Abstract
Leukocyte trafficking is a tightly regulated process essential for an appropriate inflammatory response. We now report a new adhesion pathway that allows unstimulated leukocytes to adhere to and migrate through exposed endothelial matrix or high-density ligand, a process we have termed ligand-induced adhesion. This ligand-induced adhesion is integrin mediated, but in contrast to phorbol ester-stimulated adhesion, it is not dependent on the small GTPase Rap-1 activity. Instead, we show a critical role for cyclin-dependent kinase (Cdk) 4 in ligand-induced adhesion by three independent lines of evidence: inhibition by pharmacological inhibitors of Cdk, inhibition by dominant-negative construct of Cdk4, and inhibition by Cdk4 small interfering RNA. The major substrate of Cdk4, Rb, is not required for ligand-induced adhesion, suggesting the involvement of a novel Cdk4 substrate. We also demonstrate that Cdk4−/− mice have impaired recruitment of lymphocytes to the lung following injury. The finding that Cdk inhibitors can block leukocyte adhesion and migration may expand the clinical indications for this emerging class of therapeutics.
Cyclin-dependent kinases (Cdks)5 are serine/threonine kinases that regulate progression through the cell cycle. Because of their critical role in cell proliferation and transcriptional regulation, Cdks are attractive therapeutic targets in different diseases and a number of pharmacological inhibitors have been developed to Cdks with varying degrees of specificity. All of the Cdk inhibitors to date act by competing with ATP for binding in the kinase ATP binding site (reviewed in Ref. 1). Cdk inhibitors are being evaluated for the treatment of malignancies, cardiovascular disease, and glomerulonephritis, based on the role of Cdks in cell proliferation (1, 2). However, it is increasingly clear that Cdks as well as cyclins and Cdk inhibitors are important for other functions, including cytoskeleton rearrangement (3), cell motility (4), regulation of apoptosis (5), and neurite outgrowth (6). Thus, there is increasing evidence that Cdks may have nontraditional roles in various cell behaviors, including those related to adhesion and migration.
Leukocyte trafficking from blood stream to tissue plays a key role in response to inflammation and infection. This process is a well-orchestrated series of adhesion, de-adhesion, signaling, and cytoskeletal changes that are tightly regulated. Leukocytes do not adhere to underlying endothelial cells (EC) when in a resting state. However, upon activation, that is, by cytokines or chemokines, leukocytes rapidly modulate changes in integrin conformation and/or clustering to alter integrin affinity and/or avidity that permit targeted integrin-mediated adhesion to the vascular EC and subsequent migration between EC (reviewed in Ref. 7). Following diapedesis, the leukocytes migrate through subendothelium and extravascular tissue via the interaction of integrin receptors with extracellular matrix components.
We previously demonstrated that phorbol ester-stimulated adhesion of Jurkat cells to fibronectin required activation of the small GTPase Rap1 (8). We also showed that leukocytes could adhere spontaneously to high-density fibronectin, a process we refer to as “ligand-induced adhesion.” We now further characterize the mechanism of ligand-induced adhesion in leukocytes and show that this pathway allows leukocyte adhesion to physiological relevant substrates such as the exposed endothelial matrix in the absence of exogenous stimulation. In contrast to phorbol ester-stimulated adhesion, this ligand-induced adhesion is not dependent on Rap1 but is dependent on Cdk4. Inhibition of ligand-induced adhesion and migration by Cdk inhibitors suggest that some of the in vivo effects of Cdk inhibitors may be due to blockade of leukocyte adhesion and migration, rather than, or in addition to, blockade of cell cycle.
Materials and Methods
Cells
Jurkat T, Ramos B, and THP-1 cells were obtained from the American Type Culture Collection and were cultured in RPMI 1640 (Mediatech) supplemented with glutaMAX-1 (Invitrogen Life Technologies), 1 mM sodium pyruvate (BioWhittaker), nonessential amino acids (BioWhittaker), and 10% FBS (HyClone). Peripheral blood was obtained from healthy donors with informed consent according to protocols approved by the Human Subjects Review Committee of the University of Washington. PBMC were isolated by Ficoll-Hypaque (Pharmacia) gradient centrifugation and washed with PBS. HUVEC were isolated and cultured as previously described (9) and were grown in RPMI 1640 supplemented with 2 mM glutamine, sodium pyruvate, nonessential amino acids, 10 mM HEPES, 100 U/ml penicillin, 100 U/ml streptomycin, 250 ng/ml Fungizone (Bio-Whittaker), 90 mg/ml heparin (Sigma-Aldrich), bovine hypothalamic extract, and 10% FBS (HyClone). HUVEC were cultured on surfaces coated with 2% gelatin (Sigma-Aldrich). BAEC were a gift from Helene Sage (Hope Heart Institute, Seattle WA) and were grown in DMEM (Mediatech) supplemented with glutaMAX-1, sodium pyruvate, and 8% FBS. The EC matrix was prepared by lysis of a confluent layer of EC with 20 mM NH4OH at 37°C for 5 min (10) and blocked briefly with growth medium.
Adhesion assays
Lymphocytes were labeled with 2.5 mM calcein AM (Molecular Probes) at room temperature for 20–40 min, washed, and resuspended in HBSS with calcium and magnesium (Mediatech) supplemented with 0.1% BSA and 4 mM HEPES. Lymphocytes were incubated with inhibitors or function-blocking Abs at 37°C for 10–30 min and tested for ligand-induced adhesion or phorbol ester-stimulated adhesion to EC or to the EC matrix for 15–30 min at 37°C. Adhesion was measured by a Cytofluor Series 4000 fluorescence plate reader (PerSeptive Biosystems). Plates were scanned before and after washing for total and adherent cells, respectively. Statistical significance was determined by Student’s t test. In some cases, EC were labeled with Cell Tracker Orange (Molecular Probes) per the manufacturer’s instructions before adhesion. Cells were fixed after adhesion assays and adherent cells were directly visualized by fluorescent microscopy. Underlying fibronectin fibrils were visualized with polyclonal anti-bovine fibronectin Ab (Accurate), followed by Alexa 568-conjugated secondary Ab (Molecular Probes).
