Abstract
BACKGROUND
While the size and type of a vaccine container (i.e., primary container) can have many implications on the safety and convenience of a vaccination session, another important but potentially overlooked consideration is how the design of the primary container may affect the distribution of the vaccine, its resulting cost, and whether the vial is ultimately opened.
METHODS
Using our HERMES software platform, we developed a simulation model of the World Health Organization Expanded Program on Immunization supply chain for the Republic of Benin and used the model to explore the effects of different primary containers for various vaccine antigens.
RESULTS
Replacing vaccines with presentations containing fewer doses per vial reduced vaccine availability (proportion of people arriving for vaccines who are successfully immunized) by as much as 13% (from 73% at baseline) and raised logistics costs by up to $0.06 per dose administered (from $0.25 at baseline) due to increased bottlenecks, while reducing total costs by as much as $0.15 per dose administered (from $2.52 at baseline) due to lower open vial wastage. Primary containers with a greater number of doses per vial each improved vaccine availability by 19% and reduced logistics costs by $0.05 per dose administered, while raising the total costs by up to $0.25 per dose administered due to greater vaccine procurement needs. Changes in supply chain performance were more extreme in departments with greater constraints. Implementing a vial opening threshold reversed the direction of many of these effects.
CONCLUSIONS
Our results show that one size may not fit all when choosing a primary vaccine container. Rather, the choice depends on characteristics of the vaccine, the vaccine supply chain, immunization session size, and goals of decision-makers. In fact, the optimal vial size may vary among locations within a country. Simulation modeling can help identify tailored approaches to improve availability and efficiency.
Keywords: immunization, supply chain, vial size, simulation modeling
INTRODUCTION
While the size and type of a vaccine container (i.e., primary container) can have many implications on the safety and convenience of a vaccination session, another important but potentially overlooked consideration is how the design of the primary container may affect the distribution of the vaccine, its resulting cost, and whether the vial is opened depending on the policies and session [1, 2]. The primary container is the vial or bottle in which the vaccine antigen is directly placed for storage and transport and can vary by size, shape, the number of vaccine doses it carries, and the presence or absence of an integrated administration device (e.g., a needle and syringe). These characteristics can affect the amount of storage and transport capacity required as well as vaccine wastage (i.e., if all doses in an opened container are not used during the same day, remaining doses may need to be discarded, depending on vaccine characteristics and program policies).
An immunization supply chain has three main objectives: making available the necessary vaccines and supplies, preserving vaccine potency, and utilizing resources efficiently [3]. Since a supply chain includes many different locations, storage devices, and transport vehicles, determining the combined effect of primary container characteristics on these supply chain objectives can be difficult without the use of computational simulation modeling. Therefore, using our HERMES (Highly Extensible Resource for Modeling Supply Chains) software platform, we developed a simulation model of the World Health Organization (WHO) Expanded Program on Immunization (EPI) supply chain for the Republic of Benin and used the model to explore the effects of different primary containers for various vaccines. In particular, we studied the impact of primary container choice on two major supply chain objectives: availability and efficiency.
METHODS
HERMES Model of the Benin Vaccine Supply Chain
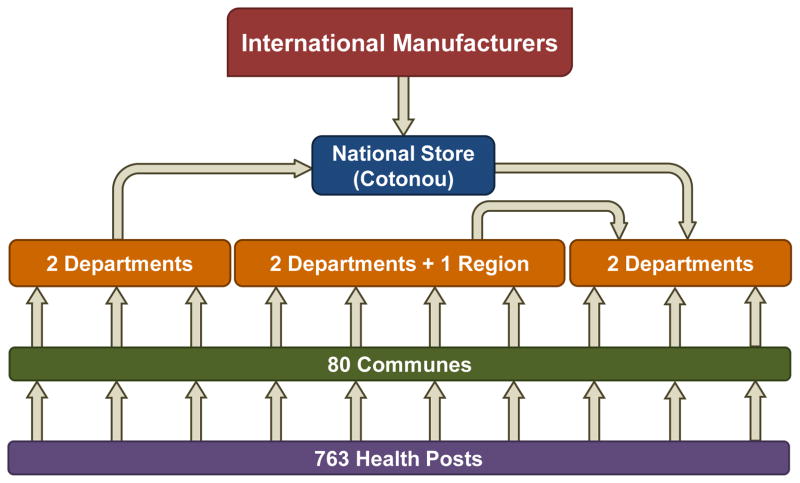
As described in a previous publication, a combined team of Agence de Médecine Préventive (AMP), PATH, and the HERMES Logistics Modeling Team developed a discrete-event simulation model of the Benin immunization supply chain [4]. Our HERMES model includes virtual representations of each vaccine vial, facility, storage equipment, transport device, route, and personnel in the supply chain as well as the anticipated demand at each immunization location. The model includes characteristics of the current EPI vaccines and one impending introduction [5, 6], summarized in Table 1. National ordering and shipping policies govern the flow of vaccines through the country’s four-level supply chain, shown in Figure 1.
