Abstract
Background
Trait anger consists of affective, behavioral, and cognitive (ABC) dimensions and may increase vulnerability for interpersonal conflict, diminished social support, and greater psychological distress. The concurrent influence anger and psychosocial dysfunction on HIV disease severity is unknown.
Purpose
Examine plausible psychosocial avenues (e.g. coping, social support, psychological distress) whereby trait anger may indirectly influence HIV disease status.
Methods
377 HIV seropositive adults, aged 18–55 years (58% AIDS-defined) completed a battery of psychosocial surveys and provided a fasting blood sample for HIV-1 viral load and T-lymphocyte count assay.
Results
A second-order factor model confirmed higher levels of the multidimensional anger trait was directly associated with elevated psychological distress and avoidant coping (p<.001) and indirectly associated with greater HIV disease severity (p<.01) (CFI=.90, RMSEA=.06, SRMR=.06).
Conclusion
The model supports ABC components of anger may negatively influence immune function through various psychosocial mechanisms; however longitudinal study is needed to elucidate these effects.
Keywords: HIV/AIDS, anger, hostility, coping, social support, psychological distress, disease severity
Introduction
In this era of combination antiretroviral therapy (cART), the morbidity and mortality of persons infected with Human Immunodeficiency Virus (HIV) has dramatically decreased (1, 2). Although HIV viremia can be reliably reduced to levels below the limit of detection, HIV cannot be eradicated with currently available cART medications and lifelong treatment is required (3). There remains large variability in the beneficial impact of cART treatment on disease severity and progression (4). Some have suggested psychological and behavioral factors may be mediating influences that contribute to HIV disease severity and progression over and above established risk factors, such as treatment adherence and illicit drug use and abuse (5–7). Numerous studies using animal models have shown that chronic stress may impact infectious disease progression through a host of immunomodulatory mechanisms (8, 9). We have previously shown that HIV viral load moderates the relationship between specific immunocellular subsets, including T-helper memory cells and B-cells, and a composite index of psychological distress, which included measures of life stress, HIV/AIDS-related anxiety and depressive symptoms (10). Subsequently, we examined this composite distress measure to evaluate its association with the cytotoxic T cell subset and with HIV disease severity, as indexed by T helper cell count and HIV-1 viral load (11). With HIV disease progression, the cytotoxic T-cell subset undergoes an expansion that is associated with an increase in cytotoxic T-cell activation, a signal that the adaptive immune system is marshaling defense mechanisms (12, 13). Our previous findings indicated that the association between greater distress and HIV disease severity was mediated by increased T cytotoxic cell subset activation (9). Thus, chronic stress and negative emotions, such as depression and anxiety, may exacerbate HIV disease severity, through inflammatory, neuroendocrine and other mediating processes (14). However, the impact of negative emotions, such as anger and hostility, on immunocellular function in the context of HIV spectrum disease is less explored.
Trait anger has been theorized to be a multidimensional construct consisting of the so-called “ABCs” of underlying psychological dimensions, affective, behavioral, and cognitive (15). The affective dimension of anger describes the tendency to experience this emotion anywhere on a continuum from mild irritation and annoyance to rage (16). The behavioral dimension of anger consists of aggressive behaviors, which may include verbal (e.g., insult, rudeness, sarcasm) or physical (e.g., badgering, harassment, assault) expressions of anger (17). Such behaviors are often displayed within the context of HIV disease as a protective measure to buffer the psychosocial effects of HIV-related stigma and discrimination (18–20). In contrast, the cognitive dimension of anger includes hostile cognitions, which may manifest in cynical worldviews and include thoughts of skepticism, alienation and mistrust (16, 21). In HIV-infected persons, skepticism and mistrust of the intentions of others has been shown to affect decisions regarding disclosure of HIV status to individuals in an extended social network, resulting in poorer social support (22).
Anger has been shown to negatively influence the quality and diminish the frequency of social interactions (23). Hostile individuals, in particular, display an increased psychosocial vulnerability and experience elevated distress due to more frequent interpersonal conflict, which results in social alienation and estrangement (23–26). The availability of social support may mediate the effect of anger on health in many ways (27–29). For example, in HIV-infected persons, suspicions regarding the role of government officials in addressing the HIV/AIDS epidemic along with greater distrust for health professionals is associated with a reluctance to participate in medically-sanctioned prevention and treatment programs (30–33). Notably, diminished social support is also associated with greater HIV-disease severity and faster disease progression (34–36).
In persons with high trait anger, social interactions tend to have a more negative tone due to their tendencies for greater negative emotional reactivity and expression (27, 37, 38). Thus, heightened stress levels from such interactions place angry individuals at greater risk for developing depression, anxiety and negative mood (39–43). Greater levels of anger and hostility also relate to major depression, dysthymia and anxiety in HIV-infected persons (44, 45). In addition, several prospective observational studies have reported that more stressful life events are associated with depressive symptomatology and a faster rate of clinical progression of HIV disease (46, 47).
In both HIV seronegative and seropositive individuals, those who report coping with life stress through the use of avoidant (e.g., disengagement, self-blame, and denial) rather than problem-focused strategies also report greater levels of anger and hostility (44, 48, 49). Moreover, recent evidence shows that HIV-infected individuals employing avoidant coping were more likely to exhibit poorer psychological (e.g., depression and anxiety), behavioral (e.g., health behaviors and medication adherence) and health outcomes (T helper cell count and HIV-1 viral load) (50–52).
In sum, existing studies broadly support the notion that trait anger, indexed by affective, behavioral, and cognitive components, may result in a vulnerability to experience interpersonal conflict, diminished social support, and greater distress (i.e., depression, anxiety and perceived stress) in persons living with HIV/AIDS. However, understanding of the complexity of these relationships is limited because this literature has focused on only subsets of these psychosocial factors in the context of HIV spectrum disease. The present study will examine how these psychosocial factors might work together to contribute to or buffer against HIV disease severity, indexed by T helper cell count and HIV-1 viral load, in HIV-infected men and women by using a quantitative method to derive a comprehensive model based on this integrative conceptual framework.
Methods
Participants
Men and women who resided in Florida’s Miami-Dade, Broward and Palm Beach counties were recruited by newspaper advertisement, flyer distribution at HIV/AIDS clinics, and support groups, as well as physician and chain referrals. Several HIV exposure categories, from asymptomatic pre-AIDS to symptomatic AIDS, were present within the sample. Study eligibility criteria required participants to: (1) provide written informed consent; (2) have documented diagnosis of HIV-1 infection; (3) provide proof of age 18–55 years; (4) be non-substance abusing as confirmed by the Structured Clinical Interview for the DSM-IV (SCID-1 Version 2.0); (5) have no indication of surgery 3 months prior to study entry; (6) have no history of diabetes or cardiovascular condition, or other major systemic diagnosis unrelated to HIV; (7) be receiving no pharmacological treatment for cardiovascular (e.g., beta-blockers, calcium antagonists, ACE inhibitors), diabetic (e.g., hypoglycemics, insulin sensitizers), psychiatric (e.g., antipsychotics), or endocrine (e.g., estrogen hormonal replacement) conditions; (8) for Hepatitis C virus (HCV) seropositive persons, have not been treated previously for this infection or if treated, to have been treated more than 6 months prior to study entry; (9) for women, not be pregnant.
Procedures
Following telephone screening, those meeting inclusion and exclusion criteria were invited for the laboratory assessments. Upon arrival to the laboratory, the HIV and HCV seropositive participants provided written confirmation from their physician verifying their infection(s). For these participants, blood was drawn for confirmation of their serostatus. HIV status was confirmed by Enzyme Immunoassay (EIA) and if a self-reported seronegative person tested positive, the EIA was confirmed by Western Blot. HCV status was determined using the anti HCV antibody EIA assay and, if positive, this status was confirmed by an RNA Quantitative PCR to obtain viral load. All current medical conditions and any preexisting medical history and physical exam information were considered through a comprehensive interview with the subject by the staff physician. The Center for Disease Control (CDC) HIV/AIDS symptom classification stage was determined using a combination of history, lab data (historical nadir and current T helper cell count), and a focused physical exam (focused on weight, fat redistribution, oral, chest, abdomen, skin, and neurological findings) (53). Adherence to prescribed cART medications was assessed with the Adult AIDS Clinical Trial Group (ACTG) structured interview (54). Sociodemographic information was obtained and then the subject underwent casual blood pressure, 12-lead ECG, height, weight, and waist and hip girth assessments. A urine sample was tested with a toxicology screen (i.e., alcohol, barbiturates, benzodiazepines, cannabinoids, LSD, PCP, THC, morphine, and amphetamines), and for women, a urine pregnancy screen. Substance use and dependence/abuse history was assessed by the SCID-1 using module E: Substance Use Disorders. Psychosocial surveys were administered to derive measures of anger, hostility, coping strategies, social support, perceived stress, HIV-related anxiety, and depression.
Blood Assays
HIV-1 viral load was determined using an in vitro nucleic acid amplification test (AMPLICOR HIV-1 Monitor Test, Roche Diagnostics, Branchburg, NJ) with ultrasensitive methods (range 50–750,000 HIV-1 RNA copies/ml) and repeated with standard methods, if viral load met the upper detection limit (range 400–10 million copies/ml). Lymphocyte subset counts, including T-helper cells (CD3+CD4+), were derived using the mean of two serially collected peripheral blood samples analyzed with flow cytometry (Epics XL-MCL flow cytometer, Coulter, Hialeah, FL). HCV antibodies were assessed using EIA (Ortho-Diagnostics, Raritan, NJ; specificity 99.5%) and positive assays (i.e., signal to cutoff ratios < 8.0) were confirmed using immunoblot analysis (CHIRON RIBA HCV v3.0 SIA method, Ortho-Diagnostics, Raritan, NJ).
