Summary
Optimal nutritional and hormonal statuses are determinants of successful ageing. The age associated decline in anabolic hormones such as testosterone and insulin-like growth factor 1 (IGF-1) is a strong predictor of metabolic syndrome, diabetes and mortality in older men. Studies have shown that magnesium intake affects the secretion of total IGF-1 and increase testosterone bioactivity. This observation suggests that magnesium can be a modulator of the anabolic/catabolic equilibrium disrupted in the elderly people. However, the relationship between magnesium and anabolic hormones in men has not been investigated. We evaluated 399 ≥65-year-old men of CHIANTI in a study population representative of two municipalities of Tuscany (Italy) with complete data on testosterone, total IGF-1, sex hormone binding globulin (SHBG), dehydroepiandrosterone sulphate (DHEAS) and serum magnesium levels. Linear regression models were used to test the relationship between magnesium and testosterone and IGF-1. Mean age of the population was 74.18 ± 6.43 (years ± SD, age range 65.2–92.4). After adjusting for age, magnesium was positively associated with total testosterone (β ± SE, 34.9 ± 10.3; p = 0.001) and with total IGF-1 (β ± SE, 15.9 ± 4.8; p = 0.001). After further adjustment for body mass index (BMI), log (IL-6), log (DHEAS), log (SHBG), log (insulin), total IGF-1, grip strength, Parkinson’s disease and chronic heart failure, the relationship between magnesium and total testosterone remained strong and highly significant (β ± SE, 48.72 ± 12.61; p = 0.001). In the multivariate analysis adjusted for age, BMI, log (IL-6), liver function, energy intake, log (insulin), log (DHEAS), selenium, magnesium levels were also still significantly associated with IGF-1 (β ± SE, 16.43 ± 4.90; p = 0.001) and remained significant after adjusting for total testosterone (β ± SE, 14.4 ± 4.9; p = 0.01). In a cohort of older men, magnesium levels are strongly and independently associated with the anabolic hormones testosterone and IGF-1.
Keywords: anabolic hormones, magnesium, older men
Introduction
Nutritional and hormonal pathways are important determinants of homeostasis during the ageing process (Morley, 2009). The two networks interact through a number of mechanisms aimed at maintaining anatomical integrity and function of the body system.
Studies have found that the age-associated decline in anabolic hormones, including testosterone and insulin-like growth factor 1 (IGF-1) (Harman et al., 2001; Maggio et al., 2006) is a strong predictor of frailty and mortality in older persons (Cappola et al., 2009; Maggio et al., 2007).
Magnesium exerts an important role in cellular functions mostly cardiovascular and neuromuscular. Symptoms of magnesium deficiency include tetany, seizures, arrhytmias and neuromuscular irritability; low magnesium levels may also contribute to hypocalcaemia and hypokaliaemia. There is evidence that magnesium deficiency is involved in many age-related phenotypes including sarcopenia and metabolic syndrome (Barbagallo & Dominguez, 2010). In older persons, serum magnesium concentration is an independent correlate of muscle performance (Dominguez et al., 2006). Intake of magnesium has been associated with a better anabolic hormonal profile. In a generally well-nourished population of middle-aged to elderly men, plasma IGF-1 levels tended to increase with higher intake of protein and minerals, including magnesium (Devine et al., 1998; Giovannucci et al., 2003). While the relationship between magnesium and IGF-1 has been investigated, little information is available on the relationship between magnesium and testosterone. Magnesium and zinc preparation increase testosterone levels in strength-trained, competitive athletes (Brilla & Conte, 2000) and 4 weeks of magnesium supplementation increase testosterone levels in healthy sedentary and young male athletes (Cinar et al., 2011). However, no epidemiological study has investigated the relationship between magnesium levels and the anabolic hormones IGF-1 and testosterone in older population. Using data from the older male population of the InCHIANTI study, we hypothesized that magnesium serum levels would be associated with higher levels of the anabolic hormones.
Materials and methods
Study sample
The study population included 455 men randomly selected from all male residents 65 years and older in the CHIANTI catchment Area, Invecchiare nel CHIANTI (InCHIANTI) study, Tuscany, Italy.
Of the initial 455 participants, 399 with complete data on total testosterone, total IGF-1 and serum magnesium levels were included in this analysis.