Table 1.
Vaccine characteristics in Benin supply chain model
| Vaccine | Presentation | Storage Location | Doses per Person | Baseline presentations | Experimental presentations | ||||
|---|---|---|---|---|---|---|---|---|---|
| Doses per Vial | Vaccine Packed Volume per Dose | Diluent Packed Volume per Dose | Doses per Vial | Vaccine Packed Volume per Dose | Diluent Packed Volume per Dose | ||||
| Diphtheria-tetanus- pertussis-haemophilus influenza type B-hepatitis B vaccine (DTP-HepB-Hib) | Liquid | Refrigerator (2°C-8°C) | 3 | 2 | 9.9 cm3 | n/a | 10 | 2.6 cm3 | n/a |
| 1 | 10.3 cm3 | n/a | |||||||
| Oral polio vaccine (OPV) | Liquid | Preferably freezer (−15°C - 0°C) | 4 | 20 | 0.7 cm3 | n/a | 10 | 1.0 cm3 | n/a |
| Bacille Calmette-Guérin tuberculosis vaccine (BCG) | Lyophilized | Refrigerator (2°C-8°C) | 1 | 20 | 1.3 cm3 | 0.6 cm3 | 10 | 2.3 cm3 | 1.0 cm3 |
| Yellow fever vaccine (YF) | Lyophilized | Refrigerator (2°C-8°C) | 1 | 10 | 2.5 cm3 | 2.5 cm3 | 2 | 7.2 cm3 | 7.2 cm3 |
| Tetanus toxoid vaccine (TT) | Liquid | Refrigerator (2°C-8°C) | 2 | 10 | 2.6 cm3 | n/a | 1 | 15.7 cm3 | n/a |
| Measles vaccine (M) | Lyophilized | Refrigerator (2°C-8°C) | 1 | 10 | 1.3 cm3 | 2.5 cm3 | 1 | 26.1 cm3 | 15.7 cm3 |
| Pneumococcal conjugate vaccine (PCV) | Liquid | Refrigerator (2°C-8°C) | 3 | 1 | 12.0 cm3 | n/a | 2 | 4.8 cm3 | n/a |
| Rotavirus vaccine (RV) | Liquid | Refrigerator (2°C-8°C) | 2 | 1 | 17.1 cm3 | n/a | n/a | n/a | n/a |
Figure 1.
Structure of Benin vaccine supply chain
In Benin, the frequency of immunization sessions at each immunizing location is determined by the size of the population served, in order to maintain approximately uniform session sizes across all health posts and open vial wastage rates that conform to national guidelines. While policy dictates that health workers open a new vaccine vial, if necessary, to immunize any patient who arrives for vaccines, national policy also requires health workers to discard all open vials at the end of every immunization session. Our model follows these stated policies, but this study also includes experiments based on anecdotal evidence of health workers turning away patients whose immunizations would lead to the wastage of most doses in a vaccine vial.
Primary Container Scenarios
We explored nine experimental scenarios which modified the baseline scenario, Benin’s current EPI with rotavirus vaccine (RV) introduced. To assess the impact of changing vaccine presentations to reduce the doses per vial, scenarios 1 through 6 each replaced one vaccine in Benin’s EPI with a presentation containing the fewest doses per vial available in the WHO pre-qualified catalogue. Scenario 7 combined the changes performed in scenarios 1–6.