Transfections
Cdk2 and Cdk4-dominant negative (DN) constructs were a generous gift from J. Roberts (Fred Hutchinson Cancer Research Center, Seattle, WA) (11). Cdk5 DN construct was a generous gift from P. Zelenka (National Eye Institute, National Institutes of Health, Bethesda, MD) (12). Generation of Rap 1 DN transfectants was previously described (8). A phosphorylation-deficient construct of pRb was a gift from Dr. J. Lukas (Institute of Cancer Biology for Center for Genotoxic Stress Research, Copenhagen, Denmark) (13, 14). Transfections were performed with TransIT-Jurkat Transfection Reagent (Mirus, Gene Transfer Specialist TM) according to the manufacturer’s instructions. Controls consisted of cells transfected with empty vector pCMV alone. Stably transfected cell lines were selected in medium containing the neomycin analog G418 (1 mg/ml). Expression was verified by Western blot analysis. Cdk and retinoblastoma (Rb) siRNAs were purchased from Invitrogen Life Technologies and transient transfections were performed using a Nucleofactor Device (Amaxa Biosystems).
Western blot analysis
Equal numbers of Jurkat cells (1 × 106) were lysed in buffer containing 50 mM HEPES (pH 7.5), 10 mM NaCl, 5 mM MgCl2, 1 mM EGTA, 1 µg/ml leupeptin, 1 µg/ml aprotinin, 1% Triton X-100, and 10% glycerol and Complete Mni Protease Inhibitor Cocktail (Roche). Equal amounts of protein were separated by SDS-PAGE, transferred to Immobilon, and blocked for 1 h. Blots were incubated with primary Ab for 1 h, followed by peroxidase-conjugated secondary Ab for 1 h and then developed with ECL (Amersham Biosciences). Anti-phosphorylated Rb (T821) and anti-phosphorylated Rb (249/252) were obtained from BioSource International. Anti-Cdk2, anti-Cdk4, and anti-Cdk5 Abs were obtained from eBioscience.
Reagents and Abs
Cdk inhibitors aminopurvalanol A, purvalanol A, and roscovitine were obtained from Tocris Bioscience. Cytochalasin D and additional inhibitors were obtained from Calbiochem. Function-blocking Abs to α4 and α5 integrin subunits were obtained from Chemicon. The β1 integrin-blocking Ab 5D1 was previously characterized (9). The β1 integrin Ab 9EG7, which recognizes the activation epitope (15), was obtained from BD Biosciences. The β1 integrin-activating Ab 8A2 was previously characterized (16). All Abs were used at saturating concentrations. Actin and tubulin Abs were obtained from Chemicon.
Flow cytometry
Integrin subunit expression levels were analyzed by a FACScan (BD Biosciences) instrument. Analyzed cells were incubated with integrin Abs for 30 min, followed by incubation with fluorescein-labeled secondary Abs for 30 min. In some cases, cells were pretreated with the β1-activating Ab 8A2 for 30 min at 37°C before analysis. PBMC cellular populations before and after adhesion were analyzed on the basis of forward and side scatter properties. Data were acquired and analyzed using CellQuest 3.3 (BD Bisociences).
Migration assays
Confluent HUVEC, grown on gelatin-coated Transwell filter (3 µm; Corning), were treated with 20 mM NH4OH for 20–30 min to remove the EC and then the remaining EC matrix was washed with PBS. Calcein-labeled PBMC were allowed to adhere to the EC matrix for 30 min, followed by washing with Percoll to remove nonadherent cells. Cells were then treated with 20 µM purvalanol or vehicle (DMSO). PBMC were allowed to migrate for 4.5 h at 37°C and the number of migrated cells in the bottom chamber was counted.
Bleomycin-induced lung injury
This animal experiment was approved by the Institutional Animal Care and Use Committee of the University of Washington. Generation of Cdk4−/− mice was previously described (17). Cdk4−/−, Cdk+/−, or Cdk+/+ litter-mates underwent intratracheal instillation with 0.033 U of bleomycin in 50 µl of saline (SICOR Pharmaceuticals) as previously described (18). Seventy-two hours later, mice were sacrificed, the right main stem bronchus was tied off, and the left lung was isolated and lavaged with 1 ml of PBS containing 0.6 mM EDTA warmed to 37°C. Bronchoalveolar lavage fluid (BALF) total cell count was determined by trypan blue exclusion and cell differential was determined on DiffQuik (Dade Behring)-stained cytospins.
Results
Leukocytes adhere spontaneously to the EC matrix
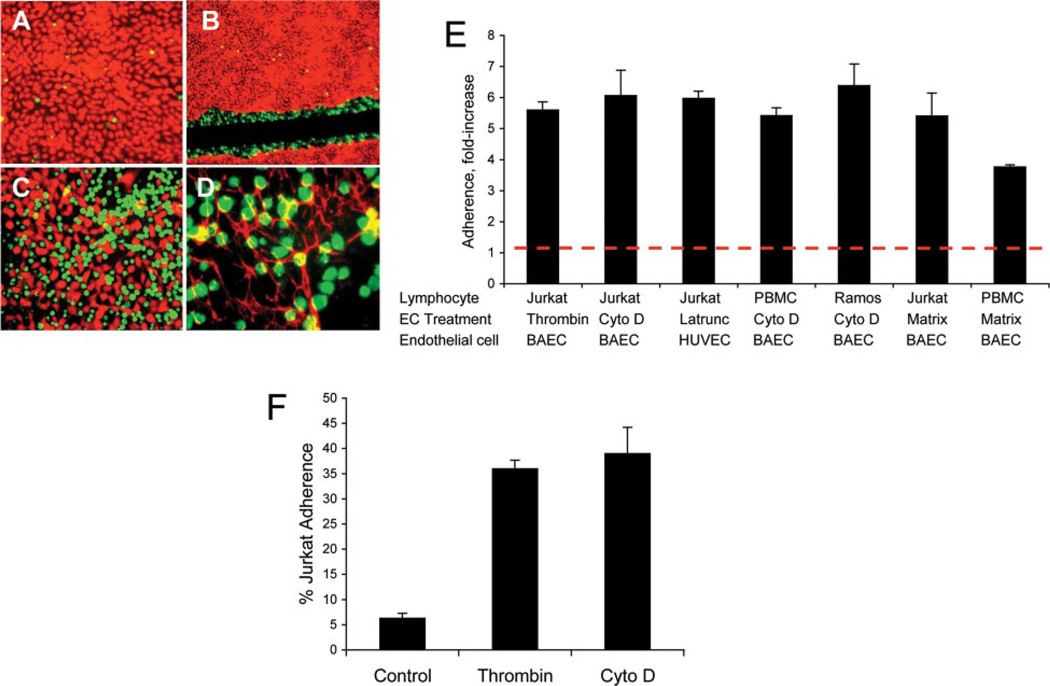
Confluent bovine aortic EC (BAEC) or HUVEC were treated with low-dose cytochalasin D to cause them to retract. Untreated Jurkat cells were allowed to adhere to cytochalasin-treated EC. We found minimal Jurkat cell adhesion to intact endothelial monolayers (Fig. 1A), but large numbers of Jurkat cells attached adjacent to the retracted EC (Fig. 1C). In addition, wounding an endothelial monolayer by scratching also caused Jurkat cells to adhere spontaneously to the margin of the retracted monolayer (Fig. 1B). When EC were removed by NH4OH treatment, the remaining underlying matrix, which contains fibronectin, also supported spontaneous Jurkat cell adhesion (Fig. 1D). The underlying EC matrix is a complex substrate that includes matrix proteins, such as fibronectin, and proteoglycans (19). High-concentration fibronectin also supported un-stimulated adhesion of Jurkat cells.