The Italian National Institute of Research and Care on Aging Institutional Review Board ratified the study protocol. Participants consented to participate and to have their blood samples analysed for scientific purposes (Ferrucci et al., 2000). Blood samples were obtained from participants after a 12-h fast and after a 15-min rest. Aliquots of serum were stored at −80 °C and were not thawed until analysed.
Laboratory measures
Serum magnesium concentration was measured by a colorimetric assay with endpoint determination and sample blank. The measure unit was expressed in mg/dL. The minimum detectable concentration (MDC) was 0.07 mg/dL, the intraassay coefficient of variation (CV) was 1.2% and the interassay CV was 1.4% (Elin, 1991).
Total testosterone and dehydroepiandrosterone sulphate (DHEAS) were assayed using commercial radioimmunologic kits (Diagnostic Systems Laboratories, Webster, TX, USA). The MDC for total testosterone was 0.03 nmol/L; intra-assay and inter-assay CVs for three different concentrations were 9.6, 8.1 and 7.8%, and 8.6, 9.1, and 8.4% respectively.
For DHEAS, the MDC was 1.7 μg/dL; intra-assay and inter-assay CVs for three different concentrations were 4.1, 5.3 and 4.7%, and 4.8, 7.0 and 4.6% respectively.
Sex hormone binding globulin (SHBG) was measured using immunoradiometric assay (Diagnostic Products, Los Angeles, CA, USA) with an MDC of 3.00 nmol/L, and inter- and intra-assay CV concentrations for three different concentrations of 3.7, 1.1 and 3.4%, and 11.5, 10.3 and 8.7% respectively.
Serum concentrations of total IGF-1 were measured in duplicate from frozen specimens by immunoradiometric assay, using commercial reagents (Diagnostic Systems Laboratories). The MDC was 0.80 ng/mL. Inter-assay and intra-assay CVs for three concentrations (low, medium, and high) were all <10%.
Serum interleukin-6 (IL-6) was measured in duplicate by high-sensitivity enzyme-linked immunsorbent assay (ELISA) (BIOSOURCE, Camarillo, CA, USA). The MDC was 0.1 pg/mL and the interassay CV was 4.5%. Plasma insulin level was determined with a double-antibody, solid-phase radioimmunoassay (intra-assay CV = 3.1 + 0.3%; Sorin Biomedica, Milan, Italy). Cross-reactivity with human proinsulin was 0.3%.
Plasma selenium was measured by graphite furnace atomic absorption spectrometry using a Perkin Elmer Analyst 600 with Zeeman background correction. Samples were diluted 1 : 4 with a triton-X (Sigma Chemical, St. Louis, MO, USA) and nitric acid solution (Fisher Scientific, Pittsburgh, PA, USA) and the matrix modifier was a palladium and magnesium nitrate solution. Within-run and between-run coefficients of variation (CV) were 3.1 and 7.1% respectively.
Liver function was evaluated by glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT).
Health behaviours
All participants were examined by a trained geriatrician and diseases were ascertained according to standard, pre-established criteria and algorithms based upon those used in the Women’s Health and Aging Study for chronic diseases (Guralnik et al., 1995).
Smoking history was determined from self-report and stratified in the analysis as ‘current smoking’ vs. ‘ever smoked’ or ‘never smoked’. Daily total energy (kilocalories) and alcohol (grams) intake were estimated by the European Prospective Investigation into Cancer and Nutrition food frequency questionnaire. Education was recorded as years of school. Cognitive function was evaluated using the validated Italian version of the Mini-Mental State Examination (MMSE), with the total score adjusted for education and age (Pisani et al., 1997).
Body size, physical activity and muscle strength
Weight and height were measured using standard techniques. Body mass index (BMI) was calculated as weight (kg) divided by the square of height (m2).
Physical activity in the year before the interview was coded as: (i) sedentary: completely inactive or light-intensity activity less than 1 h/week; (ii) light physical activity: light-intensity activity 2–4 h/week; and (iii) moderate-high physical activity: light activity at least 5 h/week or more or moderate activity at least 1–2 h/week.
Handgrip strength was measured using a handheld dynamometer (hydraulic hand ‘BASELINE’; Smith & Nephew, Agrate Brianza, Milan, Italy). Participants were asked to perform the task twice with each hand. The average of the best result obtained with each hand was used for these analyses.
