Abstract
Monoclonal antibodies (mAbs) play an increasing important role in the therapeutic armamentarium against multiple sclerosis (MS), an inflammatory and degenerative disorder of the central nervous system. Most of the mAbs currently developed for MS are immunomodulators blocking the inflammatory immune process. In contrast with mAbs targeting immune function, GNbAC1, a humanized IgG4 mAb, targets the multiple sclerosis associated retrovirus envelope (MSRV-Env) protein, an upstream factor in the pathophysiology of MS. MSRV-Env protein is of endogenous retroviral origin, expressed in MS brain lesions, and it is pro-inflammatory and toxic to the remyelination process, by preventing the differentiation of oligodendrocyte precursor cells. We present the preclinical and early clinical development results of GNbAC1. The specificity of GNbAC1 for its endogenous retroviral target is described. Efficacy of different mAb versions of GNbAC1 were assessed in MSRV-Env induced experimental allergic encephalitis (EAE), an animal model of MS. Because the target MSRV-Env is not expressed in animals, no relevant animal model exists for a proper in vivo toxicological program. An off-target 2-week toxicity study in mice was thus performed, and it showed an absence of safety risk. Additional in vitro analyses showed an absence of complement or antibody-dependent cytotoxicity as well as a low level of cross-reactivity to human tissues. The first-in-man clinical study in 33 healthy subjects and a long-term clinical study in 10 MS patients showed that GNbAC1 is well tolerated in humans without induction of immunogenicity and that it induces a pharmacodynamic response on MSRV biomarkers. These initial results suggest that the mAb GNbAC1 could be a safe long-term treatment for patients with MS with a unique therapeutic mechanism of action.
Keywords: multiple sclerosis, monoclonal antibody, drug safety, toxicology, human endogenous retrovirus, neurotoxicity, HERV-W, Syncytin, MSRV
Abbreviations
- AE
adverse events
- ADCC
antibody-dependent cell-mediated cytotoxicity
- AUC
area under the curve
- BLAST
Basic Local Alignment Search Tool
- CDC
complement-dependent cytotoxicity
- CDR
complementarity-determining regions
- ch-GNbAC1
chimeric version of mAb GNbAC1
- Cmax
maximal concentration
- Cmin
minimal concentration
- HERV-W
human endogenous retrovirus type W
- HLA
human leukocyte antigen
- mAb
monoclonal antibody
- MOG
myelin oligodendrocyte glycoprotein
- MS
multiple sclerosis
- MSRV
multiple sclerosis associated retrovirus
- MSRV-Env
multiple sclerosis associated retrovirus envelope protein
- mu-GNbAC1
murine version of mAb GNbAC1
- PBMC
peripheral blood mononuclear cell
- SU
surface domain
- SAE
serious adverse event
- TLR4
Toll-like receptor 4
Introduction
Multiple sclerosis (MS) is an inflammatory and demyelinating degenerative disorder of the central nervous system (CNS) of unknown etiology.1 Monoclonal antibodies (mAbs) play an increasing role in the treatment of MS due to their high target specificity and their biological potency.2 So far, registered mAbs and those in late clinical development have been designed to interact with molecules and cells involved in the immune response. For example, the first mAb to be registered for MS was natalizumab, which binds to α4β1/α4β7 integrin present on the surface of lymphocytes, thus preventing the lymphocytes from penetrating into the CNS.3 Alemtuzumab, recently registered in Europe, Australia and Canada, is the second mAb to be approved in MS. It is a humanized IgG1 mAb that specifically targets CD52 receptors expressed on the surface of B and T cells, thereby inducing depletion of lymphocytes over the long term.4 Several other mAbs are currently in clinical development for MS, including daclizumab, ocrelizumab, ofatumumab, tabalumab, secukinumab and BIIB033.5 Most of the mAbs currently marketed or developed in MS have an immunomodulatory or immunosuppressive mode of action impacting essentially on the immune-driven inflammatory process.
Among the mAbs in clinical development for MS is GNbAC1, which is a humanized IgG4 mAb. It is the only mAb targeting the envelope (Env) protein of an active element from the human endogenous retrovirus type W family (HERV-W), also named multiple sclerosis associated retrovirus (MSRV).6 MSRV appears to play a critical role in MS physiopathology. For example, MSRV expression is associated with distinct clinical features in MS patient populations, and its presence in the cerebrospinal fluid correlates with clinical progression and prognosis of MS.7 In post-mortem brain tissue from MS patients, the MSRV-Env protein is expressed in MS plaques, and its level of expression correlates with the plaque activity.8 MSRV-Env comprises 3 domains: the signal peptide, the surface domain or ectodomain (SU) and the transmembrane domain (TM). The SU domain is an agonist of the Toll-like receptor 4 (TLR4).9 By activating the TLR4 pathway, MSRV-Env induces the release of pro-inflammatory cytokines such as IL-1ß, IL-6 or TNF-α from peripheral blood mononuclear cells (PBMC) and microglial cells from the central nervous system (CNS).9,10 Moreover, MSRV-Env induces the blockade of the oligodendrocyte precursor cell differentiation, which is necessary for the remyelination process in MS lesions; this pathogenic effect is also mediated by TLR4 interaction.11 Both the pro-inflammatory effect and the oligodendrocyte toxicity point to MSRV-Env protein as a relevant therapeutic target for MS.
The particular therapeutic interest of the MSRV-Env target comes from the fact that this protein is highly expressed in patients, most specifically in brain demyelinating lesions, and it has no known role in the normal human physiology. MSRV proteins, and more generally proteins from the HERV-W family, appear to be immunologically tolerated in humans, thus not eliciting significant and specific antibody production when abnormally expressed.12 The gene encoding the MSRV-Env protein belongs to the HERV-W family of retroviral elements, which are widely and heterogeneously dispersed in the human genome following the integration of exogenous retroviruses that have infected the germ line of their host since nearly 25 million years ago.13,14 The HERV elements represent approximately 8% of the human genomic sequences. From a phylogenic perspective, HERV-W elements, to which MSRV belongs, shares strong homologies with other endogenous or exogenous retroviruses as it can be seen from the phylogenic tree according to Weiss et al.15 (Fig. 1).
Figure 1.
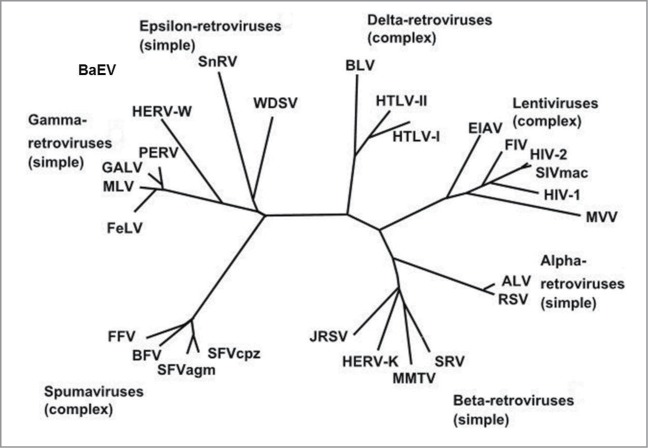
Phylogeny of endogenous and exogenous retroviruses according to Weiss et al.15 Alpha, β, gamma and epsilon retroviruses have simple genomes, lentiviruses, deltaviruses and spuma viruses have complex genomes.
Based on these considerations, a recombinant DNA-derived humanized mAb was developed that selectively binds with high affinity to the SU domain of the MSRV-Env protein and neutralizes its TLR4 binding potential without interacting with this receptor.10 This mAb, GNbAC1, may act as an innovative treatment for MS by blocking a factor that now appears to play a critical pathophysiological role.16 GNbAC1 is currently developed as a first line therapy for MS. The discovery and the results of preclinical and early clinical development studies of GNbAC1 are presented here.