FIGURE 1.
Unstimulated leukocytes adhere to disrupted EC monolayers and the endothelial matrix. A, Adhesion of unstimulated Jurkat cells (green) to a confluent monolayer of BAEC (red) after 30 min. B, Adhesion of unstimulated Jurkat cells to the edges of a wounded BAEC monolayer. A confluent endothelial monolayer was wounded by scratching and the intervening matrix was removed by scraping and aspirating. C, Adhesion of unstimulated Jurkat cells to cytochalasin-retracted BAEC monolayer. D, Adhesion of unstimulated Jurkat cells to the BAEC-derived matrix. Fibronectin fibrils are visualized in red with an Ab to bovine fibronectin. Original magnification: ×10 (A and C); ×4 (B); and X20 (D). E, PBMC, Jurkat cells, or Ramos cells were allowed to adhere to the BAEC-, HUVEC-, or EC-derived matrix for 20 min at 37°C. EC treatments were: human thrombin, 10 U/ml for 10 min; cytochalasin D (Cyto D), 1 µM for 75 min; and latrunculin A, 1 µM for 50 min. The EC matrix was prepared by treating confluent EC monolayers with 20 mM NH4OH at 37°C for 5 min. x-Axis: fold- increase in adhesion compared with adhesion to the untreated endothelial monolayer of at least six independent experiments performed in triplicate wells. Dotted line represents adhesion to untreated endothelial monolayer. Values of p< 0.01 for all compared with controls. F, A representative experiment of Jurkat cell adhesion to thrombin- or cytochalasin D-retracted BAEC or untreated BAEC monolayer (control) is shown (n = 6 independent experiments). Data are the means of triplicate wells ± SD.
To quantify adhesion, we performed cell adhesion assays as previously described (8). PBMC adhesion to cytochalasin-treated EC and the underlying EC matrix demonstrated a 5- to 6-fold increase in adhesion relative to an untreated EC monolayer (Fig. 1E). Similar results were also found with Jurkat T cells and Ramos B cells (Fig. 1, E and F). To determine which cells in PBMC were adherent, we performed flow cytometry analysis of total PBMC and adherent PBMC. By flow cytometric analysis of forward and side scatter, we found that both monocytes and lymphocytes adhered to the EC matrix (Table I). To examine a more physiological agent to provoke EC retraction, we treated BAEC with thrombin. Thrombin, like cytochalasin D, caused EC retraction and increased Jurkat cell adhesion by 4- to 5-fold (Fig. 1F). Additional reagents that caused EC retraction, including staurosporine, latrunculin, EDTA, and PBS without calcium and magnesium, showed similar enhancement of adhesion (Fig. 1E and data not shown). Of note, treatment of EC with agents that stimulated apoptosis but failed to induce retraction, such as colchicine, vinblastine, nocadozole, and paclitaxel, also failed to produce comparable leukocyte adhesion (data not shown). Similar results were found whether BAEC or HUVEC were used.
Table I.
Cell differential following ligand-induced adhesiona
| Lymphocytes (%) | Monocytes (%) | |
|---|---|---|
| Total PBMC | 67 | 31 |
| Adherent cells | 25 | 73 |
Freshly isolated PBMC and cells adherent to the EC matrix after 20 min at 37°C were evaluated by forward and side scatter to assess cell distributions. The data are the means of two separate experiments, and similar data were obtained in an additional experiment in which cell differentials were evaluated from cytospins of the two populations.
Pharmacological inhibitors of Cdks, but not other kinases, inhibit spontaneous adhesion to the matrix
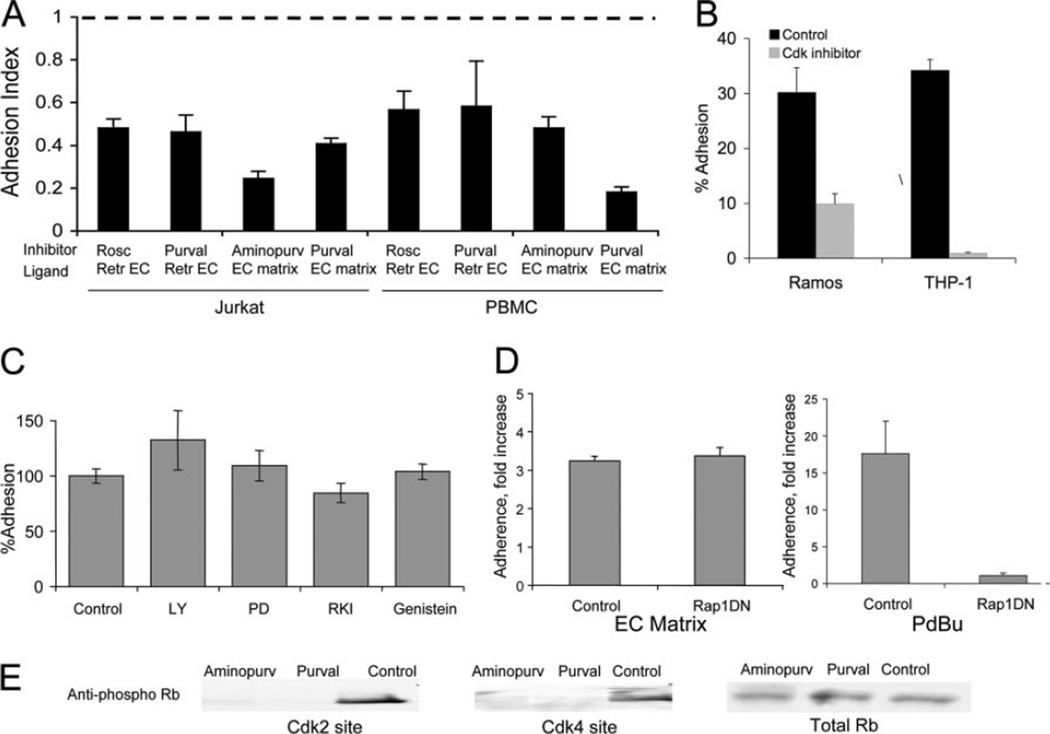
To determine the pathway(s) involved in ligand-induced adhesion, we tested the effects of pharmacological inhibitors of various pathways. Only Cdk inhibitors blocked ligand-induced adhesion to retracted EC or the EC matrix (Fig. 2A). When PBMC or Jurkat were treated with the Cdk inhibitors roscovitine, purvalanol A, or aminopurvalanol A, their adhesion to retracted BAEC or the EC matrix was significantly reduced (Fig. 2A). In contrast, the Cdk inhibitors had no effect on phorbol ester-stimulated adhesion (data not shown). Cell viability was similar in Cdk inhibitor- and vehicle-treated cells by calcein fluorescence and trypan blue exclusion (>95%). In addition to the T lymphocytic cell line (Jurkat), we showed that ligand-induced adhesion in the B lymphocytic cell line (Ramos) and monocytic cell line (THP-1) was inhibited by Cdk inhibitors (Fig. 2B). Inhibitors of other pathways, including RhoA kinase (Y-7632), MAPK (PD98059), PI3K (LY294002), and tyrosine kinases (genistein) did not inhibit ligand-induced adhesion (Fig. 2C). We previously showed that phorbol ester-stimulated Jurkat adhesion to low-density fibronectin was dependent on Rap1A activity (8). In contrast, overexpression of the DN Rap1 construct N17Rap1 had no effect on ligand-induced adhesion of Jurkat cells to the BAEC-derived matrix, whereas phorbol ester-stimulated Jurkat adhesion was almost completely inhibited (Fig. 2D).