Diseases
Diseases were ascertained by an experienced clinician according to pre-established criteria that combine information from reported physician diagnoses, current pharmacological treatment, medical records, clinical examinations and blood tests. Diseases included in the current analysis were congestive heart failure and Parkinson’s disease.
Statistical analysis
Variables are reported as means (standard deviations) and medians (interquartile ranges) for continuous variables and number and percentages for categorical variables. To approximate normal distributions, log-transformed values for fasting insulin, SHBG, DHEAS, IL-6 were used in the analysis and back transformed for data presentation. Testosterone and IGF-1 were normally distributed. To control the validity of the model, we found that the residuals were normally distributed.
Factors statistically correlated with total testosterone and total IGF-1 were identified using age-adjusted partial correlation coefficients and Spearman partial rank-order correlation coefficients, as appropriate. Linear regression models were used test the relationship of magnesium serum levels (predictor) with testosterone and IGF-1 (outcome) in analysis adjusted for age and other potential confounders. In particular, the relationship of magnesium with testosterone was first adjusted for age, BMI, log (DHEAS), log (SHBG), log (fasting insulin), grip strength, Parkinson’s Disease, Chronic Heart Failure (CHF) (Model 1) and then total IGF-1 was included in the analysis (Model 2). A Model 1 adjusted for Age, BMI, GOT, GPT, energy intake, log (fasting insulin), log (DHEAS), log (IL-6) and selenium and Model 2 (Model 1 plus total testosterone) were used to test the relationship between magnesium and total IGF-1. β-value is the increase of one unit of testosterone (express in ng/dL) per mg/dL increase in magnesium or increase IGF-1 in ng/dL per mg/dL increase in magnesium.
All the analyses were performed using the sas statistical package, version 9.1 (SAS Institute Inc., Cary, NC, USA).
Results
Table 1 shows the main characteristics of the study population. Mean age of the population studied was 74.2 ± 6.4 (mean ± SD) with an age range between 65.2 and 92.4 years.
Table 1.
Characteristics of the study population (N = 399)
| Characteristics | Mean ± SD | Medians (IQR)a |
|---|---|---|
| Age (years) | 74.18 ± 6.43 | 73.2 [68.9–78.4] |
| Body mass index (kg/m2) | 27.1 ± 3.3 | 27.0 [24.7–29.1] |
| Total testosterone (ng/dL) | 428.1 ± 130.8 | 420 [348–499] |
| Serum magnesium (mg/dL) | 2.0 ± 0.6 | 2.2 [1.7–2.4] |
| Fasting insulin (mIU/L) | 11.0 ± 6.0 | 9.6 [6.8–14.2] |
| SHBG (nmol/L) | 110.9 ± 59.8 | 98.4 [70.5–133.4] |
| DHEAS (μg/dL) | 83.8 ± 62.5 | 66.9 [41.2–108.6] |
| Total IGF-1 (ng/dL) | 126.6 ± 55.1 | 121.0 [88.6–156.9] |
| IL-6 (ng/mL) | 2.7 ± 6.0 | 1.56 [0.90–2.63] |
| Grip strength (kg) | 37.5 ± 10.7 | 38 [30–46] |
| MMSE | 25.2 ± 4.5 | 26 [24–28] |
| CES-D | 9.6 ± 7.1 | 8 [5–13] |
| Energy intake (kcal/day) | 2156.2 ± 562.9 | 2130 [1793–2499] |
| Zinc intake (mg/day) | 11.4 ± 3.1 | 11.3 [9.4–13.2] |
| Physical activity | ||
| Sedentary/light n (%) | 67 (14.8) | |
| Moderate n (%) | 347 (76.8) | |
| High n (%) | 3.8 (8.4) | |
| Smoking (never) | 130 (28.8) | |
| Education (years) | 6.2 ± 3.6 | 5 [5–7] |
| Disease n (%) | ||
| Cancer | 22 (4.9) | |
| Parkinson disease | 13 (2.8) | |
| Stroke | 41 (9.1) | |
| COPD | 17 (3.8) | |
| Chronic heart failure | 91 (20.1) |
Values (first column) are expressed as mean ± SD (second column) or medians and interquartile ranges (third column) or n (%).
IQR Interquartile ranges.
IGF-1, insulin-like growth factor 1; MMSE, mini-mental state examination; SHBG, sex hormone binding globulin; DHEAS, dehydroepiandrosterone sulphate; COPD, chronic obstructive pulmonary disease.
