Abstract
Arbuscular mycorrhiza (AM) is established by the entry of AM fungi into the host plant roots and the formation of symbiotic structures called arbuscules. The host plant supplies photosynthetic products to the AM fungi, which in return provide phosphate and other minerals to the host through the arbuscules. Both partners gain great advantages from this symbiotic interaction, and both regulate AM development. Our recent work revealed that gibberellic acids (GAs) are required for AM development in the legume Lotus japonicus. GA signaling interact with symbiosis signaling pathways, directing AM fungal colonization in host roots. Expression analysis showed that genes for GA biosynthesis and metabolism were induced in host roots around AM fungal hyphae, suggesting that the GA signaling changes with both location and time during AM development. The fluctuating GA concentrations sometimes positively and sometimes negatively affect the expression of AM-induced genes that regulate AM fungal infection and colonization.
Keywords: arbuscular mycorrhiza, gibberellin, Lotus japonicus, Rhizophagus irregularis, symbiosis
Abbreviations
- AM
arbuscular mycorrhiza
- GA
gibberellic acid
- GUS
β- glucuronidase
- CCaMK
calcium/calmodulin-dependent protein kinase
Gibberellin distribution in the host root during AM development
Gibberellins are homeostatically controlled in plants, and excess up- or downregulation of their biosynthesis or signaling causes abnormal physiological responses: for example, enhanced GA signaling causes shoot elongation, whereas inhibition induces dwarf phenotypes.1,2 Excess amounts of GAs or overloading of GA signaling inhibits AM fungal colonization in the host root, indicating that GA functions as a negative regulator in AM.3 Conversely, suppression of GA biosynthesis or signaling during AM development causes abnormal morphology of AM colonization in roots of L. japonicus.4 These results indicate that proper regulation of GA signaling is essential for AM development.
Active and inactive forms of GA accumulate in roots of L. japonicus infected with the AM fungus Rhizophagus irregularis.4 Although we could not measure actual GA amounts in tissues or cells, histochemical analyses using fusions of β-glucuronidase (GUS) with promoters of genes for GA biosynthesis (GA20ox1, GA20ox2) and metabolism (GA2ox1) indicates that GA biosynthesis/metabolism is upregulated around AM hyphae in the host root.4 During the early stage of infection, when AM hyphae of R. irregularis attached to or entered the host root, GUS staining was detected in epidermal and cortical cells near the sites of fungal attachment or entrance. Following hyphal elongation, it extended to the cortical cells, and strong staining in the arbuscule-containing cells indicated active controls of GA concentrations in these cells. This histochemical expression analysis indicates that GA levels change during AM development, causing different effects on the symbiotic responses and AM fungal colonization.
Hyphal branching in host tissues and pre-penetration apparatus
The regulation of AM fungal colonization by GA signaling is associated with interaction with the symbiosis signaling pathway and AM-induced gene expression (Fig. 1).4 We think that the negative effects of GA on hyphal entry into the host root are caused by interference with AM-induced genes such as RAM1 and RAM2, whereas the positive effect of GA on hyphal branching is closely related to the expression of AM-induced genes such as SbtM1 and the formation of the pre-penetration apparatus (PPA).5 Transgenic roots carrying gain-of-function calcium/calmodulin-dependent protein kinase (CCaMK) showed the induction of SbtM1 expression without AM fungal infection and the formation of PPA-like structures was observed in cortical cells in which SbtM1 expression was highly induced.6 The expression of SbtM1 induced by gain-of-function CCaMK was clearly reduced in the presence of a GA biosynthesis inhibitor, which decreased the cells containing a PPA-like structure.4 The decrease in the formation of PPA-like structures would be directly linked to the reduction in hyphal branching under low-GA conditions. The branched hyphae of R. irregularis elongate mainly between the cells, or sometimes penetrate the cells accompanied by PPA formation in L. japonicus.6 Therefore, the decrease in the formation of PPA-like structures induced by gain-of-function CCaMK under low-GA conditions is directly linked to the reduction in hyphal branching in the host root.
Figure 1.