FIGURE 2.
Cdk inhibitors block ligand-induced adhesion to the endothelial matrix and retracted EC, but Rap1 inhibition has no effect. A, PBMC or Jurkat cells were treated with 20 µM roscovitine, purvalanol A, or aminopurvalanol A for 30 min, then allowed to adhere to cytochalasin D-retracted BAEC or BAEC-derived matrix for 30 min. Adhesion index is reported as the ratio of adhesion with inhibitors/adhesion without inhibitors of six replicate wells ± SD. Dotted line represents adhesion to untreated EC monolayer. B, Ramos B cells or THP-1 cells with or without 10 µM purvalanol A were allowed to adhere to the BAEC-derived matrix for 30 min. Average of three replicates ± SD is shown. C, Jurkat cells were treated with PI3K inhibitor (50 µM LY294002), MAPK inhibitor (50 µM PD98059), RhoA kinase inhibitor (30 µM Y7632), or tyrosine kinase inhibitor (10 µM genistein) before adhesion to the EC matrix. Average of six replicates ± SD is shown. D, Jurkat cells were transfected with Rap1DN construct or control vector and unstimulated (left) or stimulated with phorbol dibutyrate (PDBu; right) and allowed to adhere to the EC matrix for 30 min. Values represent means of three independent experiments performed in triplicate. E, Cdk inhibitors block Rb phosphorylation. Jurkat cells were treated with 20 µM aminopurvalanol A or purvalanol A for 30 min and then lysed. Equal amounts of protein were separated by SDS-PAGE, then blotted with a phospho-specific Ab to the Cdk2-specific site on Rb (T821; left) or Cdk4-specific site on Rb (249/252; middle) or total Rb (right) as loading control.
To verify the efficacy and specificity of the Cdk inhibitors, we examined phosphorylation of Rb protein, the major substrate of Cdk. Roscovitine and aminopurvalanol A reportedly inhibit Cdk2 but not Cdk4 (1). Cdk2 and Cdk4 phosphorylate Rb at different sites: Cdk2 phosphorylation sites include threonine 821 and Cdk4 phosphorylation sites include serine 249 and threonine 252 (20). Although treatment with the Cdk inhibitors did block phosphorylation of Rb, we found that the inhibitors blocked phosphorylation of both Cdk sites (Fig. 2E). Thus, at the doses tested in leukocytes, the Cdk inhibitors function as broad-spectrum Cdk inhibitors, rather than subtype-specific inhibitors. Nevertheless, these data suggested that Cdks might be involved in ligand-induced adhesion.
Cdk4 is involved in ligand-induced adhesion
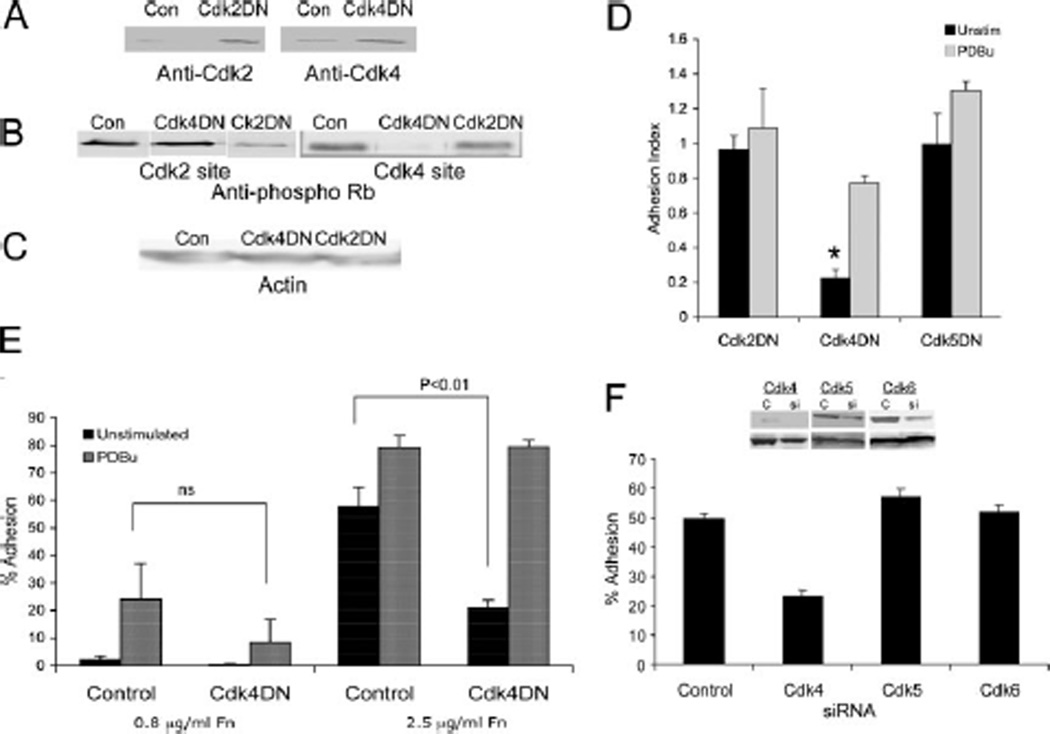
Recent studies have shown that Cdk inhibitors can target additional pathways, including MAPK (21). Therefore, to confirm our inhibitor studies and to determine which Cdk was involved in ligand-induced adhesion, we tested the ability of Cdk DN constructs to inhibit ligand-induced adhesion. We stably transfected Jurkat cells with a Cdk4 DN or Cdk2 DN expression vector that contained a mutation D145N that prevents their activity (11) or Cdk5 DN with inactivating mutation at T33 (12). Construct expression and inhibition of Rb phosphorylation was confirmed (Fig. 3, A–C, and data not shown). Only Cdk4 DN reduced the adhesion; Cdk2 DN and Cdk5 DN did not inhibit ligand-induced adhesion (Fig. 3D). We previously showed that lymphocytes were able to adhere spontaneously to high-density fibronectin in a Rap-1-independent manner. We now show that spontaneous adhesion to high-density fibronectin is inhibited by Cdk4 DN (Fig. 3E). Importantly, the Cdk DNs did not affect phorbol ester-stimulated adhesion (Fig. 3, D and E).