Magnesium levels (β ± SE, 34.9 ± 10.3; p = 0.001) were significantly and positively correlated with total testosterone (Fig. 1 and Table 2). As expected in the age-adjusted analysis SHBG, DHEAS and grip strength were significant correlates of total testosterone. BMI (r = −0.13, p = 0.02), fasting insulin (r = 0.08, p = 0.09), Parkinson disease (r = −0.13, p = 0.02) and CHF (r = −0.09, p = 0.07) were also significantly and negatively associated with total testosterone. We did not find any significant relationship between total testosterone and total IGF-1, IL-6, cognitive function assessed by MMSE, depressive status, energy intake, physical activity and the prevalence of cancer. In the age-adjusted analysis, magnesium levels were also significantly associated with total IGF-1 (β ± SE, 15.9 ± 4.8; p = 0.001) (Fig. 2). After further adjustment for multiple confounders including BMI, log (IL-6), log (DHEAS), log (SHBG), log (fasting insulin), grip strength, Parkinson’s Disease and CHF, magnesium levels were still significantly associated with total testosterone (β ± SE, 36.4 ± 10.7; p = 0.008) and the strength of the association was even stronger after adjustment for total IGF-1 (β ± SE, 48.72 ± 12.61; p = 0.001) (Table 3).
Figure 1.
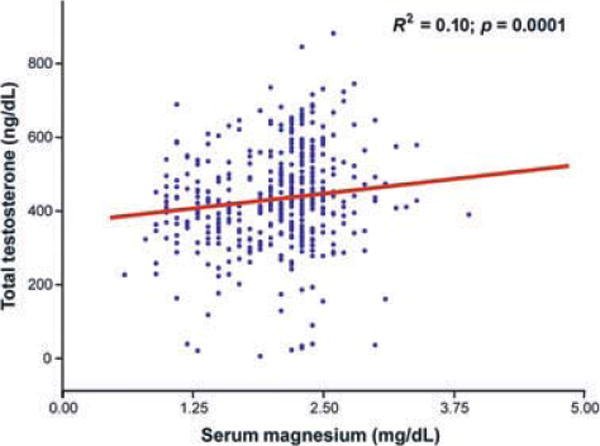
Relationship between total testosterone and serum magnesium levels adjusted for age. The beta coefficient ± SE (β ± SE) of the age-adjusted analysis was 34.9 ± 10.3 with p-value = 0.0001.
Table 2.
Age-adjusted spearman partial rank-order correlation coefficients of total testosterone including serum magnesium and other factors
| Characteristics (n = 399) | Total testosterone r (p) |
|---|---|
| Serum magnesium (mg/dL) | 0.20 (0.0002) |
| SHBG (nmol/L) | 0.36 (<.0001) |
| DHEAS (μg/dL) | 0.09 (0.09) |
| Total IGF-1 (ng/dL) | 0.02 (0.63) |
| Fasting insulin | −0.08 (0.09) |
| IL-6 (ng/mL) | −0.03 (0.54) |
| Grip strength (kg) | 0.17 (0.002) |
| MMSE | −0.06 (0.26) |
| CES-D | −0.04 (0.37) |
| Energy intake (kcal/day) | 0.03 (0.57) |
| Physical activity | 0.02 (0.68) |
| Body mass index (kg/m2) | −0.13 (0.02) |
| Smoking | 0.03 (0.59) |
| Education (years) | −0.02 (0.71) |
| Cancer n (%) | −0.06 (0.30) |
| Parkinson disease n (%) | −0.13 (0.02) |
| Stroke n (%) | −0.05 (0.34) |
| COPD n (%) | −0.003 (0.94) |
| Chronic heart failure n (%) | −0.09 (0.07) |
MMSE, mini-mental state examination; SHBG, sex hormone binding globulin; IGF-1, insulin-like growth factor 1; DHEAS, dehydroepiandrosterone sulphate; COPD, chronic obstructive pulmonary disease.
Figure 2.