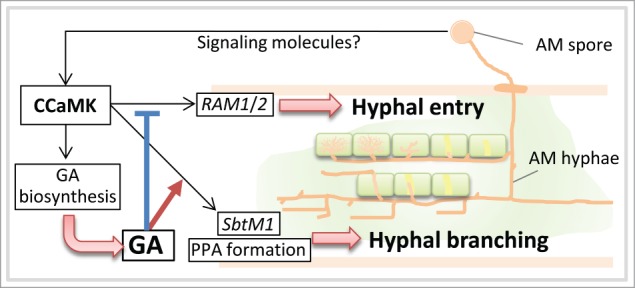
A model of the interference of GA signaling with AM-induced gene expression and AM fungal colonization. The expression of AM-induced genes SbtM1, RAM1, and RAM2 and genes for GA biosynthesis is induced by an AM signaling factor via symbiotic signaling kinase CCaMK. The same signal also induces the formation of the pre-penetration apparatus (PPA). The induction of GA biosynthesis/metabolism enzymes changes the GA concentration, which alters GA signaling in the host root. Elevated GA signaling inhibits the expression of AM-induced genes (such as RAM1 and RAM2), resulting in the inhibition of hyphal entry into the host root. On the other hand, GA signaling promotes or maintains the expression of other AM-induced genes (such as SbtM1) and PPA formation, which would promote hyphal branching in the host cortex.
Intercellular hyphal branching between the cells should also be inhibited in low-GA conditions.4 The mechanism of hyphal elongation in the intercellular spaces is largely unknown. However, a recent genomic sequence analysis of R. irregularis revealed that the fungus lacks cell-wall–degrading enzymes, which are required for invasion into the host plant and are usually abundant in pathogenic and ectomycorrhizal fungi.7 This fact suggests that the host plant should support hyphal elongation by loosening the intercellular spaces. Putative cell-wall–degrading enzymes, such as pectinesterases, galactosidases, xyloglucan endoglucanases, and proteases, were upregulated during AM formation in the host plant.4,8 GAs induce cell extension in company with reconstruction of cell walls by inducing cell-wall–degrading enzymes.9 The accumulation of GAs during AM formation might induce such enzymes, reducing cell wall stiffness around inner AM hyphae. In addition, SbtM1, whose induction is also promoted by GA signaling, is a serine protease with a secretion signal peptide and digests apoplastic proteins during AM fungal infection.10 Although substrates of SbtM1 have not been isolated, SbtM1 or other AM-induced proteases might digest structural proteins of the cell wall, facilitating the intercellular hyphal elongation of AM fungi.
Does only the host plant make and respond to GA?
In our study, we assumed that only the host plant produces GA, and considered the effects only on the host plant. However, strigolactones influence AM hyphal development inside and outside the host roots,11 indicating that plant hormones can act as symbiosis signaling factors also for the microbial symbiont. Moreover, mycorrhizal fungi produce molecules with GA-like activity.12,13 The pathogenic fungus Gibberella fujikuroi secretes a GA which reduces host resistance and enhances colonization of the host plant.14 Thus, it might be important to consider GA biosynthesis and signaling in both the host plant and AM fungi to fully understand the interrelationship between GA signaling and AM development.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank M. Nagae from National institute for Basic Biology for valuable comments on this manuscript.
Funding
This work was supported by the Japan Society for the Promotion of Science (grant no. 24770050), the Grant for Basic Science Research Projects from the Sumitomo Foundation (grant no. 110044), and the Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries, and Food Industry (grant no. 250023A).
References
- 1.Ikeda A, Ueguchi-Tanaka M, Sonoda Y, Kitano H, Koshioka M, Futsuhara Y, Matsuoka M, Yamaguchi J. slender rice, a constitutive gibberellin response mutant, is caused by a null mutation of the SLR1 gene, an ortholog of the height-regulating gene GAI/RGA/RHT/D8. Plant Cell 2001; 13:999-1010; PMID:11340177; http://dx.doi.org/ 10.1105/tpc.13.5.999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ueguchi-Tanaka M, Ashikari M, Nakajima M, Itoh H, Katoh E, Kobayashi M, Chow TY, Hsing YI, Kitano H, Yamaguchi I, Matsuoka M. GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature 2005; 437:693-8; http://www.nature.com/nature/journal/v437/n7059/full/nature04028.html; PMID:16193045; http://dx.doi.org/ 10.1038/nature04028 [DOI] [PubMed] [Google Scholar]
- 3.El Ghachtouli N, Martin-Tanguy J, Paynot M, Gianinazzi S. First report of the inhibition of arbuscular mycorrhizal infection of Pisum sativum by specific and irreversible inhibition of polyamine biosynthesis or by gibberellic acid treatment. FEBS Letts 1996; 385:189-92; http://www.sciencedirect.com/science/article/pii/0014579396003791; PMID:8647248; http://dx.doi.org/ 10.1016/0014-5793(96)00379-1 [DOI] [PubMed] [Google Scholar]