FIGURE 3.
Cdk4 DN construct and Cdk4 siRNA inhibit ligand-induced adhesion. A, Jurkat cells stably transfected with Cdk2 DN (left) or Cdk4 DN (right) or control vector were lysed and equal amounts of protein were separated by SDS-PAGE and then blotted with an Ab to Cdk2 or Cdk4. B, Cdk2 and Cdk4 DN constructs specifically inhibit phosphorylation of Rb protein. Jurkat cell transfectants were lysed, and equal amounts of protein were separated by SDS-PAGE and then blotted with a phospho-specific Ab to a Cdk2-specific phosphorylation site on Rb (T821; left) or Cdk4-specific site on Rb (249/252; right). C, Blots were probed with Ab to actin as loading control. D, Cdk4 DN, but not Cdk2 DN or Cdk5DN, inhibits ligand-induced adhesion to the EC matrix. Control, Cdk2 DN, Cdk4 DN, or Cdk5DN Jurkat cells were tested for unstimulated and phorbol ester-stimulated adhesion to the BAEC matrix. Adhesion index is the adhesion of DN transfectants compared with adhesion of controls. *, p< 0.0001 compared with control. E, Unstimulated Jurkat cell adhesion to high-density fibronectin is inhibited by Cdk4 DN. Data are means of at least six independent experiments of five replicate wells each ± SEM. F, Jurkat cells were transiently transfected with Cdk4, Cdk5, or Cdk6 siRNA or control vector. After 48 h, cells were lysed, and equal amounts of protein were separated by SDS-PAGE and then blotted with Ab to the indicated Cdk (top) or total Rb as loading control (bottom). Concurrent transfectants were allowed to adhere to the endothelial matrix for 30 min.
To further confirm the role of Cdk4, we examined the effect of Cdk4 siRNA on ligand-induced adhesion of Jurkat and Ramos cells. Maximal reduction in Cdk4 protein was achieved at 48 h after initial transfection, as indicated by Western blot analysis (Fig. 3F). At that time, ligand-induced adhesion was almost completely abolished, while control vector had no effect (Fig. 3F). In addition, Cdk5 and Cdk6 siRNA did not inhibit ligand-induced adhesion. Taken together, these results show that Cdks, specifically Cdk4, are involved in ligand-induced adhesion.
Rb phosphorylation is not required for ligand-induced adhesion
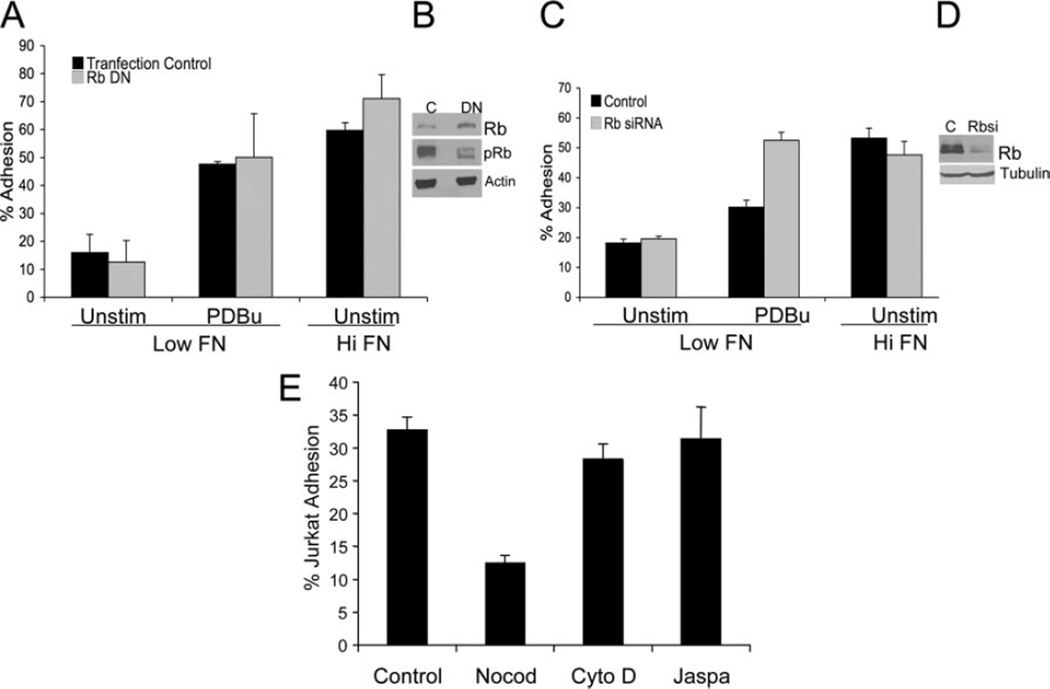
The ability of inhibitors to rapidly (within 20 min) down-regulate ligand-induced adhesion suggests that Cdk activity is required for sustained ligand-induced adhesion and is independent of transcription. In addition, transcription and translation inhibitors actinomycin and cycloheximide had no affect on ligand-induced adhesion (data not shown). Rb family members are the major substrate for Cdks (22). To determine whether Rb is involved, we transfected Jurkat cells with a phosphorylation-deficient construct of pRb (pRbcdk) lacking 10 CDK consensus sites (13). Because Rb is unable to be phosphorylated, it remains constitutively active (continues to bind transcription factors and prevents progression through the cell cycle G1) (14). If Rb phosphorylation is required for li-gand-induced adhesion, overexpression of this construct should inhibit it. However, overexpression of phosphorylation-deficient Rb did not inhibit ligand-induced adhesion (Fig. 4, A and B), suggesting that Rb is not required for ligand-induced adhesion. As further confirmation, knockdown of Rb by siRNA had no effect on ligand-induced adhesion (Fig. 4, C and D).