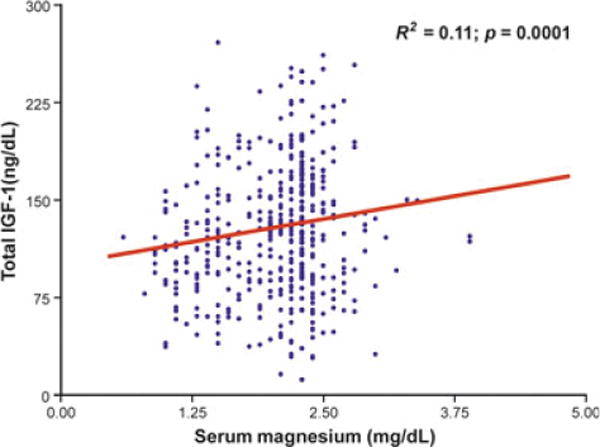
Relationship between total growth factor 1 levels and magnesium levels adjusted for age. The beta coefficient ± SE (β ± SE) of the age-adjusted analysis was 15.9 ± 4.8 with p-value = 0.001.
Table 3.
Relationship between magnesium (predictor) and total testosterone (outcome)
| Total testosterone
|
||
|---|---|---|
| Betaa ± SE | p | |
| Model 1 | ||
| Magnesium | 36.4 ± 10.7 | 0.008 |
| Model 2 | ||
| Magnesium | 48.7 + 12.6 | 0.0001 |
Each line refers the results of a separate model adjusted for indicated covariates.
Model 1: adjusted for Age, BMI, log (DHEAS), log (SHBG), log (fasting insulin), Grip strength, Parkinson’s disease, chronic heart failure.
Model 2: adjusted for Age, BMI, log (DHEAS), log (SHBG), log (fasting insulin), Grip Strength, Parkinson’s disease, chronic heart failure and for total IGF-1.
BMI, body mass index; DHEAS, dehydroepiandrosterone sulphate; IGF-1, insulin-like growth factor 1; SHBG, sex hormone binding globulin.
In a fully adjusted analysis adjusted for age, BMI, total energy intake, log (fasting insulin), log (IL-6), GOT, GPT, DHEAS and selenium, magnesium levels were also significantly associated with IGF-1 (β ± SE, 16.4 ± 5.0; p = 0.001) and this association was attenuated but remained significant even after adjusting for total testosterone (β ± SE, 14.4 ± 4.9; p = 0.01) (Table 4).
Table 4.
Relationship between magnesium (predictor) and total IGF-1 (outcome)
| Total IGF-1
|
||
|---|---|---|
| Betaa ± SE | p | |
| Model 1 | ||
| Magnesium | 16.4 ± 4.9 | 0.001 |
| Model 2 | ||
| Magnesium | 14.4 ± 4.9 | 0.01 |
Each line refers the results of a separate model adjusted for indicated covariates.
Model 1: adjusted for age, BMI, GOT, GPT, energy intake, log (fasting insulin), log (DHEAS), log (IL-6), selenium.
Model 2: adjusted for age, BMI, GOT, GPT, energy intake, log (fasting insulin), log (DHEAS), log (IL-6), selenium and total testosterone.
BMI, body mass index; DHEAS, dehydroepiandrosterone sulphate; IGF-1, insulin-like growth factor 1.
Discussion
In a cohort of older men, magnesium levels are strongly, positively and independently associated with total testosterone and total IGF-1.
This is the first population study testing the relationship between magnesium and anabolic hormones in older men. If we assume that magnesium levels are predictors of anabolic hormones, at least two different mechanisms may explain these findings, (i) the increased reactive oxygen species production observed in magnesium deficiency, and (ii) the pro-inflammatory effect of low magnesium levels.
Studies of magnesium-deficient cultured human cells and animals show evidence of decreased antioxidant capacity. Hence, magnesium seems fundamental for the control of oxidative stress (Dickens et al., 1992; Freedman et al., 1992). Interestingly both testosterone and IGF-1 are strongly related with antioxidant capacity and their concentration decrease after exposure to oxidative stress (Demirbag et al., 2005). Thus based on our findings, we can argue that magnesium by decreasing oxidative stress may possibly contribute to upregulate testosterone and IGF-1 secretion. However, given the lack of measures of oxidative stress in our population, we could not directly test this hypothesis.