- 4.Takeda N, Handa Y, Tsuzuki S, Kojima M, Sakakibara H, Kawaguchi M.. Gibberellins interfere with symbiosis signaling and gene expression, and alter colonization by arbuscular mycorrhizal fungi in Lotus japonicus. Plant Physiol 2015; 167:545-57; http://www.plantphysiol.org/content/167/2/545.full; PMID:25527715; http://dx.doi.org/ 10.1104/pp.114.247700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Genre A, Chabaud M, Timmers T, Bonfante P, Barker DG.. Arbuscular mycorrhizal fungi elicit a novel intracellular apparatus in Medicago truncatula root epidermal cells before infection. Plant Cell 2005; 17:3489-99; http://www.plantcell.org/content/17/12/3489.long; PMID:16284314; http://dx.doi.org/ 10.1105/tpc.105.035410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takeda N, Maekawa T, Hayashi M.. Nuclear-localized and deregulated calcium- and calmodulin-dependent protein kinase activates rhizobial and mycorrhizal responses in Lotus japonicus. Plant Cell 2012; 24:810-22; http://www.plantcell.org/content/24/2/810.long; PMID:22337918; http://dx.doi.org/ 10.1105/tpc.111.091827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tisserant E, Malbrei M, Kuo A, Kohler A, Symeonidi A, Balestrini R, Charron P, Duensing N, Frei dit Frey N, Gianinazzi-Pearson V, et al.. Genome of an arbuscular mycorrhizal fungus provides insight into the oldest plant symbiosis. Proc Natl Acad Sci USA 2013; 110:20117-22; http://www.pnas.org/content/110/50/20117.long; PMID:24277808; http://dx.doi.org/ 10.1073/pnas.1313452110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maldonado-Mendoza IE, Dewbre GR, Blaylock L, Harrison MJ.. Expression of a xyloglucan endotransglucosylase/hydrolase gene, Mt-XTH1, from Medicago truncatula is induced systemically in mycorrhizal roots. Gene 2005; 345:191-7; http://www.sciencedirect.com/science/article/pii/S0378111904006523; PMID:15716119; http://dx.doi.org/ 10.1016/j.gene.2004.10.028 [DOI] [PubMed] [Google Scholar]
- 9.Jan A, Yang G, Nakamura H, Ichikawa H, Kitano H, Matsuoka M, Matsumoto H, Komatsu S. Characterization of a xyloglucan endotransglucosylase gene that is up-regulated by gibberellin in rice. Plant Physiol 2004; 136:3670-81; http://www.plantphysiol.org/content/136/3/3670.long; PMID:15516498; http://dx.doi.org/ 10.1104/pp.104.052274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takeda N, Sato S, Asamizu E, Tabata S, Parniske M.. Apoplastic plant subtilases support arbuscular mycorrhiza development in Lotus japonicus. Plant J. 2009; 58:766-77; http://onlinelibrary.wiley.com/doi/10.1111/j.1365–313X.2009.03824.x/full; PMID:19220794; http://dx.doi.org/ 10.1111/j.1365-313X.2009.03824.x [DOI] [PubMed] [Google Scholar]
- 11.Akiyama K, Matsuzaki K, Hayashi H.. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 2005; 435:824-7; http://www.nature.com/nature/journal/v435/n7043/full/nature03608.html; PMID:15944706; http://dx.doi.org/ 10.1038/nature03608 [DOI] [PubMed] [Google Scholar]
- 12.Strzelczyk E, Sitek J, Kowalski S.. Production of gibberellin-like substances by fungi isolated from mycorrhizae of pine (Pinus silvestris/L.). Acta microbiologica Polonica. Series B: Microbiologia applicate 1975; 7:145-53; PMID:117264; http://link.springer.com/article/10.1007%2FBF02197150 [PubMed] [Google Scholar]
- 13.Barea JM, Azcon-Aguilar C.. Production of plant growth-regulating substances by the vesicular-arbuscular mycorrhizal fungus Glomus mosseae. Appl Env Microbiol 1982; 43:810-3; PMID:16345991; http://aem.asm.org/content/43/4/810.long [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiemann P, Sieber CMK, Von Bargen KW, Studt L, Niehaus EM, Espino JJ, Huß K, Michielse CB, Albermann S, Wagner D, et al.. Deciphering the cryptic genome: genome-wide analyses of the rice pathogen Fusarium fujikuroi reveal complex regulation of secondary metabolism and novel metabolites. PLoS Pathog 2013: 9:e1003475; http://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1003475; PMID:23825955; http://dx.doi.org/ 10.1371/journal.ppat.1003475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takeda N, Handa Y, Tsuzuki S, Kojima M, Sakakibara H, Kawaguchi M.. Gibberellins interfere with symbiosis signaling and gene expression, and alter colonization by arbuscular mycorrhizal fungi in Lotus japonicus. Plant Physiology 2015; 167:545-57; http://www.plantphysiol.org/content/167/2/545.full; PMID:25527715; http://dx.doi.org/ 10.1104/pp.114.247700 [DOI] [PMC free article] [PubMed] [Google Scholar]