-
1
Replacing 2-dose diphtheria-tetanus-pertussis-haemophilus influenza type B-hepatitis B vaccine (DTP-HepB-Hib) vial with 1-dose vial
-
2
Replacing 20-dose oral polio vaccine (OPV) vial with 10-dose vial
-
3
Replacing 20-dose Bacille Calmette-Guérin tuberculosis vaccine (BCG) vial with 10-dose vial
-
4
Replacing 10-dose yellow fever vaccine (YF) vial with 2-dose vial
-
5
Replacing 10-dose tetanus toxoid vaccine (TT) vial with 1-dose vial
-
6
Replacing 10-dose measles vaccine (M) vial with 1-dose vial
-
7
Replacing DTP-HepB-Hib, OPV, BCG, YF, TT, and M vaccines with presentations described in scenarios 1 through 6.
Of the presentations currently used in Benin, DTP-HepB-Hib and pneumococcal conjugate vaccine (PCV) contain the fewest doses per vial (two and one, respectively). Scenarios 8 and 9 each replaced one of these vaccines with a presentation containing a greater number of doses per vial.
-
8
Replacing 2-dose DTP-HepB-Hib vial with 10-dose vial
-
9
Replacing 1-dose PCV vial with 2-dose vial
To assess the implications of wastage guidelines on missed opportunities, we also ran the baseline and all experimental scenarios assuming a vial opening threshold, which required that a vial only be opened if at least half of the doses would be administered that day. The model assumed that individuals whose vaccinations would violate that policy were turned away and did not return.
Supply chain performance metrics
For each scenario, we ran 10 iterations of a single simulated year (2012) and report the mean for each output of interest. We determined vaccine availability (i.e., the proportion of people arriving at a health post for whom the necessary vaccine is available) in each scenario using the following equation:
We calculated the open vial wastage rate (OVW) for each vaccine using the following equation, and we report the average OVW across all vaccines:
We calculated the total annual logistics costs accrued (in 2012 USD) for storage equipment (energy usage, maintenance, depreciation), transportation (fuel usage, vehicle maintenance, depreciation, per diems), buildings (overhead, depreciation), and labor (personnel salaries). We determined the system costs associated with each dose administered using the following equations:
In each simulation, we observed the transport and storage capacity utilization at each location and route in the supply chain and quantified constraints.
RESULTS
Baseline System
Figure 2 presents supply chain indicators for the baseline and experimental scenarios with no vial opening threshold. The baseline scenario, in which RV was introduced to the current EPI with no improvements to the supply chain, yielded 73% overall vaccine availability with an average OVW of 25%. This was achieved at a logistics cost of $0.25 per dose administered and a total cost (including vaccine procurement) of $2.52 per dose administered. Supply chain constraints prevented many vaccines from reaching health posts and caused missed opportunities when people arrived for vaccines that were not available, as evidenced by vaccine availability below 100%. Storage bottlenecks existed at several commune-level stores. Transport bottlenecks existed at all levels, and 64% of routes had insufficient capacity to carry the quantity ordered. This led to coping, where possible, in the form of more frequent trips than prescribed by national policies. Coping was most prominent at health posts, which took an average of 20 trips per year to pick up vaccines (whereas policy dictates monthly trips).
Figure 2.
Supply chain performance metrics under each vaccine presentation scenario
Switching to Fewer-Dose Vials
In every scenario with fewer-dose vials, vaccine availability dropped. Individually, these alternative vaccine presentations reduced availability by between <1% (switching to a 1-dose DTP-HepB-Hib vaccine vial) and 6% (switching to a 1-dose TT vaccine vial). When the six fewer-dose vials were evaluated together, overall availability decreased by 13%. The larger packaged volume of these presentations worsened storage and transport constraints primarily at the commune level, where the additional storage capacity requirement increased from 327L at baseline to 928L and the average additional transport capacity needed per route doubled from 19L at baseline to 38L. The six fewer-dose vials necessitated additional coping and raised the average number of trips from health posts by 1 trip annually, as 77% of routes across all levels experienced constraints. However, results were heterogeneous among individual locations and some regions were able to accommodate certain fewer-dose presentations without significant bottlenecks.