A state of chronic inflammation has been proposed as one of the main causes of unstable homeostasis in older persons (Ferrucci & Guralnik, 2003). Poor magnesium status may trigger the development of a proinflammatory state both by causing excessive production and release of interleukin 1ß and tumour necrosis factor α (Weglicki et al., 1996). Concurrently, low-grade chronic inflammation downregulate testosterone secretion from the Leydig cells (Hong et al., 2004) with evidence of possible mechanistic link between IGF-I and inflammatory markers namely IL-6. In transgenic mice that over express IL-6, low levels of IGF-I are found relative to wild-type mice treatment of these transgenic mice with an IL-6 antibody or with an IL-6 receptor antagonist resulting in normalization of IGF-I levels (De Benedetti et al., 2001) whose levels are low in inflammatory conditions (De Benedetti et al., 1997). Contrary to this hypothesis, in our study the relationship between magnesium and total testosterone and total IGF-1 was independent of IL-6, proxy of systemic inflammation.
The relationship between nutrients and IGF-1 is not surprising as this molecule is also a sensitive nutrition marker in older individuals (Sullivan & Carter, 1994). We recently found that IGF-1 is strongly and independently associated with selenium levels in older InCHIANTI participants (Maggio et al., 2010). However, even when selenium was introduced in the model, magnesium was still significantly associated with IGF-1 suggesting that selenium and magnesium are independent determinants of IGF-1 levels. As most of IGF-1 is secreted by the liver, we also used GOT and GPT as covariates in the multivariate model but again the association between magnesium levels and total IGF-1 was unaffected. Of note, an interesting finding of our study was that magnesium is associated with each of the anabolic hormones independently also of precursor hormones (DHEAS) or binding proteins as SHBG.
The modulation of anabolic status by nutrients including magnesium in older men is an intriguing therapeutic perspective. We have previously shown that low circulating levels of multiple anabolic hormones, including testosterone, IGF-1 and DHEAS (in the lowest quartiles of the population), were an independent predictor of mortality during 6 years of follow-up in older men (Maggio et al., 2007). These findings were confirmed in patients affected by chronic heart failure where, deficiency of more than one anabolic hormone identifies groups with a higher mortality rate (Jankowska et al., 2006). As magnesium has many positive effects on muscle performance, insulin resistance and cardiovascular disease (Barbagallo & Dominguez, 2010; Dominguez et al., 2006; Champagne, 2008) it is possible that some of these effects might be mediated through the stimulation of anabolic hormones especially in older individuals. Consistently, preliminary small intervention studies suggest that magnesium supplementation with zinc or alone increase testosterone levels in healthy sedentary and strength-trained competitive young male athletes (Brilla & Conte, 2000; Cinar et al., 2011).
Given the cross-sectional nature of this study, a mechanism of reverse causality, namely, that IGF-1 and testosterone increase serum magnesium levels cannot be excluded. Dominguez et al. showed in fasting normotensive subjects that IGF-1 elevate intracellular magnesium at time dependent fashion and reverses the blunted response to insulin of hypertensive cells. However, only for insulin but not for IGF-1 the cellular magnesium responsiveness was closely and directly related to basal magnesium levels suggesting an insulin dependent effect (Dominguez et al., 1998). Interestingly, in our study the relationship between magnesium and IGF-1 was independent of fasting insulin levels suggesting an independent effect of IGF-1 on circulating serum magnesium levels. The additional hypothesis that testosterone may modulate magnesium levels has not been tested and deserves further investigation.
It is also plausible that serum levels of magnesium and anabolic hormones are both indicators of good nutritional status and general health status assessed for example by higher quality of skeletal muscle. For instance, data from the InCHIANTI Study have shown that serum magnesium concentrations were significantly associated with indexes of muscle performance including grip strength lower-leg muscle power, knee extension torque and ankle extension strength independent of several confounders (Dominguez et al., 2006), suggesting that magnesium, as testosterone and IGF-I do, may affect muscle performance. However, in our analysis magnesium and anabolic hormones are positively associated independent of energy intake, suggesting that this remains only an intriguing hypothesis.
Limitations
Our study has some limitations. This is a preliminary analysis with a cross-sectional design and therefore no causality can be determined. The analysis accounted only for a limited number of determinants of magnesium and anabolic hormones.
Strengths of the study
The limitations are offset by important strengths. This is the first attempt to test the association between magnesium and anabolic hormones in a representative sample of older population. Second, information concerning the inflammatory cytokines and the covariates used in the multivariate analysis cannot be easily found in other epidemiological studies.
Perspective
There is need of longitudinal and intervention studies to assess whether magnesium levels predict anabolic deficiency and/or magnesium supplementation is a valid strategy to slow down the decline in anabolic hormones observed in older men.