FIGURE 4.
A, Rb is not required for ligand-induced adhesion of Jurkat adhesion to high- density fibronectin (FN). Jurkat cells stably transfected with DN Rb or control vector were allowed to adhere to low- or high-density fibronectin with or without PDBu stimulation. B, Lysates of Jurkat cells transfected with DN RB or control were blotted with Ab to total Rb (top), phospho-specific Rb (middle), or actin as a loading control (bottom). C, Jurkat cells were transiently transfected with Rb siRNA or control construct. After 48 h, cells were allowed to adhere to low- or high-density fibronectin with or without PDBu stimulation. D, Concurrent Jurkat cell transfectants were lysed, and equal amounts of protein were separated by SDS-PAGE and then blotted with Ab to Rb (top) or tubulin as a loading control (bottom). E, Microtubules but not actin cytoskeleton are involved in ligand-induced adhesion. Jurkat cells were pretreated with nocodazole (20 µM), jasplakinolide (50 ng/ml), or cytochalsin D (1 µM) for 20 min, then were allowed to adhere to the EC matrix. Data are the means of triplicate wells ± SD.
We previously reported that a moderate dose of cytochalasin D (1 µM), which targets actin microfilaments, inhibited phorbol ester-stimulated adhesion (8). However, the same dose of cytochalasin, or jasplakinolide, a stabilizer of actin filaments, had no affect on ligand-induced adhesion (Fig. 4E). In contrast, treatment with nocodazole, an inhibitor of microtubules, significantly inhibited ligand-induced adhesion, but had no effect on phorbol ester-stimulated adhesion (Fig. 4E). Taken together, the data suggest a non-traditional role of the cytoskeleton in ligand-induced adhesion.
β1 integrins are involved in ligand-induced adhesion
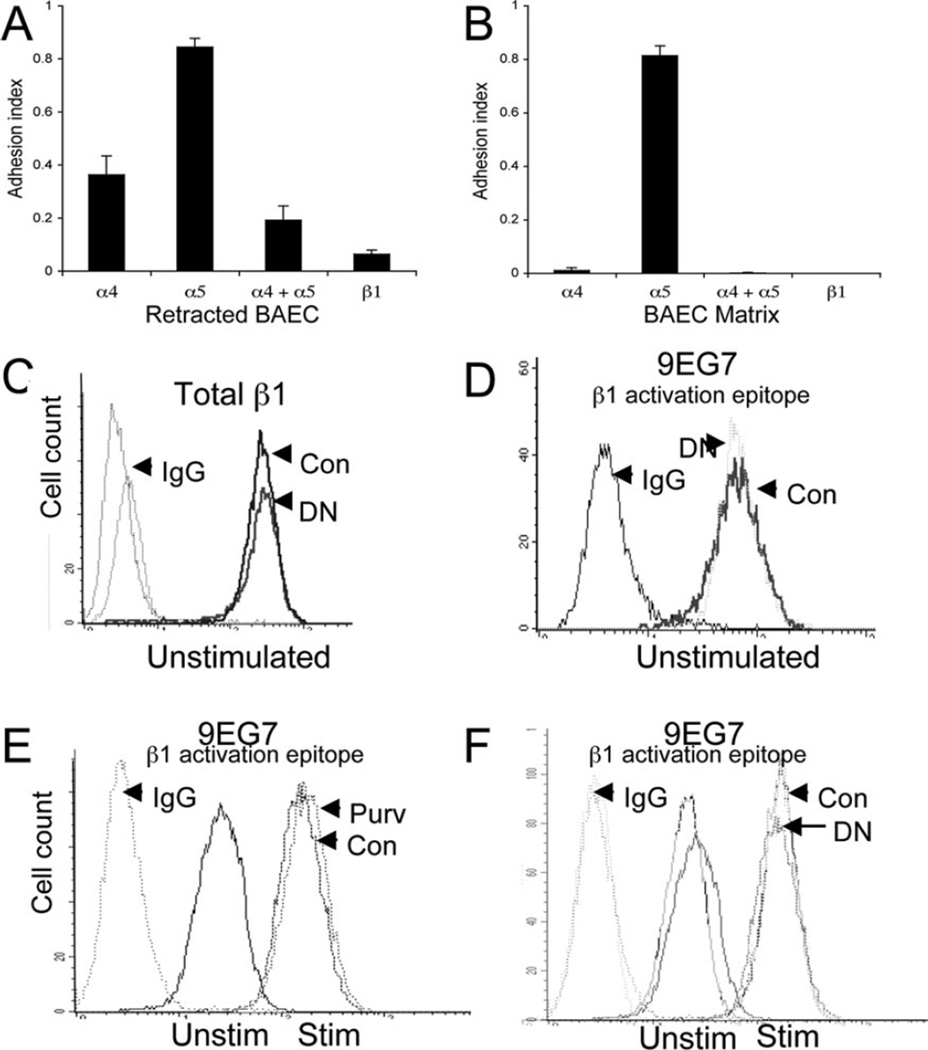
Integrins mediate binding to many components of the endothelial matrix. We previously reported that increased Jurkat adhesion to EC treated with staurosporine was β1 integrin dependent (9). To determine the cell adhesion receptors involved in ligand-induced adhesion, we pretreated Jurkat cells with function-blocking mAbs to β1, α4, and α5 integrin subunits. Pretreatment with β1-blocking mAb almost completely abrogated adhesion to retracted EC or the EC matrix (Fig. 5, A and B). Pretreatment with α4-blocking mAb also significantly decreased cell adhesion. In contrast, blockade of α5 had no significant effect on adhesion to retracted EC or the EC matrix, although blockade of both α4 and α5 further reduced cell adhesion to retracted EC. The results suggest that α4β1 is the primary integrin involved in ligand-induced adhesion of leukocytes to the EC matrix. However, α5β1 may also contribute to ligand-induced adhesion.