Results
Target identification and sequence specificity
To optimize safety and efficacy, the MSRV-Env target identification and specificity characterization were needed. Based on the Basic Local Alignment Search Tool (BLAST) sequence comparison results are shown in Figure 2, no identical protein to MSRV-Env was found in humans or animals apart from this MSRV virion-associated copy itself. Only the related HERV-W Syncytin protein, also known as Enverin,17 which is encoded by a single locus (ERVWE1) on chromosome 718,19 and expressed in human placenta, shares 81% identity with MSRV-Env.20 Syncytin is aligned and identified by its characteristic deletion of 4 amino acids in the C-terminus part (EAVK—LQMEPK, for Syncytin including 4 amino acid deletion (dots) versus EAVKLQIVLQMEPQ for MSRV-Env protein). Syncytin expression is tigthly regulated and restricted to placenta syncytio-trophoblast during pregnancy.21
Figure 2.

Sequence alignments of MSRV-Env and comparison by analysis with the Basic Local Alignment Search Tool (BLAST).
Discovery and development of GNbAC1
The parent MSRV-Env-specific mouse mAb mu-GNbAC1 (IgG1/kappa) was obtained by immunizing mice with recombinant MSRV-Env protein expressed from a cloned RT-PCR amplicon from purified extracellular MSRV virions. The lead product, mu-GNbAC1, was selected based on its ability to neutralize the induction of pro-inflammatory cytokines by MSRV-Env in PBMC cultures.10 As shown in Figure 3, it was also selected as being able to bind to the full length MSRV-Env (called Env-T, Fig. 3A), as well as to the ectodomain MSRV-Env-SU, compared to other mAb candidates that do not bind to MSRV-Env-SU but to epitopes in other domains as the pro-peptide and the transmembrane unit. (Fig. 3B). The best binding activity against the different MSRV-Env preparations was noted for the lead candidate.
Figure 3.
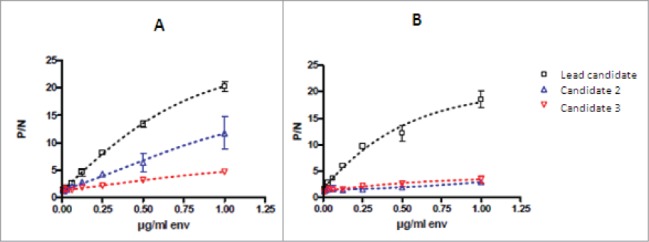
Binding activity of mAb candidates to the full length MSRV-Env-T (A) and to the surface unit MSRV-Env-SU (B). MSRV-Env-SU is a 33-kD and 293 amino acid fraction of the full-length MSRV-Env protein; serial dilutions of the MSRV-Env preparations were bound to the ApoH pre-coated microtiter wells which were then incubated with a constant amount of mAb. Results are presented as the ratio of P/N (P = signal of antibody with MSRV-Env and N = signal of antibody without MSRV-Env) showing mean values of 3 independents experiments.
Before the final humanization step, interim forms were produced consisting of a chimeric IgG1 immunoglobulin (ch-GNbAC1-IgG1) and a chimeric IgG4 immunoglobulin (ch-GNbAC1-IgG4).
Finally, a humanized version of the antibody, GNbAC1, that fully retains the binding properties of the parent murine form was developed via an in silico design based on the amino acid sequence of the murine parental antibody. GNbAC1 is a full-length antibody of the IgG4/kappa subclass. To stabilize the interchain disulfide bridges of the IgG4 molecule, site-directed mutagenesis in the core region was performed. GNbAC1 has a molecular weight of approximately 147 KDa and binds to MSRV-Env with an affinity (KD) of 2.2 nM. The specificity and the biological activity of the mAb during the humanization process were determined with in vitro assays and in vivo with experimental allergic encephalitis (EAE) models induced by MSRV-Env.22
Animal models
Assessment of therapeutic efficacy of mu-GNbAC1 and chimeric ch-GNbAC-IgG1 and ch-GNbAC1-IgG4 constructs in MSRV-Env induced EAE is presented in Figure 4. The efficacy of intermediate constructs during the mAb humanization process was assessed, and the efficacy of IgG4 vs. IgG1 was compared in this model. As shown in Figure 4, reversal of clinical score kinetics toward healing (please refer to the Methods section for clinical score description) was observed in all groups treated with the different versions of GNbAC1. All untreated animals died (or had to be euthanized because of complete paralysis) after day 28, all mice treated with ch-GNbAC1 mAbs survived, in the mu-GNbAC1 group, 2 mice did not survive, after day 28 and 35, respectively. The efficacy of ch-GNbAC1- IgG4 antibody was similar to that of the ch-GNbAC1-IgG1 antibody suggesting that IgG1 effector function was not necessary to the therapeutic efficacy in this model; therefore the IgG4 molecule was selected for humanization.
Figure 4.
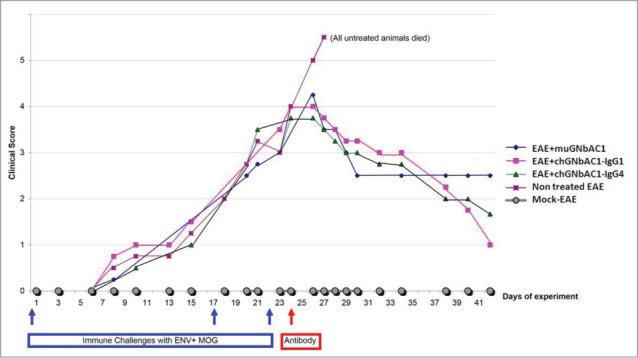
MSRV-Env experimental allergic encephalitis (EAE). Clinical scores in mock EAE negative controls, untreated EAE positive controls, mice treated with mu-GNbAC1, mice treated with ch-GNbAC1-IgG1 and mice treated with ch-GNbAC1-IgG4.
Immunoglobulin cytotoxicity
Although GNbAC1 is an IgG4 with a low likelihood of induction of antibody dependent cell-mediated cytotoxicity (ADCC) or complement-dependent cytotoxicity (CDC), these toxicities cannot be formally ruled out when MSRV-Env is expressed on the cell surface. Therefore, in vitro experiments were performed in which complement activation in the presence of transfected human cells expressing the antigen on their surface was investigated. In a similar experimental setup, PBMC or natural killer (NK) cell-mediated antibody-dependent cytotoxicity against such antigen-expressing transfectants was analyzed.
The analysis of ADCC and CDC mediated by GNbAC1 was performed using cultured HEK293 cells transiently transfected with a plasmid encoding human recombinant ENV-MSRV-ps-His-pHHB/2. The protein MSRV-Env is expressed on the surface of the transfected HEK293 cells and functions as the antigen recognized and bound by GNbAC1 antibodies. As a positive control, possibly inducing CDC or ADCC, the chimeric monoclonal ch-GNbAC1 of IgG1 isotype was used. The transfection efficiency was analyzed by flow cytometry using a fluorometric (FITC-conjugate) ch-GNbAC1 antibody. Representative results of transfection analysis are shown in Figure 5.
Figure 5.
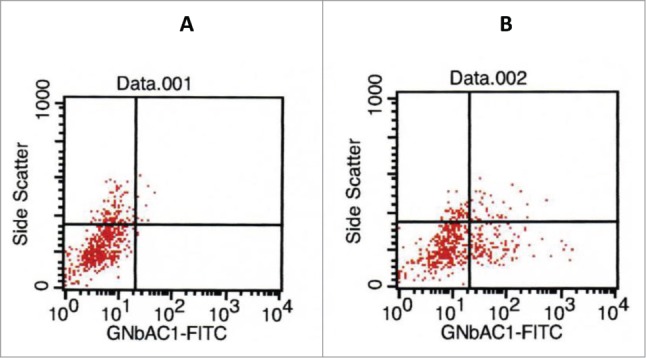
Analysis of transfection efficiency by flow cytometry. 100 μl of cells were incubated for 30 min at 4°C in the dark with 10 μl FACS flow buffer (left panel) or with 10 μl FITC-ch-GNbAC1 (right panel) and detected by flow cytometry; in this experiment 29% of transfected cells were measured for the binding of chGNbAC1.