Acknowledgments
The InCHIANTI Study was supported as a ‘targeted project’ (ICS 110.1/RS97.71) by the Italian Ministry of Health and in part by the US National Institute on Aging (Contracts N01-AG-916413 and N01-AG-821336) and by the Intramural Research Program of the US National Institute on Aging (Contracts 263 MD 9164 13 and 263 MD 821336). We thank all participants in the InCHIANTI Study. We gratefully acknowledge the important contribution of Fabrizio Ablondi for his technical assistance.
Footnotes
Author contributions
MM and GPC: conceived this specific hypothesis of the study. FL, SB: applied for the funding. FL: conducted final statistical analyses. MM: wrote the first draft of the manuscript. All authors: contributed to subsequent drafts of the manuscript and approved the final version of the manuscript. MM: had full access to all data and had final responsibility for the decision to submit the manuscript for publication.
Disclosures
None of the sponsoring institutions interfered with the collection, analysis, presentation or interpretation of the data reported here. None of the authors declared a conflict of interest.
References
- Barbagallo M, Dominguez LJ. Magnesium and aging. Curr Pharm Des. 2010;16:832–829. doi: 10.2174/138161210790883679. [DOI] [PubMed] [Google Scholar]
- Brilla LR, Conte V. Effects of a novel zinc-magnesium formulation on hormones and strength. JEPonline. 2000;3:26–36. [Google Scholar]
- Cappola AR, Xue QL, Fried LP. Multiple hormonal deficiencies in anabolic hormones are found in frail older women: the Women’s Health and Aging studies. J Gerontol A Biol Sci Med Sci. 2009;64:243–248. doi: 10.1093/gerona/gln026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne CM. Magnesium in hypertension, cardiovascular disease, metabolic syndrome, and other conditions: a review. Nutr Clin Pract. 2008;23:142–151. doi: 10.1177/0884533608314533. [DOI] [PubMed] [Google Scholar]
- Cinar V, Polat Y, Baltaci AK, Mogulkoc R. Effects of magnesium supplementation on testosterone levels of athletes and sedentary subjects at rest and after exhaustion. Biol Trace Elem Res. 2011;140:18–23. doi: 10.1007/s12011-010-8676-3. [DOI] [PubMed] [Google Scholar]
- De Benedetti F, Alonzi T, Moretta A, Lazzaro D, Costa P, Poli V, Martini A, Ciliberto G, Fattori E. Interleukin 6 causes growth impairment in transgenic mice through a decrease in insulin-like growth factor-I. A model for stunted growth in children with chronic inflammation. J Clin Invest. 1997;99:643–650. doi: 10.1172/JCI119207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedetti F, Pignatti P, Vivarelli M, Meazza C, Ciliberto G, Savino R, Martini A. In vivo neutralization of human IL-6 (hIL-6) achieved by immunization of hIL-6-transgenic mice with a hIL-6 receptor antagonist. J Immunol. 2001;166:4334–4440. doi: 10.4049/jimmunol.166.7.4334. [DOI] [PubMed] [Google Scholar]
- Demirbag R, Yilmaz R, Erel O. The association of total antioxidant capacity with sex hormones. Scand Cardiovasc J. 2005;39:172–176. doi: 10.1080/14017430510035862. [DOI] [PubMed] [Google Scholar]
- Devine A, Rosen C, Mohan S, Baylink D, Prince RL. Effects of zinc and other nutritional factors on insulin-like growth factor I and insulin-like growth factor binding proteins in postmenopausal women. Am J Clin Nutr. 1998;68:200–206. doi: 10.1093/ajcn/68.1.200. [DOI] [PubMed] [Google Scholar]
- Dickens BF, Weglicki WB, Li YS, Mak IT. Magnesium deficiency in vitro enhances free radical-induced intracellular oxidation and cytotoxicity in endothelial cells. FEBS Lett. 1992;311:187–191. doi: 10.1016/0014-5793(92)81098-7. [DOI] [PubMed] [Google Scholar]
- Dominguez LJ, Barbagallo M, Sowers JR, Resnick LM. Magnesium responsiveness to insulin and insulin-like growth factor I in erythrocytes from normotensive and hypertensive subjects. J Clin Endocrinol Metab. 1998;83:4402–4407. doi: 10.1210/jcem.83.12.5327. [DOI] [PubMed] [Google Scholar]