Psychosocial Measures
Trait Anger
To reflect the affective, behavioral, and cognitive components of trait anger, six anger and hostility scales were chosen from an exploratory analysis described previously (15). The emotional dimension of anger, Angry Affect, was measured with Spielberger’s Trait Anger Scale (TAS), a 10-item survey that includes the Angry Temperament subscale (TAS-T) and the Angry Reactivity subscale (TAS-R) (55). The TAS-T consists of four items that assess the tendency to experience anger without provocation (e.g., “I have a fiery temper”), whereas the TAS-R assesses the respondent’s perceived frequency of angry emotions that results from provocation, e.g., “I get angry when slowed down by others’ mistakes.”
The behavioral dimension of anger, Aggressive Behavior, was measured with two scales: (1) the Anger-out subscale of the Anger Expression (AX) Scale, and (2) the antagonistic behavior subscale of the Cook Medley Hostility (CM) Scale (55, 56). The AX-Out subscale consists of 8 items related to outward acts of aggression (e.g., “I strike out at whatever infuriates me”), which participants rated from 1 (almost never) to 4 (almost always). The antagonistic behavior subscale (CM-A) is derived from 17-items that assess antagonistic behavioral expression (e.g., “I have at times had to be rough with people who were rude or annoying”) (15).
The cognitive dimension of anger, Hostile Cognition, was measured using two previously described subscales from of the Cook Medley Scale (57). The Cynicism (CM-C) subscale contains 24 items that reflect negative beliefs of human nature (e.g., “I think most people would lie to get ahead”). The Cook-Medley Paranoid Alienation (CM-PA) subscale consists of 15 items that describe thoughts of persecution (e.g., “I feel like someone has it out for me”). These scales characterize the extent that weak interpersonal bonds are based on perceptions of mistrust in the intentions of others (57). The AX and the CM surveys have acceptable psychometric properties (15).
HIV-specific Coping Strategies
Three scales from the COPE were chosen to reflect approach coping, i.e., positive reappraisal and growth, acceptance, and active coping. Self-blame, denial and behavioral disengagement were selected to reflect avoidant coping. Participants were instructed to select answers that best reflected how they “dealt with HIV concerns or problems” during the last month. The items were rated on a 4-point Likert scale ranging from 1 (not at all) to 4 (a lot). These COPE subscales have demonstrated acceptable psychometric properties (58).
Social Support
Perceived social support was measured using the Social Provisions Scale (SPS) (59). The five measures of relational provisions used in this study included Attachment (emotional closeness and sense of security), Social Integration (sense of belonging to a group similar to oneself), Reassurance of Worth (recognition of one’s value by others) and Guidance (advice or information) The SPS scale demonstrates acceptable psychometric properties (59).
Distress
Indicators were selected to reflect the affective, behavioral and cognitive components of psychological distress using three surveys, as previously described (10, 11). The Impact of Events Scale (IES) is a 15-item measure of emotional disturbance and anxiety (60). Participants were asked to report on their thought intrusions and avoidances associated with life situations regarding their HIV infection. The Beck Depression Inventory (BDI) contains 21 items reflecting the affective/cognitive (13 items) and somatic (8 items) features of depression (61). The Perceived Stress Scale (PSS) is a 14-item survey that assesses the degree to which one appraises life as stressful due to unpredictable, uncontrollable and overwhelming experiences over the past month (62). The BDI, IES and PSS surveys have acceptable psychometric properties (60–62).
Statistical Analysis
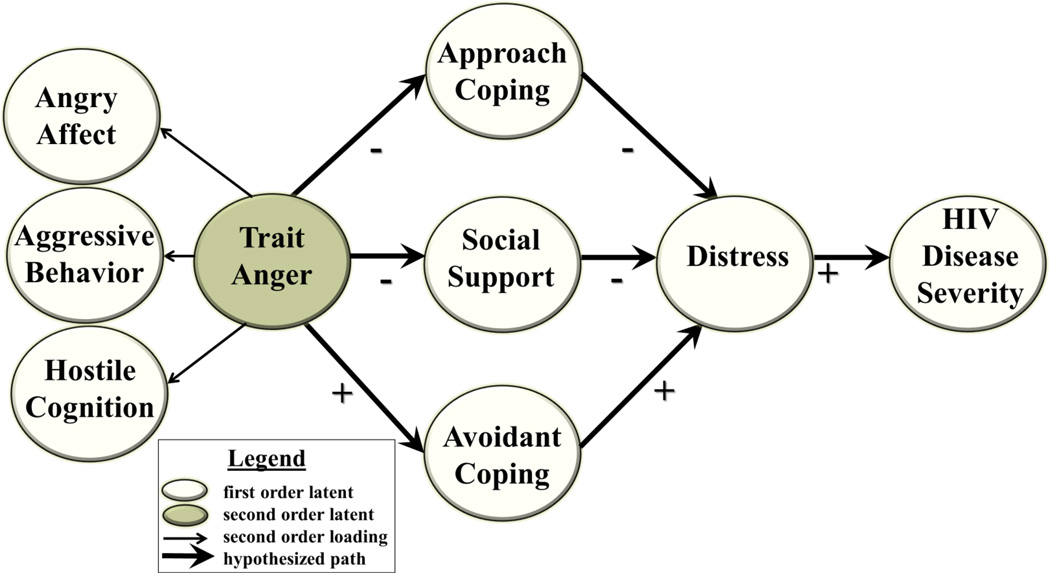
Standard preliminary data screening and variable transformations for variables violating normality were performed. Full information maximum likelihood estimates were used for data presumed to be missing at random (63). A variation of the traditional two-step approach to structural equation modeling (SEM) was followed that allowed for a measurement model, consisting of first and second order latent factors, to be tested while simultaneously examining the structure of paths within a recursive model (64). The statistical program, Mplus v.3.2 (65), was used to simultaneously run confirmatory factor analysis and produce unbiased parameter estimates of the direct and indirect relationships among the latent variables in the theorized model before and after accounting for demographic and treatment covariates. Factor loadings and residual error variance for the factors were tested for significance at the 0.05 level. Based on the extant literature summarized above, a model of the inter-relationships of anger with coping strategy, social support, distress, and HIV disease severity was hypothesized. As depicted in Figure 1, the hypothesized model included three pathways from: (1) Trait Anger to Approach Coping to Distress to HIV Disease Severity; (2) Trait Anger to Social Support to Distress to HIV Disease Severity; and (3) from Trait Anger to Avoidant Coping to Distress to HIV Disease Severity.
Figure 1. Hypothesized Model.
Model depicting hypothesized interrelationships among anger (comprised of indices of angry affect, aggressive behavior, and hostile cognition), approach coping, social support, avoidant coping, psychological distress and HIV disease severity. Open ovals represent first-order latent factors. The second order latent factor, Trait Anger, is depicted by a shaded oval. Positive hypothesized associations are indicted by “+” and inverse associations indicated by “−”.
Before evaluating model fit indices, two considerations were used to meet the criteria for an indicator to be retained in the final model. First, each observed variable needed to load on one main latent factor that it was purported to measure. Second, the factor loading for each indicator had to be equal to or greater than 0.40 (66). A reference indicator was selected for each latent factor with the loading set at 1.00 for the purpose of scaling. Measures for model fit included Bentler’s comparative fit index (CFI), root-mean-square error of approximation (RMSEA), and standardized root-mean-square residual (SRMR) (67–69). The criteria used to determine acceptable model fit included: (1) CFI ≥ .90; (2) RMSEA < .07; and (3) SRMR < .08 (69, 70). Because the χ2 fit statistic may be inflated with large sample sizes (e.g., >200), it was not included as an indicator of model fit (68, 71). Model modifications were performed based on the initial assessment of the hypothesized model. In addition, indices of the proportion of variability explained (R2) were derived for each latent and observed variable in the model. After a path model solution was derived from the data, secondary analyses regressed each latent construct on the following covariates before subsequent model trimming: age (years); sex (1 = female; 0 = male); education (years completed); ethnicity (1 = Black, 0 = other race), income, time since HIV diagnosis (months); cART (1 = on cART treatment, 0 = not ART treated), and Heptatis C Virus (HCV) co-infection (1 = HCV+, 0 = HCV−).
Results
Participant Characteristics
Demographic information for the cohort is presented in Table 1. Approximately 69% of the cohort were men and over 90% identified themselves as racial/ethnic minorities. On average, participants were early middle-aged and reported an annual household income below the U.S. poverty line (U.S. Census, 2002). Nearly half the sample exhibited an undetectable HIV-1 viral load and approximately one-quarter were also HCV seropositive. Based on conventional criteria for HIV disease staging (Centers for Disease Control and Prevention, 1993), most study participants (65%) were classified as AIDS with the majority reporting current immunodeficiency symptoms. Over 80%% of the cohort were currently taking a cART regimen that included a protease inhibitor. The self-reported antiretroviral therapy (ART) adherence among those who were treated with a cART regimen, as indexed by the ACTG measure, was reatively high.
Table 1.
Demographic and disease-related characteristics of the cohort (N = 377)
| Characteristics | Mean | (SD) |
|---|---|---|
| Age (years) | 41.4 ± | 7.4 |
| Sex (%) | ||
| Men | 68.9 | |
| Women | 30.3 | |
| Ethnicity (%) | ||
| African American | 60.5 | |
| Hispanic | 26.0 | |
| Non-Hispanic White | 6.9 | |
| Other | 6.6 | |
| Socioeconomic status | ||
| Education (years) | 12.3 ± | 2.7 |
| Household income ($/year) | 8,640.1 ± 10,075.7 | |
| Time since HIV diagnosis (months) | 111.4 ± | 70.0 |
| CD4+ count nadir (cells/µl) | 421.1 ± | 267.9 |
| Undetectable HIV viral load (%)a | 46.2 | |
| HIV disease stage classification (%) | ||
| HIV asymptomatic | 27.3 | |
| HIV symptomatic | 7.2 | |
| AIDS asymptomatic | 22.8 | |
| AIDS symptomatic | 42.7 | |
| Hepatitis C Virus seropositive (%) | 23.6 | |
| Antiretroviral medication regimen (%) | ||
| cART treatment | 82.3 | |
| Adherence (4-day) | 91.1 | |
Abbreviations: cART, combination antiretroviral therapy.