Fewer-dose vials all raised the logistics cost per dose administered. Individually, the 1-dose M vial and the 1-dose TT vial had the greatest effect, as each increased logistics costs by $0.02 per dose from baseline. Introducing the six fewer-dose vials together raised logistics costs by $0.06 per dose administered, due primarily to the reduced number of doses flowing through the system (annual logistics costs increased by approximately $8,000 from a baseline of $1,129,219).
All fewer-dose vials reduced the total cost per dose administered from the baseline. Vaccine procurement costs significantly drove total costs and were sensitive to both OVW (greater-dose vials incur higher wastage rates and necessitate the procurement of more vials) and supply chain bottlenecks (constraints can prevent locations from ordering necessary quantities if capacity is insufficient to store these vaccines). Individual fewer-dose vials reduced average OVW by between <1% and 5% and reduced the total cost per dose administered by between $0.03 (10-dose OPV and 1-dose TT) and $0.15 per dose (1-dose DTP-HepB-Hib). Introducing all six fewer-dose presentations reduced average OVW to 6% and reduced total program costs by more than $2,000,000 annually, but new system bottlenecks also reduced the number of doses administered, thereby reducing the total cost per dose administered by less than $0.01.
Switching to Greater-Dose Vials
The two alternative greater-dose vials each increased overall vaccine availability and reduced logistics costs per dose administered by alleviating supply chain bottlenecks. The two greater-dose containers each reduced the proportion of constrained routes to 52%. The 10-dose DTP-HepB-Hib vial increased overall vaccine availability by 19%, as did the 2-dose PCV vial. Each department experienced some increase in availability under these scenarios, with larger gains in the most severely constrained departments. The 10-dose DTP-HepB-Hib vial and the 2-dose PCV vial each individually reduced logistics costs by $0.05 per dose administered, and annual logistics costs decreased by nearly $7,000 in both scenarios.
Greater-dose presentations also reduced the total cost per dose administered, as increases in average OVW were relatively small. While switching to a 10-dose DTP-HepB-Hib vial raised the OVW for that vaccine by 17%, average OVW across all vaccines in the system increased by only 2%. This yielded a reduction in total costs of $0.25 per administered dose, despite a $1,331,336 increase in total annual costs as the supply chain became less constrained and allowed a greater volume of necessary vaccines to enter the system. Though approximately the same number of doses were administered in each of the two higher-dose presentation scenarios, the 2-dose PCV presentation reduced total costs from baseline by only $0.04 per dose, due to a $2,547,079 increase in annual costs from baseline. This increase was greater than that seen when switching to 10-dose DTP-HepB-Hib because the greater-dose PCV container raised OVW for PCV in particular (while average OVW across all vaccines increased by <1%), requiring procurement of additional PCV vials, which incurred the highest procurement cost of any vaccine studied.
Implementing a Vial Opening Threshold
The vial opening threshold reduced baseline OVW to 13% and vaccine availability to 71%. Evaluating all six fewer-dose vials together further reduced OVW to 4%, and availability dropped by 10% – a smaller reduction than observed without a vial opening threshold. Fewer-dose presentations for YF, BCG, and OPV moderately raised vaccine availability with the vial opening threshold, though these presentations had reduced availability when no threshold was considered. Switching to greater-dose vials for DTP-HepB-Hib and PCV each raised vaccine availability by 14%, a smaller gain than was achieved when adhering to official policy.
The vial opening threshold yielded baseline annual logistics costs that were approximately $4,000 higher than the baseline scenario without the threshold, and the logistics cost per dose administered was $0.01 higher. Fewer-dose vials of YF and OPV reduced the logistics cost per dose administered from baseline, as more doses were administered than in the baseline scenario. Substituting baseline vaccine presentations with all six fewer-dose vaccine presentations under the vial opening threshold raised average logistics costs by $0.05 per dose.
Total annual costs were approximately $600,000 lower at baseline with the vial opening threshold than under current policy but increased with three fewer-dose vial substitutions (2-dose YF, 10-dose BCG, and 10-dose OPV) as well as both greater-dose substitutions. The other fewer-dose vial scenarios yielded smaller reductions in total annual costs than seen under current policy. Similarly mixed results were seen in the total cost per dose administered, which increased from baseline in all but two fewer-dose vial scenarios (1-dose DTP-HepB-Hib and 10-dose BCG). The 10-dose DTP-HepB-Hib vial reduced the total cost per dose by $0.38, a greater reduction than achieved under current policy, but the 2-dose PCV raised the total cost per dose administered from baseline.