- Dominguez LJ, Barbagallo M, Lauretani F, Bandinelli S, Bos A, Corsi AM, Simonsick EM, Ferrucci L. Magnesium and muscle performance in older persons: the InCHIANTI study. Am J Clin Nutr. 2006;84:419–426. doi: 10.1093/ajcn/84.1.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elin RJ. Determination of serum magnesium concentration by clinical laboratories. Magnes Trace Elem. 1991;10:60–66. [PubMed] [Google Scholar]
- Ferrucci L, Guralnik JM. Inflammation, hormones, and body composition at a crossroad. Am J Med. 2003;115:501–502. doi: 10.1016/j.amjmed.2003.08.018. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, Guralnik JM. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- Freedman AM, Mak IT, Stafford RE, Dickens BF, Cassidy MM, Muesing RA, Weglicki WB. Erythrocytes from magnesium-deficient hamsters display an enhanced susceptibility to oxidative stress. Am J Physiol. 1992;262:C1371–C1375. doi: 10.1152/ajpcell.1992.262.6.C1371. [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Pollak M, Liu Y, Platz EA, Majeed N, Rimm EB, Willett WC. Nutritional predictors of insulin-like growth factor I and their relationships to cancer in men. Cancer Epidemiol Biomarkers Prev. 2003;12:84–89. [PubMed] [Google Scholar]
- Guralnik JM, Fried LP, Simonsick EM, Kasper SD, Lafferty ME. The Women’s Health and Aging Study: Health and Social Characteristics of Older Women with Disability. National Institute on Aging; Bethesda, MD: 1995. (NIH Publication No. 95-4009). [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- Hong CY, Park JH, Ahn RS, Im SY, Choi HS, Soh J, Mellon SH, Lee K. Molecular mechanism of suppression of testicular steroidogenesis by proinflammatory cytokine tumor necrosis factor alpha. Mol Cell Biol. 2004;24:2593–2604. doi: 10.1128/MCB.24.7.2593-2604.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska EA, Biel B, Majda J, Szklarska A, Lopuszanska M, Medras M, Anker SD, Banasiak W, Poole-Wilson PA, Ponikowski P. Anabolic deficiency in men with chronic heart failure: prevalence and detrimental impact on survival. Circulation. 2006;114:1829–1837. doi: 10.1161/CIRCULATIONAHA.106.649426. [DOI] [PubMed] [Google Scholar]
- Maggio M, Ble A, Ceda GP, Metter EJ. Decline in insulin-like growth factor-I levels across adult life span in two large population studies. J Gerontol A Biol Sci Med Sci. 2006;61:182–183. doi: 10.1093/gerona/61.2.182. [DOI] [PubMed] [Google Scholar]
- Maggio M, Lauretani F, Ceda GP, Bandinelli S, Ling SM, Metter EJ, et al. Relationship between low levels of anabolic hormones and 6-year mortality in older men: the aging in the Chianti Area (InCHIANTI) study. Arch Intern Med. 2007;167:2249–2254. doi: 10.1001/archinte.167.20.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio M, Ceda GP, Lauretani F, Bandinelli S, Dall’aglio E, Guralnik JM, et al. Association of plasma selenium concentrations with total IGF-1 among older community-dwelling adults: the InCHIANTI study. Clin Nutr. 2010;29:674–7. doi: 10.1016/j.clnu.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JE. Anorexia, sarcopenia, and aging. Nutrition. 2009;117:660–663. doi: 10.1016/s0899-9007(01)00574-3. [DOI] [PubMed] [Google Scholar]
- Pisani P, Faggiano F, Krogh V, Palli D, Vineis P, Berrino F. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int J Epidemiol. 1997;26(Suppl. 1):S152–S160. doi: 10.1093/ije/26.suppl_1.s152. [DOI] [PubMed] [Google Scholar]
- Sullivan DH, Carter WJ. Insulin-like growth factor I as an indicator of protein- energy undernutrition among metabolically stable hospitalized elderly. J Am Coll Nutr. 1994;13:184–191. doi: 10.1080/07315724.1994.10718393. [DOI] [PubMed] [Google Scholar]
- Weglicki WB, Dickens BF, Wagner TL, Chmielinska JJ, Phillips TM. Immunoregulation by neuropeptides in magnesium deficiency: ex vivo effect of enhanced substance P production on circulating T lymphocytes from magnesium-deficient mice. Magnes Res. 1996;9:3–11. [PubMed] [Google Scholar]