Below 50 HIV-1 RNA copies/ml
Step 1: Measurement Model Validation
The factor structure of eight first-order latent factors were operationalized as follows: (1) Angry Affect used TAS-T and TAS-R scores; (2) Aggressive Behavior used CM-A and AX-Out scores; (3) Hostile Cognitions used the CM-C and CM-PA scores; (4) Approach Coping used the COPE positive reappraisal, acceptance and active coping scores; (5) Social Support used the SPS attachment, social integration, reassurance of worth, guidance and nurturance scores; (6) Avoidant Coping used the COPE self-blame, behavioral disengagement and denial scores; (7) Distress was indicated by the IES, BDI and PSS total scores; and (8) HIV Disease Severity was indicated by HIV-1 viral load and T-helper cell count. Of additional interest was the structure of Trait Anger and whether it should be subsumed within a broader construct, “Negative Affect”, along with Distress or alternatively maintained as separate factors. These alternatives were tested using the Akaike Information Criteria (AIC) (72). The model wherein Trait Anger was subsumed with Distress within a Negative Affect factor yielded a larger AIC = 43,363 relative to the alternative model, AIC = 42,909. Thus, as hypothesized (see Figure 1), the analyses supported a hierarchical structure for Trait Anger that included the first-order latent factors, Angry Affect, Aggressive Behavior, and Hostile Cognitions independent of the Distress factor. In sum, the overall measurement model, including first- and second-order factors, demonstrated sufficient fit to proceed with path evaluation (CFI = .91, RMSEA = .07, SRMR = .07). As displayed in Table 2, adequate construct validity for the Trait Anger factor was demonstrated by a significant correlation greater than .40 between each measured indicator and the respective latent factor.
Table 2.
Means, standard deviations and factor loadings for first-order latent variables.
| First-Order Factors and Indicators | Mean | SD | Factor Loading |
|---|---|---|---|
| Angry Affect | |||
| Angry temperament | 6.2 | 2.7 | .79 |
| Angry reactivity | 7.7 | 2.8 | .79 |
| Aggressive Behavior | |||
| Antagonism | 9.1 | 3.7 | .56 |
| Anger out | 14.7 | 4.2 | .58 |
| Hostile Cognitions | |||
| Cynicism | 14.1 | 5.8 | .54 |
| Paranoidalienation | 4.5 | 3.1 | .65 |
| Avoidant Coping | |||
| Self-blame | 3.7 | 1.8 | .59 |
| Denial | 6.4 | 2.9 | .72 |
| Behavioral disengagement | 6.2 | 2.5 | .61 |
| Social Support | |||
| Attachment | 11.4 | 2.4 | .79 |
| Social integration | 11.9 | 2.4 | .87 |
| Reassurance of worth | 11.8 | 2.4 | .87 |
| Guidance | 12.3 | 2.6 | .84 |
| Nurturance | 11.2 | 2.7 | .68 |
| Approach Coping | |||
| Positive reappraisal | 12.9 | 3.2 | .81 |
| Acceptance | 13.1 | 2.9 | .68 |
| Active | 11.6 | 3.0 | .64 |
| Distress | |||
| Impact of events | 21.7 | 14.8 | .65 |
| Beck depression inventory | 7.6 | 8.1 | .69 |
| Perceived stress | 22.0 | 7.5 | .63 |
| HIV-Disease Severity | |||
| HIV-1 viral load (copies/ml) | 15,050.1 | 54,788.3 | .93 |
| T helper cell count (cells/µl) | 450.9 | 321.0 | −.49 |
The intercorrelations among the observed variables used in the latent factors are displayed in Table 3. Among latent factors, Trait Anger factor was positively associated with Avoidance Coping (r = .47, p < .001), Distress (r = .46, p < .001), and HIV Disease Severity (r = .25, p < .001). In addition, greater Distress was related to greater HIV Disease Severity (r = .25, p < .01), less Approach Coping (r = −.17, p < .05), less Social Support (r = −.21, p < .001), and more Avoidant Coping (r = .73, p < .001). Greater Social Support was associated with more Approach Coping (r = .34, p < .001) and with less Avoidant Coping (r = −.26, p < .001). In addition, greater HIV Disease Severity was related to more Avoidant Coping (r = .26, p < .01). Of note, the second-order factor structure of Trait Anger explained 66% of the variance in Angry Affect, 97% of the variance in Behavioral Aggression and 97% of the variance in Hostile Cognitions.
Table 3.
Correlations among factor indicators used in the modeling analyses.
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Angry temperament (TAS-T) | 1.00 | ||||||||||||||||||||
| 2. Angry reactivity (TAS-R) | .62** | 1.00 | |||||||||||||||||||
| 3. Antagonism (CM-A) | .29*** | .37*** | 1.00 | ||||||||||||||||||
| 4. Anger out (AX-Out) | .51*** | .37*** | .31*** | 1.00 | |||||||||||||||||
| 5. Cynicism (CM-C) | .28*** | .35*** | .87*** | .34*** | 1.00 | ||||||||||||||||
| 6. Paranoid alienation (CM-PA) | .42*** | .38*** | .68*** | .34*** | .59*** | 1.00 | |||||||||||||||
| 7. Positive reappraisal (COPE) | −.05 | .05 | −.01 | .02 | .03 | −.23** | 1.00 | ||||||||||||||
| 8. Acceptance (COPE) | −.04 | .06 | .01 | .10 | .05 | −.21** | .55*** | 1.00 | |||||||||||||
| 9. Active (COPE) | .02 | .05 | .04 | .03 | .01 | −.09 | .54*** | .45*** | 1.00 | ||||||||||||
| 10. Self-blame (COPE) | .20*** | .20*** | .28*** | .18*** | .27*** | .35*** | −.01 | .03 | .04 | 1.00 | |||||||||||
| 11. Denial (COPE) | .30*** | .24*** | .29*** | .18*** | .27*** | .40*** | −.01 | −.11* | .07 | .41*** | 1.00 | ||||||||||
| 12. Behavioral disengagement (COPE) | .19*** | .11* | .16** | .04 | .13* | .35*** | −.07 | −.14** | −.02 | .34*** | .48*** | 1.00 | |||||||||
| 13. Attachment (SPS) | −.11* | −.01 | −.08 | .03 | −.01 | −.16** | .19*** | .15** | .16** | −.17** | −.08 | −.17** | 1.00 | ||||||||
| 14. Social integration (SPS) | −.11* | −.03 | −.06 | .07 | .02 | −.17** | .25*** | .25*** | .15** | −.16** | −.16** | −.15** | .69*** | 1.00 | |||||||
| 15. Reassurance of worth (SPS) | −.22*** | −.12* | −.18** | −.07 | −.09 | −.27*** | .26*** | .23** | .15** | .20*** | −.22** | −.18 | .69*** | .75*** | 1.00 | ||||||
| 16. Guidance (SPS) | −.19*** | −.04 | −.06 | .04 | −.01 | −.19** | .24*** | .23** | .16** | −.12* | −.09 | −.13* | .75*** | .73*** | .73*** | 1.00 | |||||
| 17. Nurturance (SPS) | −.08 | −.02 | −.01 | .07 | .07 | −.13* | .22** | .18** | .18** | −.05 | .01 | −.10 | .54*** | .69*** | .58*** | .59*** | 1.00 | ||||
| 18. HIV-related Anxiety (IES) | .19*** | .22*** | .27*** | .21*** | .26*** | .30*** | .06 | .04 | .04 | .34*** | .43*** | .30*** | −.09 | −.03 | −.09 | −.07 | −.01 | 1.00 | |||
| 19. Depressive symptoms (BDI) | .28*** | .24*** | .23*** | .26*** | .25*** | .37*** | −.13* | −.13* | −.06 | .40*** | .31*** | .25*** | −.14** | −.18** | −.23*** | −.19** | −.05 | .45*** | 1.00 | ||
| 20. Perceived stress (PSS) | .28*** | .16** | .16** | .24*** | .21*** | .35*** | −.18 | −.17** | −.20** | .30*** | .23*** | .24*** | −.08 | −.10 | −.19** | −.18** | −.06 | .38*** | .48*** | 1.00 | |
| 21. HIV-1 viral load | .07 | .09 | .08 | .06 | .10 | .08 | −.04 | −.02 | −.07 | .02 | .06 | .05 | −.01 | .01 | −.05 | −.02 | −.04 | .07 | .05 | .03 | 1.00 |
| 22. T helper cell count | −.02 | −.06 | −.08 | −.01 | −.13* | −.08 | .03 | .10* | .03 | −.10 | −.09 | −.07 | .07 | .06 | .10 | .07 | .06 | −.07 | −.08 | −.01 | −.27*** |
Abbreviations: TAS-T = Trait Anger Scale-temperament; TAS-R = Trait Anger Scale-reactivity; CM-A = Cook-Medley Hostility Scale-antagonism; AX-Out = Anger Expression Scale-out; CM-C = Cook-Medley Hostility Scale-cynicism; CM-PA = Cook-Medley Hostility Scale-paranoid alienation; COPE = Coping Inventory; SPS = Social Provisions Scale; IES = Impact of Events Scale; BDI = Beck Depression Inventory; PSS = Perceived Stress Scale.
p < .05
p < .01
p < .001
Step 2: Model Evaluation
The test of the structure of the hypothesized model (see Figure 1) did not exhibit adequate fit (CFI = .89, RMSEA = .07, SRMR = .09). Multiple paths from the theoretical model were significant, including Trait Anger to Avoidant Coping (β = .46, z = 8.67, p = < .001), Avoidant Coping to Distress (β = .76, z = 14.60, p = < .001), and Distress to HIV Disease Severity (β = −.14, z = −2.15, p = < .05). However, a number of paths were not significant, including Trait Anger to Social Support, (β = −.05, z = −.81), Trait Anger to Approach Coping, (β = −.01, z = −0.02), and Social Support to Distress (β = −.05, z = −0.78); these paths were removed from the model.