FIGURE 5.
Ligand-induced adhesion is dependent on β1 integrins. A, Jurkat cells were pretreated with the indicated function-blocking Ab for 10 min, then allowed to adhere to cytochalasin D-retracted BAEC for 20 min. B, Jurkat cells were pretreated with the indicated function-blocking Ab for 10 min, then allowed to adhere to the BAEC-derived matrix for 20 min. Adhesion index is reported as the ratio of adhesion with Abs/adhesion without Abs of six replicate wells ± SD. C–F, No change in integrin expression or activation following Cdk4 blockade. Wild-type (Con) or Cdk4 DN Jurkat cells were incubated with an Ab to β1(C) or with 9EG7, a mAb that recognizes an activation epitope of β1 integrins (D) and analyzed by flow cytometry. E, Jurkat cells with or without purvalanol or (F) Cdk4 DN Jurkat cells were stimulated (Stim) for 15 min with the β1 integrin-activating mAb 8A2, then analyzed for cell surface expression of the activation epitope of β1 by incubation with 9EG7 Ab. x-Axis represents log fluorescent units; y-axis is cell count. Each experiment was independently replicated at least three times. Unstim, Unstimulated.
No change in β1 integrin expression or conformation following Cdk blockade
Because ligand-induced adhesion is spontaneous and Cdk inhibitors rapidly (within 20 min) inhibit the adhesion, it is unlikely to involve changes in surface expression of β1 integrin. As confirmation, we found no difference in total surface expression of α4 or β1 in Jurkat cells with or without Cdk4 DN or Jurkat cells with or without Cdk inhibitors (Fig. 5C and data not shown). However, integrins can assume different conformational states and many integrins at rest exhibit a low affinity state. Thus, a change in the conformation state of β1 could contribute to ligand-induced adhesion. Therefore, we determined the effect of Cdk blockade on the expression of the β1 activation epitope defined by mAb 9EG7 (15). We found no difference in baseline expression of the activation epitope by flow cytometry in Jurkat with or without Cdk inhibitors or Jurkat cells with or without Cdk4 DN (Fig. 5, D and E). Furthermore, there was no difference in the induction of β1 activation epitope by the β1-activating Ab 8A2 (16) in the presence of Cdk4 blockade (Fig. 5, E and F). Therefore, inhibition of ligand-induced adhesion by Cdk blockade is not due to changes in integrin expression or activation.
Leukocyte migration across an EC matrix is inhibited by Cdk inhibitors
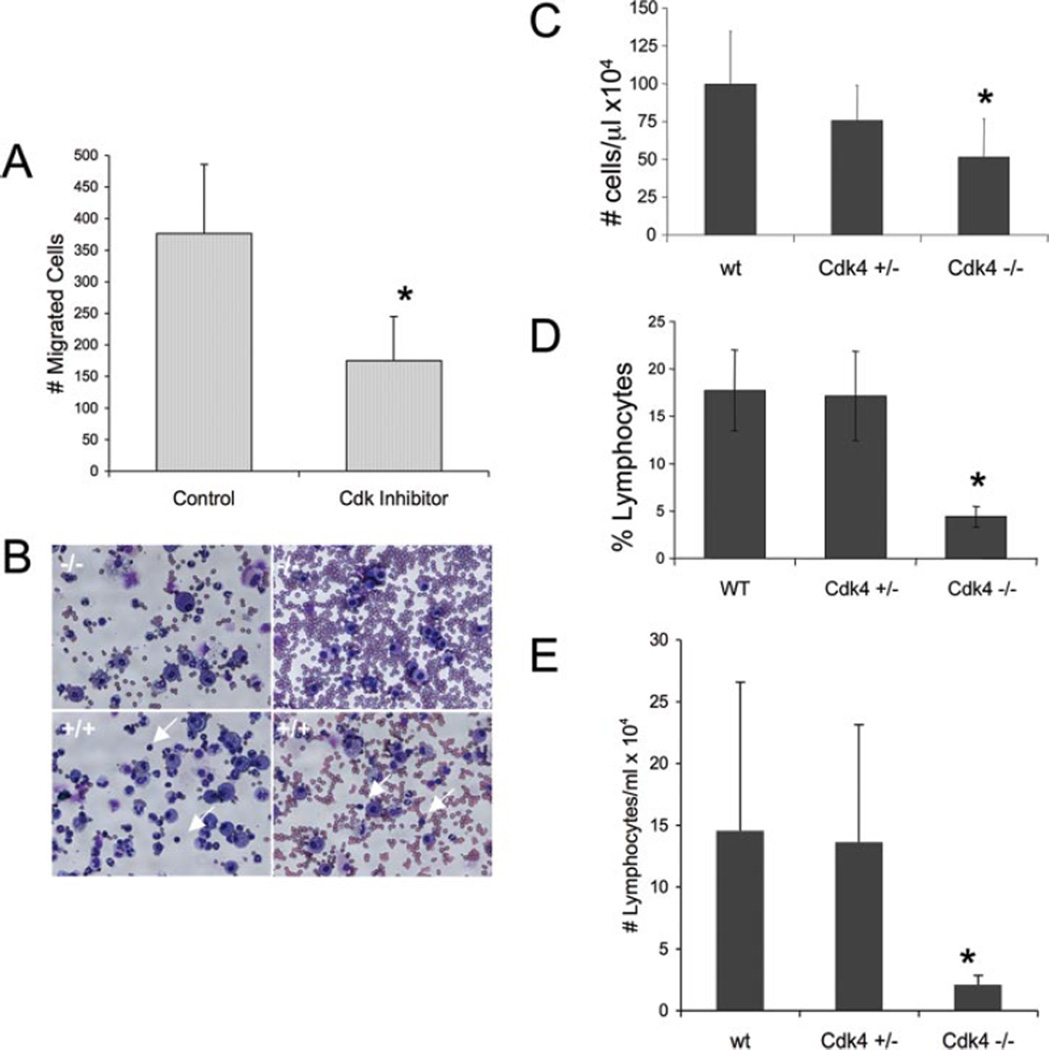
We hypothesized that ligand-induced adhesion could occur in vivo with localized retraction or loss of endothelium and during migration through the subendothelial matrix once transmigration has occurred. To test potential biological relevance of this Cdk-mediated pathway, we examined the effect of Cdk inhibitors on PBMC migration across an EC-derived matrix. Equal numbers of PBMC were allowed to adhere before treatment with Cdk inhibitor to ensure that differences in migration were not simply due to differences in the number of adherent cells. Migration in the presence of purvalanol A was inhibited by 50% (Fig. 6A). Thus, Cdk-mediated pathway plays a role in unstimulated adhesion and migration of PBMC across the exposed EC matrix.