The CDC-dependent dose-response curves of GNbAC1 isotype IgG1 (ch-GNbAC1-IgG1) and isotype IgG4 (GNbAC1), respectively, are shown in Figure 6. The isotype IgG1 induced a dose-depending signal response while isotype IgG4 did not. Maximal% cytotoxicity was calculated related to the total number of cells and to transfected cells only (14% vs 62% and 8% vs 52%, respectively). No significant change in maximal% cytotoxicity was observed when comparing different incubation times.
Figure 6.
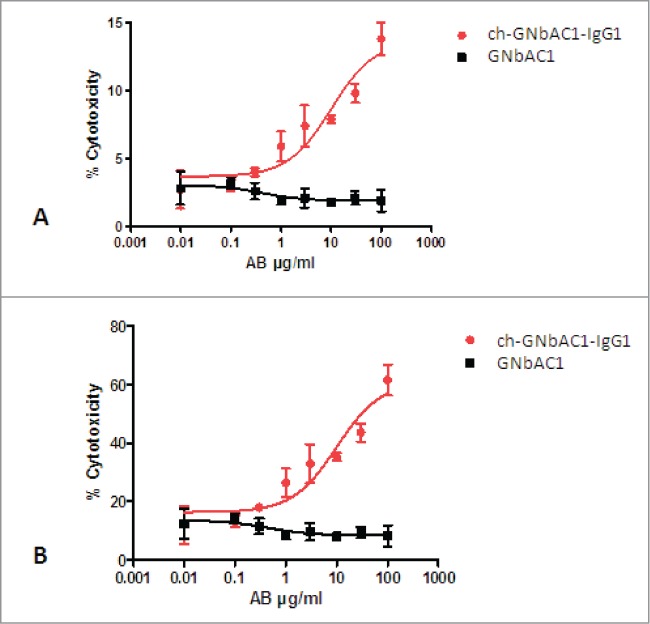
CDC-dependent dose-response curves of ch-GNbAC1-IgG1 and GNbAC1 (IgG4). (A) % cytotoxicity referring to total cells. EC50 ch-GNbAC1-IgG1: 10.12 μg/ml; EC50 GNbAC1: not calculated. (B) % cytotoxicity referring to transfected cells. EC50 ch-GNbAC1-IgG1: 10.05 μg/ml; EC50 GNbAC1: not calculated.
In conclusion, both ADCC and CDC were analyzed with both IgG1 and IgG4 isotypes of GNbAC1. Dose dependent ADCC response was not detected with any of the mAbs. A dose-dependent CDC response could be observed for the IgG1 isotype only. As expected for antibodies of IgG4 isotypes, this isotype did not show a significant CDC-mediated response or antibody-mediated cellular cytotoxicities.
In silico immunogenicity assessment
To assess potential immunogenicity, the sequence of GNbAC1 was scanned for the presence of putative human leukocyte antigen (HLA) class II restricted epitopes, also known as T helper (Th)-cell epitopes. As a general overview of the results, Table 1 shows the number of strong binders corresponding to the DRB1, DQ, DP and DRB3/4/5 genes (epitope counts). The results show that no binders were found within the constant regions or the hinge region of the antibody; overall 9 strong potential DRB1 binders were found within the variable regions VH and VL. As in the humoral response raised against an antigen, the observed Th cell activation/proliferation is generally interpreted in terms of the DRB1 specificity; we focused on this specificity. Taking into account that 10-mer overlapping peptides were used, detailed analyses of the results showed that all 9 strong potential DRB1 binders were within the complementarity-determining regions (CDR) of the antibody and none was found within the framework.
Table 1.
HLA binders corresponding to the DRB1, DQ, DP and DRB3/4/5 genes (epitope counts)
| DRB1 | DRB3/4/5 | DQ/DP | |
|---|---|---|---|
| VH | 5 | 0 | 3 |
| CH1 | 0 | 0 | 0 |
| Hinge | 0 | 0 | 0 |
| CH2 | 0 | 0 | 0 |
| CH3 | 0 | 0 | 0 |
| VL | 4 | 1 | 1 |
| CL | 0 | 0 | 0 |
| Entire Protein | 9 | 1 | 4 |
Toxicology
GNbAC1 was evaluated in a 2-week, toxicity study in mice following a single intravenous administration of GNbAC1 at 6 mg/kg and 30 mg/kg doses, representing 1× and 5×, respectively, the maximal dose administered in healthy volunteers in the Phase 1 trial. GNbAC1 serum concentrations were still quantifiable 312 hours after injection. GNbAC1 serum exposures were similar in male and female mice, and increased relatively dose‑proportionally between 6 and 30 mg/kg (Fig. 7). No GNbAC1-related clinical signs, including ophthalmological findings, were observed during the study and body weight and food consumption were considered to be unaffected by the treatment. At hematology investigations and when compared to controls, slightly higher white blood cell counts, mainly due to lymphocyte and neutrophil counts, were observed in high-dose females. The same trend was observed in some males and in low-dose females. At blood biochemistry investigations, higher glucose level was observed in high-dose males and to a lower extent in high-dose females. At pathology, no GNbAC1 treatment-related organ weight changes, macroscopic post-mortem or microscopic findings were observed. In conclusion, no effects were observed on clinical signs, body weight, food consumption or pathology; only marginally higher white blood cell counts and glucose levels were observed in animals given GNbAC1 30 mg/kg. The No Observed Adverse Effect Level (NOAEL) of GNbAC1 was established at 30 mg/kg/day.
Figure 7.
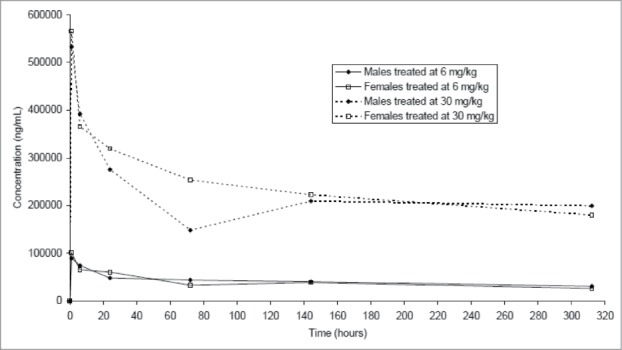
Mean GNbAC1 serum concentration-time curves after single injection of doses of 6 mg/kg and 30 mg/kg of GNbAC1 by gender in a mice toxicology study.
Human tissue cross-reactivity
Two concentrations of GNbAC1 (2 μg/ml and 10 μg/ml) were tested on 42 different human tissues. At the high (10 μg/ml) concentration, a GNbAC1-related staining considered to be specific was noted in the mature urothelium (umbrella cells) of the ureter and the urinary bladder, syncytiotrophoblasts/ trophoblasts of the placenta and superficial endometrial epithelial cells of the uterus of one single panel only. Staining of minor importance, most likely non-specific, was noted in the crypt epithelium of the intestinal tract, canaliculi of the breast and tails of spermatids in the testis. At the optimal concentration of GNbAc1 (2 μg/ml), no staining was considered to be related to the mAb.
Safety in humans
In the Phase 1 clinical study, the exposure to GNbAC1 was equivalent to 23 mAb injections or 23 subject-months. Twenty-eight adverse events (AE) were observed during this study.23 In the Phase 2a study and its 12-month extension, the exposure to GNbAC1 was equivalent to 106 mAb injections or 106 subject-months. One hundred forty AE, mostly mild to moderate and not related to treatment, were observed during this study.24,25 The total exposure to GNbAC1, cumulating both studies, was 129 injections or 10.8 subject-years. Adverse events observed with a frequency of more than 5% in the 2 studies are summarized in Table 2. Nasopharyngitis was the most frequently reported AE. Apart from this, the AEs reported were essentially associated with MS disorder or underlying medical conditions. Frequently reported AEs were gait disturbance or cystitis/leukocyturia, but were observed among MS patients and not healthy subjects. Events such as hyperglycemia, γ-glutamyl-transferase increase, sinus bradycardia and prolonged QT intervals were transient AEs of mild to moderate severity and were observed in MS patients and not in healthy subjects. Furthermore, the 2 patients presenting transient and slight hyperglycemia were known and treated for type II diabetes and both patients with bradycardia were also known for this arrhythmia. No particular changes in vital signs or ECG data apparently related to the study drug were noticed in the 2 clinical studies.