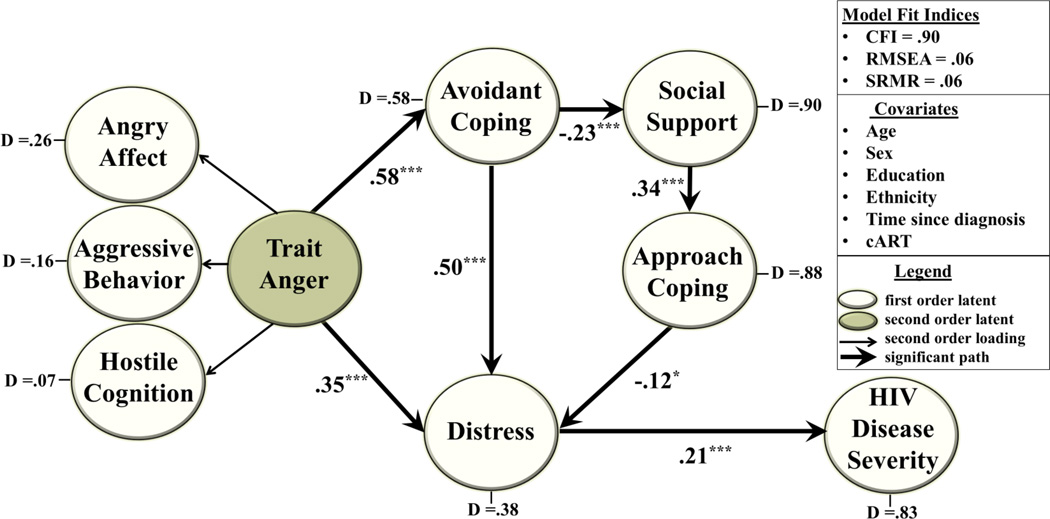
Modifications of the hypothesized model, based on the initial SEM analysis, included pathways from Avoidant Coping to Social Support, (β = −.23, z = −3.71, p < .001), and Social Support to Approach Coping (β = .34, z = 6.18, p < .001). The final model included a direct path from Trait Anger to Distress (β = .34, z = 4.02, p < .001). The model also included two indirect paths from Trait Anger to Distress; these paths were: (1) from Trait Anger to Avoidant Coping (β = .48, z = 7.30, p < .001) and from Avoidant Coping to Distress (β = .58, z = 10.56, p < .001), and; (2) from Trait Anger via Avoidant Coping to Social Support (β = −.23, z = −3.71, p < .001), and from Social Support to Approach Coping β = .34, z = 6.18, p < .001), and from Approach Coping to Distress (β = −.12, z = −1.97, p < .05). In addition, the model indicated a direct path from Distress to HIV Disease Severity, (β = .27, z = 4.23, p < .001). The cumulative association for the indirect paths linking Trait Anger to HIV Disease Severity was also significant (β = .14, z = 3.04, p < .01). This model demonstrated adequate fit to the data (CFI = .93, RMSEA = .06, SRMR = .06).
Each latent construct in the trimmed model was regressed on the covariates; HCV serostatus and income were not associated with model factors (rs were −.08 to .10 for HCV serostatus and −.03 to .03 for income). Significant paths that were retained included: a path from age (β = −.16, z = −2.94, p < .01) and ethnicity (β = .20, z = 3.60, p < .001) to Trait Anger; a path from sex (β = .16, z = 3.10, p < .01) and education (β = .13, z = 2.27, p < .05) to Social Support; paths from time since HIV diagnosis (β = −.12, z = −2.20, p < .05) and education (β = −.22, z = −4.03, p < .001) to Avoidance Coping; and from cART use to Distress (β = −.13, z = −2.56, p < .05) and Disease Severity (β = −.33, z = −6.73, p < .001). These covariates contributed to explaining an additional 4.7% of the variance in Avoidance Coping, 3.1% in Social Support, 0.7% in Distress and 10.2% in Disease Severity.
Because 16% of the cohort were not prescribed or taking anti-HIV medications, and thus were not missing medication adherence data at random, the inclusion of this measure as a covariate was inappropriate. However, to determine whether controlling for medication adherence, as indexed by ACTG, changed the significance of any pathways in the model, the model was evaluated using only the 312 participants that reported taking anti-retroviral medications. This analysis indicated model goodness of fit (CFI = .90, RMSEA = 0.06, SRMR = 0.06) and that all of the significant paths within the hypothesized model were retained.
Figure 2 shows the final trimmed model, indicating that when accounting for all significant associations among latent factors and covariates, the model demonstrated good fit to the data (CFI = .90, RMSEA = .06, SRMR = .06). Overall, this model accounted for 92.8% of the variance in Hostile Cognitions, 83.5% in Aggressive Behavior, 73.7% in Angry Affect, 11.6% in Approach Coping, 41.8% of Avoidance Coping, 9.5% in Social Support, 61.4% in Distress, and 17.2% in Disease Severity.
Figure 2. Final Model.
The final trimmed model derived using structural equation modeling methodology is depicted. The open ovals represent first-order latent factors and the shaded oval depicts the second-order latent factor, Trait Anger. The model shows that greater trait anger was linked in a direct path with elevated distress and HIV-disease severity, and in two indirect paths via increased avoidance coping, and via increased avoidance coping to decreased social support to decreased approach coping. Path coefficients are standardized β weights. Solid arrows depict statistically significant associations (***p < .001; **p < .01). Non-significant paths are not shown. Disturbance (D) terms reflect the proportion of unexplained variance in each latent factor after accounting for all other latent variables and covariates. The model has good fit to the data as indicated by the fit indices.
Discussion
Being HIV seropositive creates social stigma and isolation, and numerous chronic stressors (e.g., interpersonal, financial and others) that are often unpredictable and uncontrollable (19, 73). Besides the threat of mortality, these stressors are associated with increased dysphoria, anxiety, anger, hostility, the feeling of being overwhelmed and may be associated with negative coping strategies including avoidant coping, denial and distancing (5, 74). The present study used a quantitative modeling methodology to examine putative inter-relationships among factors reflecting these influences in the context of HIV disease severity while controlling for demographic (age, sex, education, and ethnicity) and disease-related (time since HIV diagnosis, and cART treatment) measures. In addition, the study extends these findings by demonstrating that higher levels of trait anger, both directly and indirectly, were associated with greater psychological distress. Specifically, the model indicated one direct and two indirect pathways between trait anger and increased psychological distress accounting for 68% of the variance in distress and 18% of the variance in HIV disease severity. The indirect model pathways indicated that greater anger was associated in one path with elevated distress through increased avoidant coping, whereas in a second path greater anger was associated with increased avoidant coping, which was related to decreased social support and in turn associated with decreased approach coping.
This study has replicated previous lines of research pertaining to the structure of the Trait Anger factor and its relationship with distress and HIV disease severity. The literature suggests a considerable overlap among the constructs of anger, anxiety and depression in psychological regulation and in their association with chronic disease (42, 75). The present model, however, suggested that Trait Anger and Distress better fit the data when included as distinct factors, rather than as subsumed within an umbrella construct of negative affect. The final model (see Figure 2) shows the hierarchical factor structure for Trait Anger. Although this is the first study to demonstrate the multidimensionality of this trait in HIV-infected persons, others have demonstrated the affective, behavioral and cognitive dimensions of this trait in non-clinical samples (15, 76, 77). In addition, the observed association between Trait Anger and Distress derives support from a previous report citing positive associations between hostility, anxiety, life stress and major depression in HIV seropositive men (44). The final model also shows greater symptoms of depression, HIV-related anxiety and perceived stress, indexed by the Distress factor, were related to more severe HIV Disease Severity, indexed by greater HIV-1 viral load and lower T helper cell count. This finding replicates our research describing psychoimmune models in HIV-positive men and women (11). Collectively, these findings coincide with an extensive body of research suggesting that depressive symptoms, anxiety and HIV-related stress lead to accelerated HIV disease progression in the post-cART era (50, 78, 79). However, it should be noted that worsening HIV disease severity may result in greater distress and consequently greater avoidant coping and anger. Thus, it is possible that some components of the model operate in an opposite direction from that tested in the model or may even be bi-directionally linked (80, 81).
The present study extends previous findings by providing specification for the linkage of Trait Anger with the Distress-Disease Severity pathway vis a vis the specification of one direct pathway to Distress and two indirect pathways to Distress through coping and social support mechanisms. The linkage between Trait Anger and Distress that occurs indirectly via Avoidant Coping is plausible and well-supported by theoretical and empirical studies linking negative coping behaviors with anger as well as with depression, anxiety and perceived stress (82–86). Another study finding was the lack of replication of previous reports showing that approach coping mediates the direct association between greater anger with greater psychological distress. Instead, the present results suggest a more nuanced inter-relationship among these psychosocial variables, wherein trait anger was positively related to distress indirectly via greater use of avoidant coping which, in turn, was related to both lower social support and approach coping. Numerous studies have provided support for the association between maladaptive coping strategies and diminished social support in persons with HIV (36, 45, 87–89). Furthermore, the use of approach coping strategies, such as positive reappraisal, acceptance and direct action, have been associated with lower levels of psychological distress in HIV positive persons (45, 90–92). Thus, these findings suggest that anger influences distress more proximally via its effect on negative coping strategies, which then may influence distress either directly or indirectly via its effect on social support and positive coping strategies.
