995; 16:1311-7; PMID:7788848; http://dx.doi.org/ 10.1093/carcin/16.6.1311 [DOI] [PubMed] [Google Scholar]
- 25. Ofner P, Bosland MC, Vena RL. Differential effects of diethylstilbestrol and estradiol-17 β in combination with testosterone on rat prostate lobes. Toxicol Appl Pharmacol 1992; 112:300-9; PMID:1539166; http://dx.doi.org/ 10.1016/0041-008X(92)90200-C [DOI] [PubMed] [Google Scholar]
- 26. Bernoulli J, Yatkin E, Laakso A, Anttinen M, Bosland M, Vega K, Kallajoki M, Santti R, Pylkkanen L. Histopathological evidence for an association of inflammation with ductal pin-like lesions but not with ductal adenocarcinoma in the prostate of the noble rat. Prostate 2008; 68:728-39; PMID:18302197; http://dx.doi.org/ 10.1002/pros.20719 [DOI] [PubMed] [Google Scholar]
- 27. Chughtai B, Lee R, Te A, Kaplan S. Role of inflammation in benign prostatic hyperplasia. Rev Urol 2011; 13:147-50; PMID:22110398 [PMC free article] [PubMed] [Google Scholar]
- 28. Sfanos KS, De Marzo AM. Prostate cancer and inflammation: the evidence. Histopathology 2012; 60:199-215; PMID:22212087; http://dx.doi.org/ 10.1111/j.1365-2559.2011.04033.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hayashi N, Sugimura Y, Kawamura J, Donjacour AA, Cunha GR. Morphological and functional heterogeneity in the rat prostatic gland. Biol Reprod 1991; 45:308-21; PMID:1786296; http://dx.doi.org/ 10.1095/biolreprod45.2.308 [DOI] [PubMed] [Google Scholar]
- 30. Huang L, Pu Y, Alam S, Birch L, Prins GS. The role of Fgf10 signaling in branching morphogenesis and gene expression of the rat prostate gland: lobe-specific suppression by neonatal estrogens. Dev Biol 2005; 278:396-414; PMID:15680359; http://dx.doi.org/ 10.1016/j.ydbio.2004.11.020 [DOI] [PubMed] [Google Scholar]
- 31. Suzuki T, Fujimoto N, Kitamura S, Ohta S. Quantitative determination of lobe specificity of mRNA expression of androgen-dependent genes in the rat prostate gland. Endocr J 2007; 54:123-32; PMID:17146147; http://dx.doi.org/ 10.1507/endocrj.K06-142 [DOI] [PubMed] [Google Scholar]
- 32. Banerjee PP, Banerjee S, Brown TR. Increased androgen receptor expression correlates with development of age-dependent, lobe-specific spontaneous hyperplasia of the brown Norway rat prostate. Endocrinology 2001; 142:4066-75; PMID:11517186; http://dx.doi.org/ 10.1210/endo.142.9.8376 [DOI] [PubMed] [Google Scholar]
- 33. Li LC, Dahiva R. MethPrimer: desiging primers for methylation PCRs. Bioinformatics 2002; 18:1427-31; PMID:12424112; http://dx.doi.org/ 10.1093/bioinformatics/18.11.1427 [DOI] [PubMed] [Google Scholar]
- 34. Claessens F, Rushmere NK, Davies P, Celis L, Peeters B, Rombauts WA. Sequence-specific binding of androgen-receptor complexes to prostatic binding protein genes. Mol Cell Endocrinol 1990; 74:203-12; PMID:2095354; http://dx.doi.org/ 10.1016/0303-7207(90)90225-W [DOI] [PubMed] [Google Scholar]
- 35. Adib TR, Henderson S, Perrett C, Hewitt D, Bourmpoulia D, Ledermann J, Boshoff C. Predicting biomarkers for ovarian cancer using gene-expression microarrays. Br J Cancer 2004; 90:686-92; PMID:14760385; http://dx.doi.org/ 10.1038/sj.bjc.6601603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tassi RA, Bignotti E, Rossi E, Falchetti M, Donzelli C, Calza S, Ravaggi A, Bandiera E, Pecorelli S, Santin AD. Overexpression of mammaglobin B in epithelial ovarian carcinomas. Gynecol Oncol 2007; 105:578-85; PMID:17343903; http://dx.doi.org/ 10.1016/j.ygyno.2007.01.043 [DOI] [PubMed] [Google Scholar]
- 37. Tassi RA, Calza S, Ravaggi A, Bignotti E, Odicino FE, Tognon G, Donzelli C, Falchetti M, Rossi E, Todeschini P, et al. Mammaglobin B is an independent prognostic marker in epithelial ovarian cancer and its expression is associated with reduced risk of disease recurrence. BMC Cancer 2009; 9:253; PMID:19635143; http://dx.doi.org/ 10.1186/1471-2407-9-253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bellone S, Tassi R, Betti M, English D, Cocco E, Gasparrini S, Bortolomai I, Black JD, Todeschini P, Romani C, et al. Mammaglobin B (SCGB2A1) is a novel tumour antigen highly differentially expressed in all major histological types of ovarian cancer: implications for ovarian cancer immunotherapy. Br J Cancer 2013; 109:462-71; PMID:23807163; http://dx.doi.org/ 10.1038/bjc.2013.315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tassi RA, Bignotti E, Falchetti M, Calza S, Ravaggi A, Rossi E, Martinelli F, Bandiera E, Pecorelli S, Santin AD. Mammaglobin B expression in human endometrial cancer. Int J Gynecol Cancer 2008; 18:1090-6; PMID:18021217; http://dx.doi.org/ 10.1111/j.1525-1438.2007.01137.x [DOI] [PubMed] [Google Scholar]