Table 2.
Consolidated overview of adverse events (AE) with GNbAC1 or placebo with a frequency >5% per group in healthy subjects and multiple sclerosis patients (SAE serious AE)
| Placebo (n = 12, 12 inj) | <2 mg/kg (n = 15, 15 inj) | 2 mg/kg (n = 9, 51inj) | 6 mg/kg (n = 9, 63inj) | All GNbAC1 (n = 33, 129 inj) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| System Organ Classes | Preferred Terms | n | % | n | % | n | % | n | % | n | % | |
| SAE | Gastrointestinal disorder | pancreatitis | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 1 | (11.1) | 1 | (3.0) |
| AE > 5% | Infections and infestations | Cystitis | 0 | (0.0) | 0 | (0.0) | 2 | (22.2) | 1 | (11.1) | 3 | (9.1) |
| Nasopharyngitis | 1 | (8.3) | 2 | (13.3) | 4 | (44.4) | 2 | (22.2) | 8 | (24.2) | ||
| Rhinitis | 0 | (0.0) | 1 | (6.7) | 1 | (11.1) | 3 | (33.3) | 5 | (15.2) | ||
| Metabolism and nutrition disorders | Hyperglycaemia | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 2 | (22.2) | 2 | (6.1) | |
| Nervous system disorder | Headache | 1 | (8.3) | 1 | (6.7) | 3 | (33.3) | 2 | (22.2) | 5 | (15.2) | |
| Spasm | 0 | (0.0) | 0 | (0.0) | 2 | (22.2) | 1 | (11.1) | 3 | (9.1) | ||
| Cardiac disorders | Sinus bradycardia | 0 | (0.0) | 0 | (0.0) | 2 | (22.2) | 2 | (22.2) | 4 | (12.1) | |
| Respiratory, thoracic and mediastinal dis. | Oropharyngeal pain | 0 | (0.0) | 1 | (6.7) | 1 | (11.1) | 2 | (22.2) | 4 | (12.1) | |
| Gastrointestinal disorders | Nausea | 0 | (0.0) | 1 | (6.7) | 0 | (0.0) | 1 | (11.1) | 2 | (6.1) | |
| Renal and urinary disorders | Leukocyturia | 0 | (0.0) | 0 | (0.0) | 1 | (11.1) | 2 | (22.2) | 3 | (9.1) | |
| Proteinuria | 0 | (0.0) | 0 | (0.0) | 1 | (11.1) | 2 | (22.2) | 3 | (9.1) | ||
| General disorders and admin.site cond. | Chest Pain | 0 | (0.0) | 0 | (0.0) | 2 | (22.2) | 0 | (0.0) | 2 | (6.1) | |
| Fatigue | 0 | (0.0) | 0 | (0.0) | 1 | (11.1) | 3 | (33.3) | 4 | (12.1) | ||
| Gait disturbance | 0 | (0.0) | 0 | (0.0) | 2 | (22.2) | 1 | (11.1) | 3 | (9.1) | ||
| Influenza-like illness | 0 | (0.0) | 0 | (0.0) | 2 | (22.2) | 0 | (0.0) | 2 | (6.1) | ||
| Investigations | ECG QT prolonged | 0 | (0.0) | 0 | (0.0) | 1 | (11.1) | 1 | (11.1) | 2 | (6.1) | |
| Hyperglycemia | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 2 | (22.2) | 2 | (6.1) | ||
| gGT increased | 0 | (0.0) | 0 | (0.0) | 2 | (22.2) | 1 | (11.1) | 3 | (9.1) | ||
| injury, poisoning, proced. complication | Hematoma | 0 | (0.0) | 0 | (0.0) | 1 | (11.1) | 1 | (11.1) | 2 | (6.1) | |
| Thermal burn | 0 | (0.0) | 0 | (0.0) | 1 | (11.1) | 1 | (11.1) | 2 | (6.1) | ||
In vivo immunogenicity assessment
In the Phase 1 study in healthy volunteers, 2 subjects treated with GNbAC1 showed reactivity prior and after dosing. One subject (dosed at 0.0025 mg/kg GNbAC1) had positive results at all time points, and the other subject (dosed at 0.15 mg/kg GNbAC1) had positive results at Baseline and at Day 64. Hence, 2 out of the 33 study subjects had pre-existing reactivity against GNbAC1. Due to the low antibody titers, clinical relevance is not likely, although an assay for the detection of the neutralizing capacity was not performed. A correlation of the measured antibodies with the in silico data is speculative currently since no clinical or pharmacokinetic (PK) effects of the measured antibodies were detectable, targeting epitopes other than the CDRs (as predicted by the in silico tools) is conceivable. Since no significant anti-drug antibody (ADA) titer was measured, and therefore no drug induced ADA were observed, no treatment-emergent antibodies against GNbAC1 appeared in any of the treated subjects throughout the entire study period of 64 d. In the Phase 2a study, in 10 MS patients followed over 12 administrations of GNbAC1 at 2 mg/kg or at 6 mg/kg, all the monthly measurements were negative.
Pharmacokinetic evaluation
Serum concentrations of GNbAC1 measured in patients in the Phase 2a study up to 63 d after the first administrations at 2 and 6 mg/kg are summarized in Figure 8 with semi-logarithmic scale presentations. The shape of the mean PK profiles suggests 2 exponential decline components. The PK of GNbAC1 after intravenous administration in humans is characterized by a rather long apparent terminal elimination half-life of approximately 27–37 d. Similarly, observed geometric mean mean residence time (MRT) values by dose range from 34 to 50 d. Mean maximal concentrations (Cmax) after the first dose for the 2 mg/kg and 6 mg/kg dose groups were 59.2 μg/ml and 165.5 μg/ml, respectively, and mean minimal concentrations (Cmin) for the same dose groups after multiple dosing were 27.3 μg/ml and 78.9 μg/ml, respectively. A single cerebro-spinal fluid (CSF) GNbAC1 concentration was obtained one month after dosing at steady state in the 6 mg/kg group; the CSF/plasma concentration ratio was 0.02%. Geometric mean clearance by dose ranged from 0.08 to 0.10 mL/kg/h. Geometric mean volume of distribution by dose ranges from 88.6 to 91.5 mL/kg. The ratios of area under the curve (AUC) and Cmax between the 2 and 6 mg/kg dose levels were between 2.2 and 2.8, supporting a linear PK of GNbAC1 within the evaluated dose range.
Figure 8.
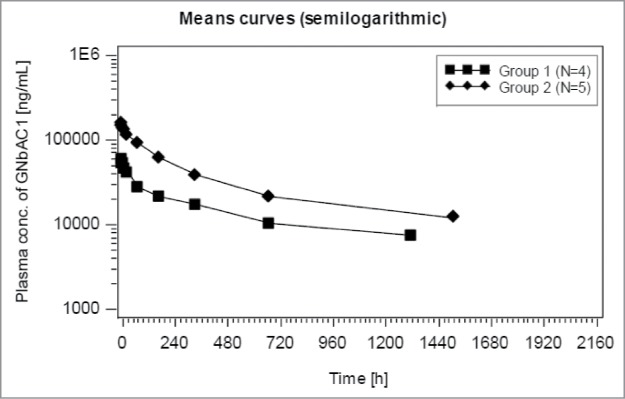
GNbAC1 blood concentration after first administration for group 1 (2 mg/kg) and group 2 (6 mg/kg) in MS patients in study GNC002; semi-logarithmic presentation.