It is pertinent to note that our research in HIV-infected individuals has shown that group-based cognitive behavioral interventions, which taught coping skills, and relaxation and interpersonal skills, such as anger management (93), may modulate coping strategies (decrease avoidance coping and increase approach coping (94), increase social support (95), and reduce distress states (94, 96–99). Of note, such interventions have been shown to improve potential neuroendocrine biomediators (i.e., cortisol, norepinephrine) that may influence immune system function (97, 100). In addition, in other reports, we have shown that cognitive behavioral interventions in HIV-infected men and women can have a demonstrable impact on immune system reconstitution (101), and result in decreased HIV viral load (98) and reduced incidence of opportunistic neoplasias (99).
The present study controlled for the influence of several demographic in an effort to evaluate the unique effects of the latent constructs. In so doing, the model accounted for a greater percentage of explained variance in Trait Anger, Social Support and Avoidance Coping. Greater trait anger was associated with younger age, a finding that conflicts with previous studies of HIV negative cohorts (102, 103). However, study findings are supported by previous HIV studies, which have shown that seropositive individuals, with advancing age, report feeling less cheated by life, have more patience dealing with others, and experience less anger and frustration when confronting personal functional limitations (104, 105). The analyses also indicated that greater psychological distress was associated with self-identifying as Black or African American. Previous literature supports the notion that perceived stress, anxiety and depression has greater prevalence in low SES African American communities (79, 106, 107). Similarly, studies have shown that African Americans endorse higher rates of anger and hostility than their Caucasian counterparts (108, 109). Although these findings are likely related to factors such as education and access to resources, evidence continues to mount suggesting higher levels of anger and hostility may further increase risk for severe chronic disease in members of the African Americans community (21, 109, 110).
There is no question that anti-retroviral medication adherence plays an important role in influencing CD4 count, HIV viral load and ultimately HIV disease severity (111). Moreover psychosocial functioning may have a marked influence on adherence to HIV medication regimens (112, 113). Medication regimen adherence, as indexed by the ACTG measure, averaged about 91% among the cohort. This self-report retrospective measure is commonly used in the HIV literature (54). Notably, when the cohort was restricted to those taking anti-retroviral medications, the analysis showed that the model fit adequately, when the ACTG was controlled. Although HCV coinfection did not account for significant variance in the model factors, the analyses indicated significant relationships between other HIV-related covariates and the latent factors. Time since HIV diagnosis was inversely related to Avoidant Coping and Distress suggesting greater psychosocial adaptation to the disease over time (114–116). The use of cART was also associated with levels of Distress and HIV Disease Severity. Others have shown cART use to predict slower HIV disease progression and reductions in depression, anxiety and chronic stress over time (117–119). Overall, the present study findings replicated established associations of relevant HIV-related factors with key model constructs and controlled for these factors in the model derivation.
Limitations
Although the inter-relationships among coping strategies, social support and distress measures used in this study have been previously evaluated to varying extents in studies of HIV/AIDS cohorts, this study provided evaluation of trait anger multidimensionality and its putative linkages with psychological distress and HIV disease severity. Any conclusions regarding the derived model reported in this study must be restricted to the operational definitions of the constructs employed. Notably, the study cohort demographics, in terms of age range, and sex and minority composition, were comparable to the general HIV/AIDS population in the U.S.(120). The model plausibility is supported by the use of key sociodemographic and disease-related variables including age, sex, education, ethnicity, time since HIV diagnosis and cART treatment. Notably, study entry criteria were stringent with regard to excluding those with comorbid systemic and psychiatric conditions; hence, potential confounding influences related to these factors were minimized. Nevertheless, the study findings may only generalize to those persons with comparable demographic, psychological and disease-related characteristics as present in the study cohort. Sample size considerations prohibited inclusion of other variables associated with HIV disease, such as illicit substance use, dependence and abuse, indices of immunocellular enumerative or functional relevance other than T helper cell count and HIV-1 viral load, or measures of coinfection other than HCV. In addition, factors such as job status (121, 122) and sexual orientation (123, 124), which have been associated with poorer psychosocial functioning and HIV disease severity, were not controlled in the present assessments. Although the present study provided a meaningful conceptual model, the cross-sectional design does not permit conclusions regarding directionality. Hence, it is possible that model pathways may operate in the reverse direction or that the model pathways include bidirectionality. Longitudinal observations of disease progression would permit more definitive conclusions regarding directional influences of the examined psychosocial and disease-related factors in HIV spectrum disease.
Conclusion
In sum, the present study describes a hierarchical structure of trait anger and suggests conceptually reasonable psychosocial avenues by which this trait may influence perceived distress and ultimately HIV disease severity. The derived model is consistent with the behavioral science literature pertaining to the inter-relationships among anger, coping strategies and social support and explains a substantial amount of variance in psychological distress and its covariance with HIV disease severity, independent of numerous demographic and HIV-related factors. Negative emotions, such as anger, in the context of HIV spectrum disease, may induce an environment where adequate social provisions are not received. The consequences of which, in addition to its deleterious impact on psychosocial factors, may extend to an acceleration of pathophysiological mechanisms that drive HIV disease progression. To this point, these mechanisms have not yet been elucidated. Some have suggested that the psychoneuroimmunological transaction associated with negative emotionality is mediated by inducing an exacerbation of inflammatory status directly or indirectly via a neuroendocrine axis dysregulation of cortisol (14, 125–127). Furthermore, heightened proinflammatory status has been associated with an imbalance of oxidative metabolism that results in the expression of reactive oxygen species, greater HIV replication, destruction of the T helper cell subset and further disease progression (128, 129). Some have suggested that a similar set of psychoneuroimmunological mechanisms play a role in facilitating comorbidities commonly found in persons with HIV such as the metabolic syndrome, diabetes and cardiovascular (CV) disease (130–133). Indeed, elevated CV risk has been associated with anger and hostility in HIV negative populations and may be an appropriate area for further research given the incidence of coronary artery disease in HIV positive persons. Although the present study underscores the potential avenues by which trait anger and its linkages with psychosocial factors influence HIV disease severity, further research will be needed to evaluate the extent to which these factors are central or more tangential in their influence on HIV pathophysiological mechanisms and disease progression.
Acknowledgments
Grant Support:
T32 MH018917; R01 HL072712; P30 NIAID1073961
Footnotes
‘Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards:
Authors Roger C. McIntosh, Barry E. Hurwitz, Michael Antoni, Alex Gonzalez, Julia Seay and Neil Schneiderman declare that they have no conflict of interest. All procedures, including the informed consent process, were conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.’