DISCUSSION
Our results show that one size may not fit all when choosing a primary vaccine container. Rather, the choice depends on the characteristics of the vaccine, the vaccine supply chain, immunization session size, and the goals of policy-makers and immunization program managers. Some stakeholders may prioritize certain measures over others (e.g., cost of immunizing a child over vaccine wastage). In Benin, prioritizing minimizing costs over reducing wastage would suggest that switching to greater-dose vials for DTP-HepB-Hib and PCV may be optimal. An alternative set of priorities could produce a different optimal container size. Simulation modeling can identify how various presentations affect supply chain metrics to inform stakeholders. Many industries utilize computational simulation to forecast the potential impact of changing product characteristics and to help choose the characteristics that best fit a country’s diverse needs. While not routinely performed for vaccines, our study shows such simulation could help identify more tailored approaches to improve availability and efficiency throughout a system.
We observed the complexity of such decision-making when we raised the doses per DTP-HepB-Hib vaccine vial from 2 to 10 and subsequently replaced it with a 1-dose vial. The effects on overall vaccine availability and OVW were as expected: Increasing the doses per vial raised both indicators, and reducing the doses per vial reduced both indicators. Similarly, due primarily to the changes in vaccine availability, the logistics cost per dose administered increased when the doses per vial decreased. However, the effects on total cost per dose did not follow such a clear trend. Increasing the doses per vial for the DTP-HepB-Hib vaccine reduced the total cost per dose administered by $0.25 from baseline, while reducing the doses per vial also reduced the total cost per dose administered, by $0.15 from baseline. Thus, even the direction of the effect on cost per dose when changing vial size can be difficult, or even impossible, to predict without a model that captures the dynamics of the supply chain. Switching to a 2-dose PCV vial demonstrated how sensitive these trends are to procurement costs, and simulation modeling can help determine the cost-effectiveness of new vaccine presentations at various prices.
Further adding to the complexity of primary container choice is the possibility that one vial size may not serve all regions equally well, even within the same country. Each of the seven departments in Benin faced different degrees of capacity constraints, and the vaccine availability at baseline ranged from 35% to 98% across different departments. In the department with the highest availability, switching to all six fewer-dose vials reduced availability by 4%. In contrast, making the same switch in the department with the lowest baseline performance reduced availability by 14%. For all experiments, departments with lower baseline availability generally experienced more extreme changes. While managing multiple presentations of each vaccine antigen may add complexity to immunization program logistics, tools such as computational simulation can help forecast and potentially overcome these challenges and tradeoffs as it has in other industries. Targeted investments to alleviate system bottlenecks may help maintain vaccine availability and diminish the increased logistics costs associated with introducing fewer-dose vials in all regions. Generally, incremental investments in cold chain could be viewed as relatively small when compared to the benefits of an efficiently run system.
A vial opening threshold revealed how health worker behavior could impact supply chain performance metrics and how various vaccine presentations might exacerbate or mitigate such effects. The vial opening threshold reduced overall vaccine availability at baseline and reversed the direction of some effects seen with alternative vial sizes, suggesting that deviation from stated policies can yield unintended – but impactful – consequences for vaccine supply chain performance. However, modeling may help diminish this real-world practice of health workers deviating from official vial opening policies. Other work suggests that predicting expected open vial wastage in an immunization system could not only help decision-makers to select appropriate vial sizes and session frequencies but could also provide realistic wastage targets, alleviating pressure on health workers to reduce open vial wastage below values that are unavoidable when following stated policies [7].
Safety is a critical factor to consider in decision-making for vaccine presentations but is challenging to capture in models and was not addressed in this study. Generally, greater-dose vials are thought to be more prone to user errors [8]. Inappropriate vaccine administration can potentially place children at risk of non-sterile injections and injections with expired vaccines [9]. This may be of particular concern in countries which are more risk averse, even in perceptions relating to safety of vaccine programs, versus countries whose goal is to minimize costs. Investments to train health workers in using new vaccine presentations would need to be considered when deciding whether to switch.