Discussion
The IgG4 mAB GNbAC1, developed as a treatment for MS, targets the MSRV-Env protein, which appears to play a critical role in the inflammatory and demyelination processes central to the disease. The advantage of this new targeted therapeutic approach is the unique pathophysiological role of the MSRV-Env target in the disease, in the absence of any known physiological action. Notably, this approach does not modulate the immune system, which is currently the focus of most MS therapeutics development.5 Neutralization of MSRV-Env should not induce a perturbation of the normal physiology of the treated patients, and, consequently, GNbAC1 should have a favorable safety profile, which is critical for long-term treatments. The preclinical and early clinical data support this view.
In vivo preclinical models mimicking MS showed that the different versions of GNbAC1 could treat and prevent exacerbations of MSRV-Env induced EAE. In particular, the IgG4 version of the mAb appeared equally effective as the IgG1 version; this result supported the humanization of a ch-GNbAC1-IgG4 which may present a better safety profile.
Because the target is translated from endogenous retrovirus genes that are specific to humans,16 there are no valid animal models to assess toxicity. MSRV-Env is a protein translated from genes located in the human genome, but of retroviral origin showing unfixed position in the human genome and population,26 and, to a certain extent, their translation product can be considered as a foreign target. As stated in the addendum to the International Conference on Harmonisation (ICH) S6 (R1) guideline 27 “for monoclonal antibodies and other related antibody products directed at foreign targets (i.e., bacterial, viral targets etc.), a short-term safety study in one species […] can be considered.” Discussion with regulatory authorities supported the view that toxicology studies in animals would not provide valuable data leading to a meaningful outcome. Therefore a minimal toxicology program in line with ICH guidelines was chosen before Phase 1 study. The off-target acute toxicity study showed an absence of clinical or pathological effects with non-significant marginal laboratory perturbations. The No Observed Adverse Effect Level (NOAEL) for GNbAC1 was established at 30 mg/kg. In the absence of a suitable animal model expressing the native target antigen for assessment of the consequences of target modulation, no further toxicology studies in animals were performed.
Due to this limitation, and to reinforce the preclinical safety assessment, the Fc effector function of GNbAC1 was assessed. Both ADCC and CDC were analyzed with GNbAC1 IgG4, in comparison to an IgG1 isotype control (ch-GNbAC1 IgG1). Dose-dependent ADCC response was not detected in any of the mAbs. A dose-dependent CDC response could be observed for the IgG1 isotype control only. As expected for antibodies of IgG4 isotypes, this isotype did not show a significant CDC-mediated response. We conclude that GNbAC1 does not induce a complement-dependent or antibody-mediated cellular cytotoxicities. The absence of ADCC and CDC is an important aspect of the therapeutic strategy with GNbAC1 because it is designed to block MSRV-Env and its toxic action, but not to destroy cells expressing the target, such as monocytes, lymphocytes B or microglial cells.16,28 This is another critical element supporting the safety of the molecule.
In a tissue cross-reactivity study, GNbAC1 was found to bind at high concentration to mature superficial urothelium (umbrella cells), syncytiotrophoblast /trophoblast of the placenta, and superficial endometrial cells of a single subject only. At the optimal concentration, no staining was considered to be related to GNbAC1. It should be noted that the clinical concentration of GNbAC1 in the upper layer of the urothelium whose cells are connected with tight junctional complexes would be probably low. The staining of syncytiotrophoblast might be due to binding to syncytin, a protein related to MSRV-Env, and motivates a contraindication for pregnant women as a precaution.
Early clinical data representing about 10.8 subject-years of exposure show an absence of serious adverse drug reactions associated with GNbAC1. The safety profile in terms of AEs does not show any particular association with safety signals according to the duration of treatment or to the dose.23,25 The AE pattern was mainly associated with the MS disease and with pre-existing pathological conditions.25 Notably, there was no evidence for either infusion reactions or hypersensitivity, a frequent safety issue with biologicals,29 in the early clinical trials of GNbAC1. Overall, the initial safety profile of GNbAC1 is in line with the expectation raised during the preclinical development and appears to be favorable.
The immunogenicity profile of GNbAC1 was tested before administration to humans and the in-silico analyses showed that the potential binders were localized in the CDR regions, and, in a clinical setting, an anti-idiotypic response is considered more likely than an anti-isotype response. So far in humans, GNbAC1 has not shown an induction of ADA, which is in line with its sequence structure. An alteration of the PK profile due to the presence of pre-existing antibodies was not observed.
The PK of GNbAC1 appears linear in the tested dose range with half-lives between 27 to 37 d. The PK parameters of GNbAC1, computed in a small population and over a relatively short period, correspond to expected values for IgG4 mAbs.30 The CSF/plasma ratio of concentration observed in one subject is in line with the values of between 1% and 0.1% observed with immunoglobulins and therapeutic mAbs in MS.31-33 This point is important as the target is expressed in the blood on monocytes and lymphocytes B, as well as on brain microglial cells. The concentrations observed at Cmax and Cmin at steady-states are in line with plasma concentrations between 100 μg/ml to 300 μg/ml needed to bind not only targets in the plasma, but in particular those expressed in the CNS taking into account the blood-brain barrier penetration reflected by the above-mentioned CSF/plasma ratios. The metabolism of the MSRV-Env target after binding is currently unknown, but a mix of receptor-mediated clearance for target expressed on cells and of FcRn process for soluble MSRV-Env is expected.30,34 Derfuss et al showed that binding to the target induces a statistically significant decline of the MSRV transcripts for Env as well as for Pol25; declines of MSRV Env transcripts have been observed with MS reference drugs such as interferon β35 and natalizumab.36 It will be interesting to test in further clinical development whether this biomarker response will be predictive of efficacy response.
Overall, GNBAC1 presents a favorable preclinical and early clinical safety profile, and shows promise as a mAb against an innovative target relevant in MS as shown in animal models. This observation is supported by promising pharmacodynamic data in patients, showing clinical stabilization and MSRV biomarker decrease.24,25 For a chronic disorder like MS starting in young patients, there is a need for drugs for which the safety risk can be managed over the long-term.37 By targeting MSRV-Env, with its unique role in MS, we have developed a mAb that should demonstrate maximal efficacy with an optimal safety profile. Although the safety profile and the clinical efficacy need to be confirmed during the further clinical development of GNbAC1, these results are encouraging for the therapeutic armamentarium of MS.
Methods
Target sequence specificity
To identify potential analogies with other protein sequence, the sequence of the protein encoded by MSRV-Env reference sequence (Genbank: AF 331500) was aligned by the method of Basic Local Alignment Search Tool (Blast search) on worldwide protein databases accessible through the National Center for Biotechnology Information (NCBI) website (http://www.ncbi.nlm.nih.gov).
Target binding specificity
The binding activity of GNbAC1 to the surface unit (Env-SU) of MSRV-Env protein (Env-SU 0.2 mg/ml) compared to the full-length MSRV-Env protein (Env-T; 0.5 mg/ml) was determined. Env-SU is a 33-kDa and 293-amino acid fraction of the full-length MSRV-Env protein. For this purpose, serial dilutions of the Env preparations were bound to the ApoH pre-coated microtiter wells, which were then incubated with a constant amount of mAb (10 μg/ml). The capture by APOH is based on its properties to bind non-physiological molecular motifs on proteins with one domain and to have another strong binding to associated lipids (from cellular membranes or in viral particles), which stabilizes the capture of viral glycoproteins; after a washing step, the detection is feasible with a labeled mAb specifically binding to the antigen, as previously described for MSRV and other viral glycoproteins.38,39 Results are presented as the ratio of P/N (P = signal of antibody with MSRV-Env and N = signal of antibody without MSRV-Env) showing mean values of 3 independents experiments.