References
- 1.Volberding PA, Deeks SG. Antiretroviral therapy and management of HIV infection. The Lancet. 2010;376:49–62. doi: 10.1016/S0140-6736(10)60676-9. [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. New England Journal of Medicine. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 3.May MT, Sterne J, Costagliola D, et al. HIV treatment response and prognosis in Europe and North America in the first decade of highly active antiretroviral therapy: a collaborative analysis. Lancet. 2006;368:451–458. doi: 10.1016/S0140-6736(06)69152-6. [DOI] [PubMed] [Google Scholar]
- 4.Lodi S, Phillips A, Touloumi G, et al. Time from human immunodeficiency virus seroconversion to reaching CD4+ cell count thresholds <200, <350, and <500 cells/mm3: assessment of need following changes in treatment guidelines. Clinical infectious diseases. 2011;53:817–825. doi: 10.1093/cid/cir494. [DOI] [PubMed] [Google Scholar]
- 5.Penedo FJ, Gonzalez JS, Davis C, et al. Coping and psychological distress among symptomatic HIV+ men who have sex with men. Annals of Behavioral Medicine. 2003;25:203–213. doi: 10.1207/S15324796ABM2503_06. [DOI] [PubMed] [Google Scholar]
- 6.Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychological bulletin. 2004;130:601. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glaser R, Rabin B, Chesney M, Cohen S, Natelson B. Stress-induced immunomodulation. JAMA: the journal of the American Medical Association. 1999;281:2268–2270. doi: 10.1001/jama.281.24.2268. [DOI] [PubMed] [Google Scholar]
- 8.Biondi M, Zannino L-G. Psychological stress, neuroimmunomodulation, and susceptibility to infectious diseases in animals and man: a review. Psychotherapy and Psychosomatics. 1997;66:3–26. doi: 10.1159/000289101. [DOI] [PubMed] [Google Scholar]
- 9.Yang E, Glaser R. Stress-induced immunomodulation: impact on immune defenses against infectious disease. Biomedicine & pharmacotherapy. 2000;54:245–250. doi: 10.1016/S0753-3322(00)80066-9. [DOI] [PubMed] [Google Scholar]
- 10.Motivala SJ, Hurwitz BE, Llabre MM, et al. Psychological distress is associated with decreased memory helper T-cell and B-cell counts in pre-AIDS HIV seropositive men and women but only in those with low viral load. Psychosomatic medicine. 2003;65:627–635. doi: 10.1097/01.psy.0000041549.72780.5b. [DOI] [PubMed] [Google Scholar]
- 11.Greeson JM, Hurwitz BE, Llabre MM, et al. Psychological distress, killer lymphocytes and disease severity in HIV/AIDS. Brain, behavior, and immunity. 2008;22:901–911. doi: 10.1016/j.bbi.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Giorgi JV, Lyles RH, Matud JL, et al. Predictive value of immunologic and virologic markers after long or short duration of HIV-1 infection. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2002;29:346–355. doi: 10.1097/00126334-200204010-00004. [DOI] [PubMed] [Google Scholar]
- 13.Sousa AE, Carneiro J, Meier-Schellersheim M, Grossman Z, Victorino RM. CD4 T cell depletion is linked directly to immune activation in the pathogenesis of HIV-1 and HIV-2 but only indirectly to the viral load. The Journal of Immunology. 2002;169:3400–3406. doi: 10.4049/jimmunol.169.6.3400. [DOI] [PubMed] [Google Scholar]
- 14.Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Emotions, morbidity, and mortality: new perspectives from psychoneuroimmunology. Annual review of psychology. 2002;53:83–107. doi: 10.1146/annurev.psych.53.100901.135217. [DOI] [PubMed] [Google Scholar]
- 15.Martin R, Watson D, Wan CK. A Three-Factor Model of Trait Anger: Dimensions of Affect, Behavior, and Cognition. Journal of personality. 2000;68:869–897. doi: 10.1111/1467-6494.00119. [DOI] [PubMed] [Google Scholar]
- 16.Smith TW. Concepts and methods in the study of anger, hostility, and health. Anger, hostility, and the heart. 1994:23–42. [Google Scholar]
- 17.Buss AH. The psychology of aggression. New York, US: Wiley; 1961. [Google Scholar]
- 18.Viney LL, Henry R, Walker BM, Crooks L. The emotional reactions of HIV antibody positive men. British journal of medical psychology. 1989;62:153–161. doi: 10.1111/j.2044-8341.1989.tb02822.x. [DOI] [PubMed] [Google Scholar]
- 19.Earnshaw VA, Kalichman SC. Stigma, Discrimination and Living with HIV/AIDS. Springer; 2013. Stigma experienced by people living with HIV/AIDS; pp. 23–38. [Google Scholar]
- 20.Brook DW, Brook JS, Richter L, et al. Coping strategies of HIV-positive and HIV-negative female injection drug users: A longitudinal study. AIDS education and prevention. 1999 [PubMed] [Google Scholar]
- 21.Barefoot JC, Peterson BL, Dahlstrom WG, et al. Hostility patterns and health implications: correlates of Cook-Medley Hostility Scale scores in a national survey. Health Psychology. 1991;10:18. doi: 10.1037//0278-6133.10.1.18. [DOI] [PubMed] [Google Scholar]
- 22.Bravo P, Edwards A, Rollnick S, Elwyn G. Tough decisions faced by people living with HIV: a literature review of psychosocial problems. Aids Rev. 2010;12:76–88. [PubMed] [Google Scholar]
- 23.Gallo LC, Smith TW. Patterns of hostility and social support: Conceptualizing psychosocial risk factors as characteristics of the person and the environment. Journal of Research in Personality. 1999;33:281–310. [Google Scholar]
- 24.Hardy JD, Smith TW. Cynical hostility and vulnerability to disease: Social support, life stress, and physiological response to conflict. Health Psychology. 1988;7:447. doi: 10.1037//0278-6133.7.5.447. [DOI] [PubMed] [Google Scholar]
- 25.Hart KE. Perceived availability of different types of social support among cynically hostile women. Journal of clinical psychology. 1996;52:383–387. doi: 10.1002/(SICI)1097-4679(199607)52:4<383::AID-JCLP2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 26.Watkins PL, Ward CH, Southard DR, Fisher EB. The Type A belief system: Relationships to hostility, social support, and life stress. Behavioral Medicine. 1992;18:27–32. doi: 10.1080/08964289.1992.10544238. [DOI] [PubMed] [Google Scholar]
- 27.Benotsch EG, Christensen AJ, McKelvey L. Hostility, social support, and ambulatory cardiovascular activity. Journal of Behavioral Medicine. 1997;20:163–176. doi: 10.1023/a:1025530711432. [DOI] [PubMed] [Google Scholar]
- 28.Guyll M, Contrada RJ. Trait hostility and ambulatory cardiovascular activity: responses to social interaction. Health Psychology. 1998;17:30. doi: 10.1037//0278-6133.17.1.30. [DOI] [PubMed] [Google Scholar]
- 29.Uchino BN. Social support and health: a review of physiological processes potentially underlying links to disease outcomes. Journal of behavioral medicine. 2006;29:377–387. doi: 10.1007/s10865-006-9056-5. [DOI] [PubMed] [Google Scholar]
- 30.Bogart LM, Thorburn S. Are HIV/AIDS conspiracy beliefs a barrier to HIV prevention among African Americans? JAIDS Journal of Acquired Immune Deficiency Syndromes. 2005;38:213–218. doi: 10.1097/00126334-200502010-00014. [DOI] [PubMed] [Google Scholar]
- 31.Corbie-Smith G, Thomas SB, Williams MV, Moody-Ayers S. Attitudes and beliefs of African Americans toward participation in medical research. Journal of General Internal Medicine. 1999;14:537–546. doi: 10.1046/j.1525-1497.1999.07048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klonoff EA, Landrine H. Do blacks believe that HIV/AIDS is a government conspiracy against them? Preventive Medicine. 1999;28:451–457. doi: 10.1006/pmed.1999.0463. [DOI] [PubMed] [Google Scholar]
- 33.Sengupta S, Strauss RP, DeVellis R, et al. Factors affecting African-American participation in AIDS research. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2000;24:275–284. doi: 10.1097/00126334-200007010-00014. [DOI] [PubMed] [Google Scholar]
- 34.Ashton E, Vosvick M, Chesney M, et al. Social support and maladaptive coping as predictors of the change in physical health symptoms among persons living with HIV/AIDS. AIDS Patient Care & STDs. 2005;19:587–598. doi: 10.1089/apc.2005.19.587. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez JS, Penedo FJ, Antoni MH, et al. Social support, positive states of mind, and HIV treatment adherence in men and women living with HIV/AIDS. Health Psychology. 2004;23:413. doi: 10.1037/0278-6133.23.4.413. [DOI] [PubMed] [Google Scholar]
- 36.Ironson G, Hayward HS. Do positive psychosocial factors predict disease progression in HIV-1? A review of the evidence. Psychosomatic medicine. 2008;70:546–554. doi: 10.1097/PSY.0b013e318177216c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brownley KA, Light KC, Anderson NB. Social support and hostility interact to influence clinic, work, and home blood pressure in Black and White men and women. Psychophysiology. 1996;33:434–445. doi: 10.1111/j.1469-8986.1996.tb01069.x. [DOI] [PubMed] [Google Scholar]
- 38.Hart KE, Hope CW. Cynical hostility and the psychosocial vulnerability model of disease risk: Confounding effects of neuroticism (negative affectivity) bias. Personality and individual differences. 2004;36:1571–1582. [Google Scholar]
- 39.Felsten G. Hostility, stress and symptoms of depression. Personality and Individual Differences. 1996;21:461–467. [Google Scholar]
- 40.Heponiemi T, Elovainio M, Kivimäki M, et al. The longitudinal effects of social support and hostility on depressive tendencies. Social Science & Medicine. 2006;63:1374–1382. doi: 10.1016/j.socscimed.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 41.Nabi H, Singh-Manoux A, Ferrie JE, et al. Hostility and depressive mood: results from the Whitehall II prospective cohort study. Psychological medicine. 2010;40:405. doi: 10.1017/S0033291709990432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suls J, Bunde J. Anger, anxiety, and depression as risk factors for cardiovascular disease: the problems and implications of overlapping affective dispositions. Psychological bulletin. 2005;131:260. doi: 10.1037/0033-2909.131.2.260. [DOI] [PubMed] [Google Scholar]
- 43.Vandervoort D. Depression, anxiety, hostility, and physical health. Current Psychology. 1995;14:69–82. [Google Scholar]