- 40. Sjodin A, Guo D, Sorhaug S, Bjermer L, Henriksson R, Hedman H. Dysregulated secretoglobin expression in human lung cancers. Lung Cancer 2003; 41:49-56; PMID:12826312; http://dx.doi.org/ 10.1016/S0169-5002(03)00126-0 [DOI] [PubMed] [Google Scholar]
- 41. Munakata K, Uemura M, Takemasa I, Ozaki M, Konno M, Nishimura J, Hata T, Mizushima T, Haraguchi N, Noura S, et al. SCGB2A1 is a novel prognostic marker for colorectal cancer associated with chemoresistance and radioresistance. Int J Oncol 2014; 44:1521-8; PMID:24585249 [DOI] [PubMed] [Google Scholar]
- 42. Aihara T, Fujiwara Y, Miyake Y, Okami J, Okada Y, Iwao K, Sugita Y, Tomita N, Sakon M, Shiozaki H, et al. Mammaglobin B gene as a novel marker for lymph node micrometastasis in patients with abdominal cancers. Cancer Lett 2000; 150:79-84; PMID:10755390; http://dx.doi.org/ 10.1016/S0304-3835(99)00378-X [DOI] [PubMed] [Google Scholar]
- 43. Okami J, Dohno K, Sakon M, Iwao K, Yamada T, Yamamoto H, Fujiwara Y, Nagano H, Umeshita K, Matsuura N, et al. Genetic detection for micrometastasis in lymph node of biliary tract carcinoma. Clin Cancer Res 2000; 6:2326-32; PMID:10873083 [PubMed] [Google Scholar]
- 44. Aihara T, Fujiwara Y, Ooka M, Sakita I, Tamaki Y, Monden M. Mammaglobin B as a novel marker for detection of breast cancer micrometastases in axillary lymph nodes by reverse transcription-polymerase chain reaction. Breast Cancer Res Treat 1999; 58:137-40; PMID:10674878; http://dx.doi.org/ 10.1023/A:1006335817889 [DOI] [PubMed] [Google Scholar]
- 45. Bergenheim AT, Henriksson R. Pharmacokinetics and pharmacodynamics of estramustine phosphate. Clin Pharmacokinet 1998; 34:163-72; PMID:9515186; http://dx.doi.org/ 10.2165/00003088-199834020-00004 [DOI] [PubMed] [Google Scholar]
- 46. Walz PH, Bjork P, Gunnarsson PO, Edman K, Hartley-Asp B. Differential uptake of estramustine phosphate metabolites and its correlation with the levels of estramustine binding protein in prostate tumor tissue. Clin Cancer Res 1998; 4:2079-84; PMID:9748122 [PubMed] [Google Scholar]
- 47. Ravery V, Fizazi K, Oudard S, Drouet L, Eymard JC, Culine S, Gravis G, Hennequin C, Zerbib M. The use of estramustine phosphate in the modern management of advanced prostate cancer. BJU Int 2011; 108:1782-6; PMID:21756277; http://dx.doi.org/ 10.1111/j.1464-410X.2011.10201.x [DOI] [PubMed] [Google Scholar]
- 48. Greathouse KL, Bredfeldt T, Everitt JI, Lin K, Berry T, Kannan K, Mittelstadt ML, Ho SM, Walker CL. Environmental estrogens differentially engage the histone methyltransferase EZH2 to increase risk of uterine tumorigenesis. Mol Cancer Res 2012; 10:546-57; PMID:22504913; http://dx.doi.org/ 10.1158/1541-7786.MCR-11-0605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Padmanabhan V, Siefert K, Ransom S, Johnson T, Pinkerton J, Anderson L, Tao L, Kannan K. Maternal bisphenol-A levels at delivery: a looming problem? J Perinatol 2008; 28:258-63; PMID:18273031; http://dx.doi.org/ 10.1038/sj.jp.7211913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ozten N, Horton L, Lasano S, Bosland MC. Selenomethionine and α-tocopherol do not inhibit prostate carcinogenesis in the testosterone plus estradiol-treated NBL rat model. Cancer Prev Res 2010; 3:371-80; PMID:20179302; http://dx.doi.org/ 10.1158/1940-6207.CAPR-09-0152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shappell SB, Thomas GV, Roberts RL, Herbert R, Ittmann MM, Rubin MA, Humphrey PA, Sundberg JP, Rozengurt N, Barrios R, et al. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res 2004; 64:2270-305; PMID:15026373; http://dx.doi.org/ 10.1158/0008-5472.CAN-03-0946 [DOI] [PubMed] [Google Scholar]
- 52. Hajkova P, el-Maarri O, Engemann S, Oswald J, Olek A, Walter J. DNA-methylation analysis by the bisulfite-assisted genomic sequencing method. Methods Mol Biol 2002; 200:143-54; PMID:11951649 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