Kinetic analysis
Kinetic constants including the Kd values of GNbAC1 were determined utilizing the technology from Attana (Sweden). The Attana A200 system is a dual channel, continuous-flow system for automated analysis based on the quartz crystal microbalance technology. A direct assay was used to measure the interaction between immobilized GNbAC1 and the MSRV-Env protein (glycosylated form expressed in HEK293 cells) in solution.
Animal models
Pathogen free female C57BL/6 mice (6–8 weeks old) were purchased from the Charles River laboratories and maintained at the animal facilities for one week before immunization. EAE induction was obtained under previously validated conditions for MSRV-Env induced EAE.22 Clinical assessment: animals were weighed and clinically scored 5 d per week according to the following criteria: 0 = no signs; 1 = tail paralysis or hyper-reflex of hind limb(s) or unilateral hind limb weakness; 2 = bilateral hind limb or forelimb weakness; 3 = plus unilateral paralysis or major deficit; 4 = complete hind limb or forelimb paralysis; 5 = plus partial paralysis or major deficit of opposite limbs; 6 = moribund or dead. These were adapted from standard criteria, in order to reflect the more rapid induction of brain and cervical cord lesions with this model. A comparison of therapeutic efficacy was made among the parental mu-GNbAC1 and 2 chimeric forms, ch-GNbAC1-IgG1 and ch-GNbAC1-IgG4. All mice (n = 4 per group) received 3 injections of myelin oligodendrocyte glycoprotein (MOG) emulsion (200 μg/mouse in incomplete Freund Adjuvant (IFA)) with or without MSRV-Env s.c. in the dorsal neck at day 1, on the dorsal flank at day 17 and day 22. Mice from “Mock-Control” Group were injected with MOG in IFA without MSRV-Env and received no antibody, thus constituting a negative control group without EAE in the present series. The positive control group with untreated EAE in the present series is represented by “Non-treated EAE” Group without antibody injection. In addition to MOG emulsion with MSRV-Env, mu-GNbAC1 Group, ch-GNbAC1-IgG1 and ch-GNbAC1-IgG4 groups received 100 μg of their respective immunoglobulin at day 24.
Immunoglobulin cytotoxicity
For ADCC and CDC, complement activation in the presence of transfected human cells expressing the MSRV-Env antigen on their surface was investigated in vitro. The analysis of ADCC and CDC was performed using cultured HEK293 cells transfected with the recombinant plasmid ENV-MSRV-ps-His-pHHB/2 leading to a transient expression of MSRV-Env on the surface of HEK293 cells. The transfection rate was analyzed by flow cytometry using fluorometric ch-GNbAC1-IgG1 antibody. Rituximab, which is also a chimeric monoclonal IgG1 antibody but with another specificity (anti-CD20), was used as an isotype and negative control. The antibody was also used as a positive control for both the ADCC and CDC assays, which were measured by using the CytoTox-Glo™ assay from Promega (Madison, WI, USA). In parallel, the cytotoxic capacity of humanized GNbAC1 (IgG4 isotype) was analyzed. The transfection rate was analyzed by flow cytometry revealing a transfection rate of 28.9% of gated cells. For the analysis of ADCC, freshly prepared PBMCs and MACS-purified CD16+-NK-cells from a normal healthy volunteer were used as effector cells. An effector:target cell ratio of 10:1 and 25:1 for PBMC:HEK293 and 10:1 for NK cells:HEK293 were used for ADCC analyses. Target cells were incubated with GNbAC1 for 30 min at 37°C. Effector cells were added and incubated for additional 5 hours at 37°C. In a similar experimental setup, NK cell-mediated ADCC against such antigen-expressing transfectants was analyzed. CDC was measured using human serum from a normal healthy blood donor (with a normal total complement activity). Target cells were incubated with GNbAC1 for 30 min at 37°C. In all sets of experiments, 10% human serum was added and incubated for additional 5 hours at 37°C. As a control experiment the maximal cytotoxicity was measured after saponin-mediated total cell lysis. The antibody mediated% cytotoxicity was calculated compared with the transfected cells only and total cells, respectively.
Toxicological study
GNbAC1 was evaluated in a 2-week, toxicity study in mice following a single intravenous administration of GNbAC1 at 6 mg/kg and 30 mg/kg doses, representing 1× and 5×, respectively, the maximal dose administered in healthy volunteers in the Phase 1 trial. The drug was injected intravenously. Two groups of 10 male and 10 female Swiss mice received GNbAC1 by a single intravenous bolus administration at dose-levels of 6 or 30 mg/kg. The dosage forms were administered under a constant dosage-volume of 3 mL/kg. A further group of 10 males and 10 females received a control injection alone. The animals were kept for a 2-week observation period. Satellite animals (4 males and females in the control group and 18 males and females in treated groups) were allocated for toxicokinetics. Clinical signs, food consumption and body weight were monitored. Ophthalmological examinations were performed on all animals in pre-test and on control and high-dose animals at the end of the observation period. Hematology and blood biochemistry investigations were performed on designated animals at the end of the observation period. Serum levels of GNbAC1 were determined in samples collected pre-dose and 1 hour after dosing for control animals and 1, 6, 24 hours, then 4, 7 and 14 d after dosing for test item-treated animals. At the end of the observation period, the animals were sacrificed and submitted for macroscopic and microscopic post-mortem examination.
Human tissue cross-reactivity study
The potential tissue cross-reactivity of GNbAC1 in human tissues was evaluated by characterizing the immunohistochemical staining patterns of a panel of 42 human frozen tissues from different organs and one blood smear from 3 unrelated individuals. Tissues were used unfixed, except for pancreas, gall bladder and bone marrow, which were fixed in neutral buffered formalin for 10 minutes following fixation. The test item was FITC-labeled GNbAC1 with a Human IgG-FITC conjugate as control; positive and negative controls were HEK293 cells expressing MSRV-Env, HEK293 wild type cells.
Clinical studies
A Phase 1, first-in-man, clinical study performed in a double-blind, placebo-controlled, dose-escalating titration design was performed in 33 healthy subjects who do not express the MSRV-Env target.23 In dose cohort zero at dose 0.0025 mg/kg, 3 subjects received GNbAC1 i.v infusion and one subject received placebo. In dose cohort 1 at dose 0.025 mg/kg, 4 subjects received GNbAC1 i.v infusion and one subject received placebo. For the following 4 dose cohorts (respective doses of 0.15 mg/kg, 0.6 mg/kg, 2 mg/kg, 6 mg/kg), 4 subjects received GNbAC1 intravenously and 2 subjects received placebo (randomization ratio 2:1) in a sequential manner. All the 33 healthy subjects received the scheduled injections.
A Phase 2a study was performed as a single-blind, placebo-controlled dose-escalating randomized study in 10 MS patients.24 In each of 2 dose cohorts (2 mg/kg and 6 mg/kg), 4 patients received GNbAC1 and one patient received placebo (randomization ratio 4:1) in a sequential manner. Then, the patients entered in a repeated dose extension study where all 10 patients received GNbAC1 in an open-label setting for 11 additional administrations.
Study protocols and informed consents for the 2 clinical studies were approved by the local ethics committees and the Swiss Medicine Agency, Swissmedic. All subjects and patients enrolled in the studies had signed written informed consent form prior to study entry.
Pharmacokinetic analysis
The analysis of GNbAC1 in plasma and CSF in Phase 2a study was based on a competitive electrochemiluminescence (ECL)-based immunoassay using an anti-idiotypic mAb (Mab1E4F7H6) against GNbAC1 as capture antibody and ruthenylated GNbAC1 as competitive drug.23 The pharmacokinetic analysis was performed using NC_PKP.sas using SAS Version 9.2 (SAS Institute, Cary, NC, USA).
In silico immunogenicity assessment
In silico T-cell profiling for GNbAC1 was performed based on Epibase® technique. The sequence of GNbAC1 was scanned for the presence of putative human leukocyte antigen (HLA) class II restricted epitopes, also known as Th-epitopes. HLA binding specificities of all possible 10-mer peptides derived from a target sequence were analyzed. Profiling was done at the allotype level for 47 HLA class II receptors representative of the total Caucasian population.