- 44.Blaney NT, Morgan RO, Feaster D, et al. Cynical Hostility: A Risk Factor in HIV-1 Infection? 1. Journal of Applied Social Psychology. 1991;21:668–695. [Google Scholar]
- 45.Weaver KE, Llabre MM, Durán RE, et al. A stress and coping model of medication adherence and viral load in HIV-positive men and women on highly active antiretroviral therapy (HAART) Health psychology. 2005;24:385. doi: 10.1037/0278-6133.24.4.385. [DOI] [PubMed] [Google Scholar]
- 46.Hartzell JD, Janke IE, Weintrob AC. Impact of depression on HIV outcomes in the HAART era. Journal of Antimicrobial Chemotherapy. 2008;62:246–255. doi: 10.1093/jac/dkn193. [DOI] [PubMed] [Google Scholar]
- 47.Leserman J, Petitto JM, Golden RN, et al. Impact of stressful life events, depression, social support, coping, and cortisol on progression to AIDS. American Journal of Psychiatry. 2000;157:1221–1228. doi: 10.1176/appi.ajp.157.8.1221. [DOI] [PubMed] [Google Scholar]
- 48.Maan Diong S, Bishop GD, Enkelmann HC, et al. Anger, stress, coping, social support and health: Modelling the relationships. Psychology & Health. 2005;20:467–495. [Google Scholar]
- 49.Vandervoort DJ. Hostility and health: Mediating effects of belief systems and coping styles. Current Psychology. 2006;25:50–66. [Google Scholar]
- 50.Chida Y, Vedhara K. Adverse psychosocial factors predict poorer prognosis in HIV disease: A meta-analytic review of prospective investigations. Brain, behavior, and immunity. 2009;23:434–445. doi: 10.1016/j.bbi.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 51.McIntosh RC, Rosselli M. Stress and Coping in Women Living with HIV: A Meta-Analytic Review. AIDS and Behavior. 2012;16:2144–2159. doi: 10.1007/s10461-012-0166-5. [DOI] [PubMed] [Google Scholar]
- 52.Moskowitz JT, Hult JR, Bussolari C, Acree M. What works in coping with HIV? A meta-analysis with implications for coping with serious illness. Psychological Bulletin. 2009;135:121. doi: 10.1037/a0014210. [DOI] [PubMed] [Google Scholar]
- 53.Castro KG, Ward JW, Slutsker L, et al. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. Clinical Infectious Diseases. 1993;17:802–810. [Google Scholar]
- 54.Chesney MA, Ickovics J, Chambers D, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. AIDS care. 2000;12:255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 55.Spielberger CD, Johnson EH, Russell SF, et al. The experience and expression of anger: Construction and validation of an anger expression scale. Anger and hostility in cardiovascular and behavioral disorders. 1985:5–30. [Google Scholar]
- 56.Cook WW, Medley DM. Proposed hostility and pharisaic-virtue scales for the MMPI. Journal of Applied Psychology. 1954;38:414. [Google Scholar]
- 57.Costa PT, Zonderman AB, McCrae RR, Williams RB. Cynicism and paranoid alienation in the Cook and Medley HO Scale. Psychosomatic medicine. 1986;48:283–285. doi: 10.1097/00006842-198603000-00014. [DOI] [PubMed] [Google Scholar]
- 58.Carver CS, Scheier MF, Weintraub JK. Assessing coping strategies: a theoretically based approach. Journal of personality and social psychology. 1989;56:267. doi: 10.1037//0022-3514.56.2.267. [DOI] [PubMed] [Google Scholar]
- 59.Cutrona CE, Russell DW. The provisions of social relationships and adaptation to stress. Advances in personal relationships. 1987;1:37–67. [Google Scholar]
- 60.Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosomatic medicine. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 61.Beck AT, Ward C, Mendelson M. Beck depression inventory (BDI) Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 62.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of health and social behavior. 1983:385–396. [PubMed] [Google Scholar]
- 63.Enders CK, Bandalos DL. The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Structural Equation Modeling. 2001;8:430–457. [PubMed] [Google Scholar]
- 64.Kline RB. Principles and practice of structural equation modeling. Guilford press; 2011. [Google Scholar]
- 65.Muthén L, Muthén B. Mplus. Statistical analysis with latent variables. Version. 2007;3 [Google Scholar]
- 66.Byrne BM. Structural equation modeling with Mplus: Basic concepts, applications, and programming. Routledge Academic New York; 2011. [Google Scholar]
- 67.Bentler PM. Comparative fit indexes in structural models. Psychological bulletin. 1990;107:238. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- 68.Jöreskog KG, Sörbom D. Lisrel 8: Structured equation modeling with the Simplis command language. Scientific Software International; 1993. [Google Scholar]
- 69.Steiger JH. Structural model evaluation and modification: An interval estimation approach. Multivariate behavioral research. 1990;25:173–180. doi: 10.1207/s15327906mbr2502_4. [DOI] [PubMed] [Google Scholar]
- 70.Hu Lt, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 1999;6:1–55. [Google Scholar]
- 71.Hooper D, Coughlan J, Mullen M. Structural equation modelling: guidelines for determining model fit. Articles. 2008:2. [Google Scholar]
- 72.Schermelleh-Engel K, Moosbrugger H, Müller H. Evaluating the fit of structural equation models: Tests of significance and descriptive goodness-of-fit measures. Methods of psychological research online. 2003;8:23–74. [Google Scholar]
- 73.Alonzo AA, Reynolds NR. Stigma, HIV and AIDS: An exploration and elaboration of a stigma trajectory. Social Science & Medicine. 1995;41:303–315. doi: 10.1016/0277-9536(94)00384-6. [DOI] [PubMed] [Google Scholar]
- 74.Leserman J. Role of depression, stress, and trauma in HIV disease progression. Psychosomatic Medicine. 2008;70:539–545. doi: 10.1097/PSY.0b013e3181777a5f. [DOI] [PubMed] [Google Scholar]
- 75.Vandervoort DJ. Depression, anxiety, hostility, and physical health. Current Psychology. 1995;14:69–82. [Google Scholar]
- 76.Barefoot JC, Dodge KA, Peterson BL, Dahlstrom WG, Williams RB. The Cook-Medley hostility scale: item content and ability to predict survival. Psychosomatic medicine. 1989;51:46–57. doi: 10.1097/00006842-198901000-00005. [DOI] [PubMed] [Google Scholar]
- 77.Giancola PR, Saucier DA, Gussler-Burkhardt NL. The Effects of Affective, Behavioral, and Cognitive Components of Trait Anger on the Alcohol-Aggression Relation. Alcoholism: Clinical and Experimental Research. 2003;27:1944–1954. doi: 10.1097/01.ALC.0000102414.19057.80. [DOI] [PubMed] [Google Scholar]
- 78.Evans DL, Leserman J, Perkins DO, et al. Severe life stress as a predictor of early disease progression in HIV infection. American Journal of Psychiatry. 1997;154:630–634. doi: 10.1176/ajp.154.5.630. [DOI] [PubMed] [Google Scholar]
- 79.Rabkin JG. HIV and depression: 2008 review and update. Current HIV/AIDS Reports. 2008;5:163–171. doi: 10.1007/s11904-008-0025-1. [DOI] [PubMed] [Google Scholar]
- 80.Del Guerra F, Fonseca J, Figueiredo V, Ziff E, Konkiewitz EC. Human immunodeficiency virus-associated depression: contributions of immuno-inflammatory, monoaminergic, neurodegenerative, and neurotrophic pathways. Journal of neurovirology. 2013:1–14. doi: 10.1007/s13365-013-0177-7. [DOI] [PubMed] [Google Scholar]
- 81.Schuster R, Bornovalova M, Hunt E. The Influence of Depression on the Progression of HIV Direct and Indirect Effects. Behavior modification. 2012;36:123–145. doi: 10.1177/0145445511425231. [DOI] [PubMed] [Google Scholar]
- 82.Beck AT. Prisoners of hate. HarperCollins: 2010. [Google Scholar]
- 83.Deffenbacher JL, Oetting ER, Lynch RS, Morris CD. The expression of anger and its consequences. Behaviour Research and Therapy. 1996;34:575–590. doi: 10.1016/0005-7967(96)00018-6. [DOI] [PubMed] [Google Scholar]
- 84.Martin RC, Dahlen ER. Cognitive emotion regulation in the prediction of depression, anxiety, stress, and anger. Personality and Individual Differences. 2005;39:1249–1260. [Google Scholar]
- 85.Vandervoort DJ. Belief systems and coping styles as mediating variables in the relationship between hostility and illness. Current Psychology. 1992;11:226–235. [Google Scholar]
- 86.Fitzgerald GJ. Hostility now, depression later? Longitudinal associations among emotional risk factors for coronary artery disease. Annals of Behavioral Medicine. 2010;39:258–266. doi: 10.1007/s12160-010-9185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gonzalez JS, Penedo FJ, Llabre MM, et al. Physical symptoms, beliefs about medications, negative mood, and long-term HIV medication adherence. Annals of Behavioral Medicine. 2007;34:46–55. doi: 10.1007/BF02879920. [DOI] [PubMed] [Google Scholar]
- 88.Llabre MM, Weaver KE, Durán RE, et al. A measurement model of medication adherence to highly active antiretroviral therapy and its relation to viral load in HIV-positive adults. AIDS Patient Care & STDs. 2006;20:701–711. doi: 10.1089/apc.2006.20.701. [DOI] [PubMed] [Google Scholar]
- 89.Penedo FJ, Gonzalez JS, Dahn JR, et al. Personality, quality of life and HAART adherence among men and women living with HIV/AIDS. Journal of Psychosomatic Research. 2003;54:271–278. doi: 10.1016/s0022-3999(02)00482-8. [DOI] [PubMed] [Google Scholar]
- 90.Gore-Felton C, Koopman C, Spiegel D, et al. Effects of quality of life and coping on depression among adults living with HIV/AIDS. Journal of health psychology. 2006;11:711–729. doi: 10.1177/1359105306066626. [DOI] [PubMed] [Google Scholar]
- 91.McIntosh RC, Seay JS, Antoni MH, Schneiderman N. Cognitive vulnerability for depression in HIV. Journal of affective disorders. 2013 doi: 10.1016/j.jad.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 92.van der Veek SM, Kraaij V, Van Koppen W, Garnefski N, Joekes K. Goal disturbance, cognitive coping and psychological distress in HIV-infected persons. Journal of health psychology. 2007;12:225–230. doi: 10.1177/1359105307074249. [DOI] [PubMed] [Google Scholar]
- 93.Antoni M, Ironson G, Schneiderman N. Stress management for persons with HIV infection. New York: Oxford University Press; 2007. [Google Scholar]