LIMITATIONS
Models, by their nature, are simplified representations of reality and cannot capture every factor that could affect the delivery of vaccines. This study focused on routine, facility-based immunization in Benin and did not include outreach immunization activities. Our model also did not capture the cost or safety implications of waste disposal. Incorrect waste disposal has been associated with risk of blood borne diseases among community members and health workers [10, 11].
CONCLUSIONS
One size may not fit all when choosing a primary vaccine container. The choice depends on vaccine characteristics, the supply chain, immunization session size, and the goals of stakeholders. In fact, for a given antigen, the optimal vial size may vary among different locations within the same country. When selecting primary container sizes, dynamic simulation modeling can help identify tailored approaches to improve availability and efficiency.
HIGHLIGHTS.
Primary container size affects many aspects of vaccine delivery and administration.
We used HERMES to simulate different primary vaccine containers in the Benin WHO EPI.
The ideal choice for vaccine administration may not be optimal for the whole system.
Deviation from vial opening policies may impact the optimal vial size.
The optimal vial size may be different for various locations within the same country.
Acknowledgments
This work was supported by the Bill and Melinda Gates Foundation via the HERMES grant and the LogiVac Project, the Agency for Healthcare Research and Quality (AHRQ) via grant R01HS023317, the National Institute of Child Health and Human Development (NICHD) and the Global Obesity Prevention Center (GOPC) via grant U54HD070725. A Vaccine Primary Container Roundtable, held on May 9-10, 2012 in Washington, DC, was supported by a series of donations from industry, PATH, and John Snow, Inc. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee BY, Assi T-M, Rookkapan K, Connor DL, Rajgopal J, Sornsrivichai V, et al. Replacing the measles ten-dose vaccine presentation with the single-dose presentation in Thailand. Vaccine. 2011;29:3811–7. doi: 10.1016/j.vaccine.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee BY, Norman BA, Assi T-M, Chen S-I, Bailey RR, Rajgopal J, et al. Single versus multi-dose vaccine vials: an economic computational model. Vaccine. 2010;28:5292–300. doi: 10.1016/j.vaccine.2010.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thornton D, Schreiber B. GAVI Alliance immunisation supply chain strategy. Report to the GAVI Alliance Board. 2014 [Google Scholar]
- 4.Brown ST, Schreiber B, Cakouros BE, Wateska AR, Dicko HM, Connor DL, et al. The benefits of redesigning Benin's vaccine supply chain. Vaccine. 2014;32:4097–103. doi: 10.1016/j.vaccine.2014.04.090. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. WHO prequalified vaccines. 2012 Available from: http://www.who.int/immunization_standards/vaccine_quality/PQ_vaccine_list_en/en/index.html.
- 6.UNICEF. Vaccine price data. 2012 Available from: http://www.unicef.org/supply/index_57476.html.
- 7.WHO Immunization Practices Advisory Committee (IPAC) Final meeting report and recommendations. World Health Organization Department of Immunizations, Vaccines, and Biologicals; 2013. [Google Scholar]
- 8.Pereira CC, Bishai D. Vaccine presentation in the USA: economics of prefilled syringes versus multidose vials for influenza vaccination. Expert Review of Vaccines. 2010;9:1343–9. doi: 10.1586/erv.10.129. [DOI] [PubMed] [Google Scholar]
- 9.DeBaun B. Transmission of infection with multi-dose vials. Infection Control Resource. 2005;3:1–7. [Google Scholar]
- 10.Babanyara Y, Ibrahim D, Garba T, Bogoro A, Abubakar M. Poor medical waste management (MWM) practices and its risks to human health and the environment: a literature review. International Journal of Environmental, Ecological, Geological and Mining Engineering. 2013;7:512–9. [Google Scholar]
- 11.Sagoe-Moses C, Pearson RD, Perry J, Jagger J. Risks to health care workers in developing countries. New England Journal of Medicine. 2001;345:538–41. doi: 10.1056/NEJM200108163450711. [DOI] [PubMed] [Google Scholar]