In vivo immunogenicity assessment
For in vivo immunogenicity assessment, blood samples were taken before GNbAC1 administration and at days 29 and 64 post infusion in the Phase 1 study and before each GNbAC1 administration in the Phase 2 study to determine the immunogenic potential of GNbAC1. The screening for binding antibodies against GNbAC1 was performed by an ADA assay using a homogeneous bridging assay format utilizing the electrochemiluninescence (ECL) technology. For this assay GNbAC1 was labeled with biotin and with a ruthenium label (Sulfo-Tag™), respectively, to be used as capture antigens. After binding to anti-GNbAC1 antibodies the complex was immobilized on streptavidin coated ECL-specific microtiter plates and was detected. This approach enables the detection of all isotypes. Affinity purified monoclonal anti-idiotypic GNbAC1 antibodies served as controls. The assay screening cut point of 246 RLU was determined after analysis of serum from 51 normal blood donors by the calculation of the mean reactivity plus 1.645 standard deviation (SD) resulting in a specificity of 95%. A confirmatory assay cut point was determined by immune depletion experiments using the same 51 serum samples as used for the determination of the screening assay cut point. Applying a 99.9% confidence interval a confirmatory cut point of 38.2% was calculated. A quasi-quantitation of positive study samples was performed by titer analysis.
Disclosure of Potential Conflicts of Interest
FC, HPo, HPe, and ABL are employees of GeNeuro SA; FC, HPe, and ABL are shareholders of GeNeuro SA.
Funding
The studies to develop GNbAC1 were funded by GeNeuro SA, Geneva, Switzerland. The studies were funded by GeNeuro SA, Switzerland.
Author Contributions
FC wrote the manuscript and supervised the clinical studies, HPo supervised the clinical studies, HPe supervised the preclinical studies, ABL supervised the molecule development. All co-authors reviewed the paper. AK performed the immunogenicity and PK analysis.
References
- 1. McFarland HF, Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol 2007; 8:913-9; PMID:17712344; http://dx.doi.org/ 10.1038/ni1507 [DOI] [PubMed] [Google Scholar]
- 2. Fontoura P. Monoclonal antibody therapy in multiple sclerosis. mAbs 2010; 2:670-81; PMID:21124072; http://dx.doi.org/ 10.4161/mabs.2.6.13270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hoepner R, Faissner S, Salmen A, Gold R, Chan A. Efficacy and side effects of natalizumab therapy in patients with multiple sclerosis. J Cent Nerv Syst Dis 2014; 6:41-9; PMID:24855407; http://dx.doi.org/ 10.4137/JCNSD.S14049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coles AJ. Alemtuzumab therapy for multiple sclerosis. Neurotherapeutics 2013; 10:29-33; PMID:23184314; http://dx.doi.org/ 10.1007/s13311-012-0159-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Curtin F, Hartung HP. Novel therapeutic options for multiple sclerosis. Expert Rev Clin Pharmacol 2014; 7:91-104; PMID:24325127; http://dx.doi.org/ 10.1586/17512433.2014.865517 [DOI] [PubMed] [Google Scholar]
- 6. Perron H, Garson JA, Bedin F, Beseme F, Paranhos-Baccala G, Komurian-Pradel F, Mallet F, Tuke PW, Voisset C, Blond JL, et al. Molecular identification of a novel retrovirus repeatedly isolated from patients with multiple sclerosis. The collaborative research group on multiple sclerosis. Proc Natl Acad Sci U S A 1997; 94:7583-8; PMID:9207135; http://dx.doi.org/ 10.1073/pnas.94.14.7583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sotgiu S, Mameli G, Serra C, Zarbo IR, Arru G, Dolei A. Multiple sclerosis-associated retrovirus and progressive disability of multiple sclerosis. Mult Scler 2010; 16:1248-51; PMID:20685761; http://dx.doi.org/ 10.1177/1352458510376956 [DOI] [PubMed] [Google Scholar]
- 8. Mameli G, Astone V, Arru G, Marconi S, Lovato L, Serra C, Sotgiu S, Bonetti B, Dolei A. Brains and peripheral blood mononuclear cells of multiple sclerosis (MS) patients hyperexpress MS-associated retrovirusHERV-W endogenous retrovirus, but not Human herpesvirus 6. J Gen Virol 2007; 88:264-74; PMID:17170460; http://dx.doi.org/ 10.1099/vir.0.81890-0 [DOI] [PubMed] [Google Scholar]
- 9. Rolland A., Jouvin-Marche E, Saresella M, Ferrante P, Cavaretta R, Créange A, Marche P, Perron H. Correlation between disease severity and in vitro cytokine production mediated by MSRV (multiple sclerosis associated retroviral element) envelope protein in patients with multiple sclerosis. J Neuroimmunol 2005; 160:195-203; PMID:15710473; http://dx.doi.org/ 10.1016/j.jneuroim.2004.10.019 [DOI] [PubMed] [Google Scholar]
- 10. Rolland A, Jouvin-Marche E, Viret C, Faure M, Perron H, Marche PN. The envelope protein of a human endogenous retrovirus-W family activates innate immunity through CD14TLR4 and promotes Th1-like responses. J Immunol 2006; 176:7636-44; PMID:16751411; http://dx.doi.org/ 10.4049/jimmunol.176.12.7636 [DOI] [PubMed] [Google Scholar]
- 11. Kremer D1, Schichel T, Förster M, Tzekova N, Bernard C, van der Valk P, van Horssen J, Hartung HP, Perron H, Küry P. Human endogenous retrovirus type W envelope protein inhibits oligodendroglial precursor cell differentiation. Ann Neurol 2013; 74:721-32; PMID:23836485; http://dx.doi.org/ 10.1002/ana.23970 [DOI] [PubMed] [Google Scholar]
- 12. Ruprecht K, Gronen F, Sauter M, Best B, Rieckmann P, Mueller-Lantzsch N. Lack of immune responses against multiple sclerosis-associated retrovirushuman endogenous retrovirus W in patients with multiple sclerosis. J Neurovirol 2008; 14:143-51; PMID:18444086; http://dx.doi.org/ 10.1080/13550280801958922 [DOI] [PubMed] [Google Scholar]
- 13. Voisset C, Blancher A, Perron H, Mandrand B, Mallet F, Paranhos-Baccala G. Phylogeny of a novel family of human endogenous retrovirus sequences, HERV-W, in humans and other primates. AIDS Res Hum Retroviruses 1999; 15:1529-33; PMID:10580403; http://dx.doi.org/ 10.1089/088922299309810 [DOI] [PubMed] [Google Scholar]
- 14. Belshaw R, Pereira V, Katzourakis A, Talbot G, Paces J, Burt A, Tristem M. Long-term reinfection of the human genome by endogenous retroviruses. Proc Natl Acad Sci U S A 2004; 101:4894-9; PMID:15044706; http://dx.doi.org/ 10.1073/pnas.0307800101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weiss RA. The discovery of endogenous retroviruses. Retrovirology 2006; 3:67; PMID:17018135; http://dx.doi.org/ 10.1186/1742-4690-3-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Perron H, Germi R, Bernard C, Garcia-Montojo M, Deluen C, Farinelli L, Faucard R, Veas F, Stefas I, Fabriek BO, et al. Human endogenous retrovirus type W envelope expression in blood and brain cells provides new insights into multiple sclerosis disease. Mult Scler 2012; 18:1721-36; PMID:22457345; http://dx.doi.org/ 10.1177/1352458512441381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alliel PM, Perin JP, Goudou D, Bitoun M, Robert B, Rieger F. The HERV-W7q family in the human genome. Potential for protein expression and gene regulation. Cell Mol Biol (Noisy-le-Grand) 2002; 48:213-17; PMID:11990458 [PubMed] [Google Scholar]