- 94.Lutgendorf SK, Antoni MH, Ironson G, et al. Changes in cognitive coping skills and social support during cognitive behavioral stress management intervention and distress outcomes in symptomatic human immunodeficiency virus (HIV)-seropositive gay men. Psychosomatic Medicine. 1998;60:204–214. doi: 10.1097/00006842-199803000-00017. [DOI] [PubMed] [Google Scholar]
- 95.Cruess S, Antoni M, Cruess D, et al. Reductions in herpes simplex virus type 2 antibody titers after cognitive behavioral stress management and relationships with neuroendocrine function, relaxation skills, and social support in HIV-positive men. Psychosomatic Medicine. 2000;62:828–837. doi: 10.1097/00006842-200011000-00013. [DOI] [PubMed] [Google Scholar]
- 96.Antoni M, Cruess D, Wagner S, et al. Cognitive behavioral stress management effects on anxiety, 24-hour urinary catecholamine output, and T-cytotoxic/suppressor cells over time among symptomatic HIV-infected gay men. Journal of Consulting and Clinical Psychology. 2000;68:31–45. doi: 10.1037//0022-006x.68.1.31. [DOI] [PubMed] [Google Scholar]
- 97.Antoni MH, Cruess S, Cruess DG, et al. Cognitive-behavioral stress management reduces distress and 24-hour urinary free cortisol output among symptomatic HIV-infected gay men. Annals of Behavioral Medicine. 2000;22:29–37. doi: 10.1007/BF02895165. [DOI] [PubMed] [Google Scholar]
- 98.Antoni MH, Carrico AW, Durán RE, et al. Randomized clinical trial of cognitive behavioral stress management on human immunodeficiency virus viral load in gay men treated with highly active antiretroviral therapy. Psychosomatic Medicine. 2006;68:143–151. doi: 10.1097/01.psy.0000195749.60049.63. [DOI] [PubMed] [Google Scholar]
- 99.Antoni MH, Pereira DB, Marion I, et al. Stress management effects on perceived stress and cervical neoplasia in low-income HIV-infected women. Journal of psychosomatic research. 2008;65:389–401. doi: 10.1016/j.jpsychores.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Antoni MH, Cruess DG, Klimas N, et al. Increases in a marker of immune system reconstitution are predated by decreases in 24-h urinary cortisol output and depressed mood during a 10-week stress management intervention in symptomatic HIV-infected men. Journal of psychosomatic research. 2005;58:3–13. doi: 10.1016/j.jpsychores.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 101.Antoni MH, Cruess DG, Klimas N, et al. Stress management and immune system reconstitution in symptomatic HIV-infected gay men over time: Effects on transitional naïve T cells (CD4+ CD45RA+ CD29+) American Journal of Psychiatry. 2002;159:143–145. doi: 10.1176/appi.ajp.159.1.143. [DOI] [PubMed] [Google Scholar]
- 102.Schieman S. Age and anger. Journal of health and Social Behavior. 1999:273–289. [PubMed] [Google Scholar]
- 103.Phillips L, Henry J, Hosie J, Milne A. Age, anger regulation and well-being. Aging and Mental Health. 2006;10:250–256. doi: 10.1080/13607860500310385. [DOI] [PubMed] [Google Scholar]
- 104.Singh N, Squier C, Sivek C, Wagener MM, Victor LY. Psychological stress and depression in older patients with intravenous drug use and human immunodeficiency virus infection: implications for intervention. International journal of STD & AIDS. 1997;8:251–255. doi: 10.1258/0956462971920000. [DOI] [PubMed] [Google Scholar]
- 105.LeBlanc AJ. Handbook of Sociology of Aging. Springer; 2011. Aging with HIV/AIDS; pp. 495–512. [Google Scholar]
- 106.Catz SL, Gore-Felton C, McClure JB. Psychological distress among minority and low-income women living with HIV. Behavioral Medicine. 2002;28:53–60. doi: 10.1080/08964280209596398. [DOI] [PubMed] [Google Scholar]
- 107.Watkins DC. Depression Over the Adult Life Course for African American Men Toward a Framework for Research and Practice. American Journal of Men's Health. 2012;6:194–210. doi: 10.1177/1557988311424072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mabry JB, Kiecolt KJ. Anger in black and white: Race, alienation, and anger. Journal of Health and Social Behavior. 2005;46:85–101. doi: 10.1177/002214650504600107. [DOI] [PubMed] [Google Scholar]
- 109.Scherwitz L, Perkins L, Chesney M, Hughes G. Cook-Medley Hostility scale and subsets: relationship to demographic and psychosocial characteristics in young adults in the CARDIA study. Psychosomatic Medicine. 1991;53:36–49. doi: 10.1097/00006842-199101000-00004. [DOI] [PubMed] [Google Scholar]
- 110.Turner J, Kelly B. Emotional dimensions of chronic disease. Western Journal of Medicine. 2000;172:124. doi: 10.1136/ewjm.172.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Annals of internal medicine. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 112.Gonzalez JS, Batchelder AW, Psaros C, Safren SA. Depression and HIV/AIDS treatment nonadherence: a review and meta-analysis. Journal of acquired immune deficiency syndromes (1999) 2011:58. doi: 10.1097/QAI.0b013e31822d490a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Springer SA, Dushaj A, Azar MM. The impact of DSM-IV Mental Disorders on adherence to combination antiretroviral therapy among adult persons living with HIV/AIDS: a systematic review. AIDS and Behavior. 2012;16:2119–2143. doi: 10.1007/s10461-012-0212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Burgoyne R, Renwick R. Social support and quality of life over time among adults living with HIV in the HAART era. Social Science & Medicine. 2004;58:1353–1366. doi: 10.1016/S0277-9536(03)00314-9. [DOI] [PubMed] [Google Scholar]
- 115.Jia H, Uphold CR, Wu S, Chen GJ, Duncan PW. Predictors of changes in health-related quality of life among men with HIV infection in the HAART era. AIDS Patient Care & STDs. 2005;19:395–405. doi: 10.1089/apc.2005.19.395. [DOI] [PubMed] [Google Scholar]
- 116.Pakenham KI, Dadds MR, Terry DJ. Relationship between adjustment to HIV and both social support and coping. Journal of Consulting and Clinical Psychology. 1994;62:1194. doi: 10.1037//0022-006x.62.6.1194. [DOI] [PubMed] [Google Scholar]
- 117.Hogg RS, Yip B, Chan KJ, et al. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA: the journal of the American Medical Association. 2001;286:2568–2577. doi: 10.1001/jama.286.20.2568. [DOI] [PubMed] [Google Scholar]
- 118.Paredes R, Mocroft A, Kirk O, et al. Predictors of virological success and ensuing failure in HIV-positive patients starting highly active antiretroviral therapy in Europe: results from the EuroSIDA study. Archives of internal medicine. 2000;160:1123. doi: 10.1001/archinte.160.8.1123. [DOI] [PubMed] [Google Scholar]
- 119.Murphy EL, Collier AC, Kalish LA, et al. Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Annals of internal medicine. 2001;135:17–26. doi: 10.7326/0003-4819-135-1-200107030-00005. [DOI] [PubMed] [Google Scholar]
- 120.Paltiel AD, Weinstein MC, Kimmel AD, et al. Expanded screening for HIV in the United States—an analysis of cost-effectiveness. New England Journal of Medicine. 2005;352:586–595. doi: 10.1056/NEJMsa042088. [DOI] [PubMed] [Google Scholar]
- 121.Kass NE, Munoz A, Chen B, et al. Changes in employment, insurance, and income in relation to HIV status and disease progression. JAIDS Journal of Acquired Immune Deficiency Syndromes. 1994;7:86–91. [PubMed] [Google Scholar]
- 122.Rueda S, Raboud J, Mustard C, et al. Employment status is associated with both physical and mental health quality of life in people living with HIV. AIDS care. 2011;23:435–443. doi: 10.1080/09540121.2010.507952. [DOI] [PubMed] [Google Scholar]
- 123.White L, Cant B. Social networks, social support, health and HIV-positive gay men. Health & social care in the community. 2003;11:329–334. doi: 10.1046/j.1365-2524.2003.00431.x. [DOI] [PubMed] [Google Scholar]
- 124.Carrico AW, Antoni MH, Durán RE, et al. Reductions in depressed mood and denial coping during cognitive behavioral stress management with HIV-positive gay men treated with HAART. Annals of Behavioral Medicine. 2006;31:155–164. doi: 10.1207/s15324796abm3102_7. [DOI] [PubMed] [Google Scholar]
- 125.Gallo LC, Matthews KA. Understanding the association between socioeconomic status and physical health: do negative emotions play a role? Psychological bulletin. 2003;129:10. doi: 10.1037/0033-2909.129.1.10. [DOI] [PubMed] [Google Scholar]
- 126.Brummett BH, Boyle SH, Ortel TL, et al. Associations of depressive symptoms, trait hostility, and gender with C-reactive protein and interleukin-6 response after emotion recall. Psychosomatic medicine. 2010;72:333–339. doi: 10.1097/PSY.0b013e3181d2f104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Stewart JC, Janicki-Deverts D, Muldoon MF, Kamarck TW. Depressive symptoms moderate the influence of hostility on serum interleukin-6 and C-reactive protein. Psychosomatic medicine. 2008;70:197–204. doi: 10.1097/PSY.0b013e3181642a0b. [DOI] [PubMed] [Google Scholar]
- 128.Gandhi RT, Walker BD. Immunologic control of HIV-1. Annual review of medicine. 2002;53:149–172. doi: 10.1146/annurev.med.53.082901.104011. [DOI] [PubMed] [Google Scholar]
- 129.Dinarello CA. Interleukin-1 and its biologically related cytokines. Advances in immunology. 1989;44:153–205. doi: 10.1016/s0065-2776(08)60642-2. [DOI] [PubMed] [Google Scholar]
- 130.Porter KM, Sutliff RL. HIV-1, reactive oxygen species, and vascular complications. Free Radical Biology and Medicine. 2012;53:143–159. doi: 10.1016/j.freeradbiomed.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Maggi P, Maserati R, Antonelli G. Atherosclerosis in HIV patients: a new face for an old disease. Aids Rev. 2006;8:204–209. [PubMed] [Google Scholar]
- 132.Grinspoon SK. Metabolic syndrome and cardiovascular disease in patients with human immunodeficiency virus. The American Journal of Medicine Supplements. 2005;118:23–28. doi: 10.1016/j.amjmed.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 133.Hurwitz BE, Klimas NG, Llabre MM, et al. HIV, metabolic syndrome X, inflammation, oxidative stress, and coronary heart disease risk. Cardiovascular toxicology. 2004;4:303–315. doi: 10.1385/ct:4:3:303. [DOI] [PubMed] [Google Scholar]