- 18. Blond JL, Beseme F, Duret L, Bouton O, Bedin F, Perron H, Mandrand B, Mallet F. Molecular characterization and placental expression of HERV-W, a new human endogenous retrovirus family. J Virol 1999; 73:1175-85; PMID:9882319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Voisset C, Bouton O, Bedin F, Duret L, Mandrand B, Mallet F, Paranhos-Baccala G. Chromosomal distribution and coding capacity of the human endogenous retrovirus HERV-W family. AIDS Res Hum Retroviruses 2000; 16:731-40; PMID:10826480; http://dx.doi.org/ 10.1089/088922200308738 [DOI] [PubMed] [Google Scholar]
- 20. Perron H, Rolland A, Marche P, Jouvin-Marche E, Inventors; Biomerieux, INSERM Assignees. Composition for treating pathology associated with MSRV/HERV-W. US patent US7666420B2 2010 Feb 23 [Google Scholar]
- 21. Trejbalová K, Blazková J, Matousková M, Kucerová D, Pecnová L, Vernerová Z, Herácek J, Hirsch I, Hejnar J. Epigenetic regulation of transcription and splicing of syncytins, fusogenic glycoproteins of retroviral origin. Nucleic Acids Res 2011; 39:8728-39; PMID:21771862; http://dx.doi.org/ 10.1093/nar/gkr562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Perron H, Dougier-Reynaud HL, Lomparski C, Popa I, Firouzi R, Bertrand JB, Marusic S, Portoukalian J, Jouvin-Marche E, Villiers CL, et al. Human endogenous retrovirus protein activates innate immunity and promotes experimental allergic encephalomyelitis in mice. PLoS One 2013; 8:e80128; PMID:24324591; http://dx.doi.org/ 10.1371/journal.pone.0080128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Curtin F, Lang AB, Perron H, Laumonier M, Vidal V, Porchet HC, Hartung HP. GNbAC1, a humanized monoclonal antibody against the envelope protein of multiple sclerosis-associated endogenous retrovirus: a first-in-humans randomized clinical study. Clin Ther 2012; 34:2268-78; PMID:23200102; http://dx.doi.org/ 10.1016/j.clinthera.2012.11.006 [DOI] [PubMed] [Google Scholar]
- 24. Derfuss T, Curtin F, Lang AB, Perron H, Kappos L, Lalive P. GNbAC1, a humanised monoclonal antibody against the multiple sclerosis associated retrovirus envelope protein is well tolerated in patients with multiple sclerosis. Poster session presented at: 66th Annual Meeting of the American Academy of Neurology, 26 April – 3 May 2014, Philadelphia, PA. [Google Scholar]
- 25. Derfuss T, Curtin F, Guebelin C, Bridel C, Rasenack M, Matthey A, Du Pasquier R, Schluep M, Desmeules J, Lang AB, et al. A phase IIa randomized clinical study of GNbAC1, a humanized monoclonal antibody against the envelope protein of multiple sclerosis associated endogenous retrovirus in multiple sclerosis patients. Multiple Scler 2014 Nov 12; pii: 1352458514554052 [Epub ahead of print] [Google Scholar]
- 26. Marchi E, Kanapin A, Magiorkinis G, Belshaw R. Unfixed endogenous retroviral insertions in the human population. J Virol 2014; 88:9529-37; In press; PMID:24920817; http://dx.doi.org/ 10.1128/JVI.00919-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Preclinical safety evaluation of biotechnology-derived pharmaceuticals S6(R1). June 2011;. http://www.ich.orgfileadminPublic_Web_SiteICH_ProductsGuidelinesSafetyS6_R1Step4S6_R1_Guideline.pdf">http:www.ich.orgfileadminPublic_Web_SiteICH_ProductsGuidelinesSafetyS6_R1Step4S6_R1_Gui-deline.pdf [Google Scholar]
- 28. Mameli G, Poddighe L, Mei A, Uleri E, Sotgiu S, Serra C, Manetti R, Dolei A. Expression and activation by Epstein Barr virus of human endogenous retroviruses-W in blood cells and astrocytes: inference for multiple sclerosis. PLoS One 2012; 7:e44991; PMID:23028727; http://dx.doi.org/ 10.1371/journal.pone.0044991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brennan FR, Morton LD, Spindeldreher S, Kiessling A, Allenspach R, Hey A, Muller PY, Frings W, Sims J. Safety and immunotoxicity assessment of immunomodulatory monoclonal antibodies. MAbs 2010; 2:233-55; PMID:20421713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang W1, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther 2008 Nov; 84(5):548-58; PMID:18784655; http://dx.doi.org/ 10.1038/clpt.2008.170 [DOI] [PubMed] [Google Scholar]
- 31. Kaschka WP, Theilkaes L, Eickhoff K, Skvaril F. Disproportionate elevation of the immunoglobulin G1 concentration in cerebrospinal fluids of patients with multiple sclerosis. Infect Immun 1979 Dec; 26(3):933-41; PMID:528058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Petereit HF, Rubbert-Roth A. Rituximab levels in cerebrospinal fluid of patients with neurological autoimmune disorders. Mult Scler 2009 Feb; 15(2):189-92; PMID:18971221; http://dx.doi.org/ 10.1177/1352458508098268 [DOI] [PubMed] [Google Scholar]
- 33. Tran J, Palaparthy R, Zhao J, Brosofsky K, Ray S, Rana J, Cadavid D. Safety, tolerability and pharmacokinetics of the anti-lingo-1 monoclonal antibody BIIB033 in healthy volunteers and subjects with multiple sclerosis . Poster session presented at: 64th Annual Meeting of the American Academy of Neurology, 22-27 April 2012, New Orleans, LA. [Google Scholar]
- 34. Mould DR, Green B. Pharmacokinetics and pharmacodynamics of monoclonal antibodies: concepts and lessons for drug development. BioDrugs 2010 Feb 1; 24(1):23-39; PMID:20055530; http://dx.doi.org/ 10.2165/11530560-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 35. Mameli G, Serra C, Astone V, Castellazzi M, Poddighe L, Fainardi E, Neri W, Granieri E, Dolei A. Inhibition of multiple-sclerosis-associated retrovirus as biomarker of interferon therapy. J Neurovirol 2008; 14:73-77; PMID:18300077; http://dx.doi.org/ 10.1080/13550280701801107 [DOI] [PubMed] [Google Scholar]
- 36. Arru G, Leoni S, Pugliatti M, Mei A, Serra C, Delogu LG, Manetti R, Dolei A, Sotgiu S, Mameli G. Natalizumab inhibits the expression of human endogenous retroviruses of the W family in multiple sclerosis patients: a longitudinal cohort study. Mult Scler 2014; 20:174-182; PMID:23877972; http://dx.doi.org/ 10.1177/1352458513494957 [DOI] [PubMed] [Google Scholar]
- 37. Riminton DS, Hartung HP, Reddel SW. Managing the risks of immunosuppression. Curr Opin Neurol 2011; 24:217-23; PMID:21519254; http://dx.doi.org/ 10.1097/WCO.0b013e328346d47d [DOI] [PubMed] [Google Scholar]
- 38. Stefas E, Rucheton M, Graafland H, Moynier M, Sompeyrac C, Bahraoui EM, Veas F. Human plasmatic apolipoprotein H binds human immunodeficiency virus type 1 and type 2 proteins. AIDS Res Hum Retroviruses 1997; 13:97-104; PMID:8989432; http://dx.doi.org/ 10.1089/aid.1997.13.97 [DOI] [PubMed] [Google Scholar]
- 39. Stefas I, Rucheton M, D’Angeac AD, Morel-Baccard C, Seigneurin JM, Zarski JP, Martin M, Cerutti M, Bossy JP, Missé D, et al. Hepatitis B virus Dane particles bind to human plasma apolipoprotein H. Hepatology 2001; 33:207-17; PMID:11124838; http://dx.doi.org/ 10.1053/jhep.2001.20531 [DOI] [PubMed] [Google Scholar]