FIGURE 6.
A, Leukocyte migration across the EC matrix is inhibited by Cdk inhibitors. PBMC were allowed to adhere to the endothelial matrix for 30 min, nonadherent cells were removed, then control diluent (DMSO) or purvalanol (20 µM) was added. Cells that migrated through the Transwell filters after 4.5 h were counted. Data are presented as the mean of two independent experiments performed in triplicate ±SD and expressed as a migration index (cell number/average of control DMSO). The number of migrated cells for the control ranged from 285 to 467 in the two experiments. *, p< 0.001 compared with control. B–D, Decreased lymphocyte recruitment in Cdk4−/− mice. Wild-type (wt, WT) (n = 9), Cdk4+/−(n = 4), and Cdk4−/−(n = 5) mice were treated with bleomycin administered intratracheally. Bronchoalveolar lavage was performed 72 h later B, Cytospin demonstrating relative paucity of lymphocytes (arrows) in Cdk4−/− mice compared with wild-type mice (two mice per condition). Original magnification, X40. C, Total BALF cell count. D and E, Percent and absolute number of lymphocytes in BALF. *, p< 0.04 −/− compared with wild type.
Impaired cell recruitment in Cdk4 knockout mice following lung injury
The response of Cdk4−/− mouse to injury and inflammation has not been examined. Based on our data showing a novel role of Cdk4 in leukocyte adhesion and migration, we asked whether the absence of Cdk4 would affect the recruitment of leukocytes following injury. To test this, we examined the response of Cdk4−/− mice to intratracheal instillation of bleomycin. Bleomycin lung injury response involves an initial inflammatory influx of leukocytes, followed by fibroblast proliferation and fibrosis (23). We examined BALF cell count and cell differential 72 h after bleomycin-induced lung injury. We found that Cdk4−/− mice had significantly decreased total cell count compared with wild-type mice (p < 0.04; Fig. 6C). Although Cdk4+/− mice appeared to have an intermediate phenotype, Cdk4+/− cell counts were not statistically significantly different from wild-type or Cdk4−/− mice. When we analyzed the cell populations in BALF, we found a selective decrease in lymphocytes in Cdk4−/− mice, while the absolute number of BALF neutrophils was similar in all groups (Fig. 6, B, D, and E). This suggests that the decrease in total BALF cell number is due to a selective blockade of lymphocyte recruitment. There were no differences in peripheral blood counts of Cdk4−/− mice compared with Cdk4+/− and wild-type littermates at baseline or following bleomycin injury (data not shown), suggesting that differences in BALF were not simply reflecting alterations in peripheral cell counts. These results suggest that Cdk4 plays a role in lymphocyte trafficking in vivo.
Discussion
We report a new pathway that regulates leukocyte adhesion to the endothelial matrix and migration of leukocytes through the matrix in the absence of exogenous cytokine or chemokine stimulation. Many of the features of ligand-induced adhesion described in this report do not fit into classic integrin-mediated adhesion paradigms, including lack of dependence on Rap1, lack of inhibition by cytochalasin D, and a role for Cdks in leukocyte adhesion (Table II). Only Cdk inhibitors were able to block ligand-induced adhesion, demonstrating a new mechanism of action of Cdk inhibitors. Inhibitors of other signaling pathways (e.g., RhoA kinase, MAPK, PI3K) failed to block ligand-induced adhesion. The role of Cdk4, specifically in ligand-induced adhesion, is supported by two independent lines of evidence: inhibition by a DN construct of Cdk4, but not Cdk2, or Cdk5 and inhibition by Cdk4 siRNA but not Cdk5 or Cdk6 siRNA.
Table II.
Comparison of two pathways of leukocyte adhesion
| Stimulated Adhesiona | Ligand-Induced Adhesion |
|---|---|
| Cdk-independent | Cdk dependent |
| Rap1 dependent | Rap1 independent |
| RhoA kinase dependent | RhoA kinase independent |
| Integrin dependent | Integrin dependent |
| Actin filaments involved (inhibited by cytochalasin D) | Actin filaments not involved |
| Microtubules not involved | Microtubules involved (inhibited by nocodazole) |
By soluble agonists.
Although the rationale for use of Cdk inhibitors in many diseases is blockade of cell cycle progression and limitation of cell proliferation, our study and others suggest that Cdks have additional functions independent of cell cycle regulation. Cdks 4 and 6 were reported to regulate neuronal cell death (5). Cdk5 plays an important role in neuronal cell function and contributes to neurite outgrowth and axonal regeneration (6, 24). In non-neuronal cells, Cdk5 also regulates cytoskeletal remodeling, cell motility, and adhesion (4, 12, 25). Cdk1 (cdc2) localized to membrane ruffles of migrating cells and was noted to be a downstream effector of the integrin αvβ3, leading to cell migration (26). In murine models of glomerular disease (27, 28) and polycystic kidney disease (29), treatment with Cdk inhibitors improved outcomes. Furthermore, blockade of Cdks during injury resulted in increased neutrophil apoptosis and enhanced resolution of inflammation (30).
Our study shows that a Cdk inhibitor blocks leukocyte adhesion and migration in vitro and knockout Cdk4 activity blocks lymphocyte recruitment to lung following injury in vivo. This may expand potential therapeutic indications for Cdk inhibitors to include diseases characterized by abnormal lymphocyte infiltration, such as psoriasis, or multiple sclerosis. The effect of Cdks on migration could also lead to unexpected outcomes. For example, the presence of tumor-infiltrating lymphocytes in several malignancies is associated with a better outcome (31–33). Cdk inhibitors could potentially decrease lymphocyte homing to tumors, which might lead to poorer outcomes unrelated to its effect on cell cycle.
Circulating leukocytes constitutively express α4β1 in a low-affinity conformation. We now show that these cells are adhesion-competent without stimulation by chemokines or cytokines when presented with an appropriate substrate. In addition to our studies demonstrating that ligand (i.e., high-density fibronectin or EC matrix) regulates spontaneous adhesion of lymphocytes (8, 34), other examples of regulation of adhesion by ligand have been noted. For example, eosinophil spreading and migration is inhibited by high density, but not low-density fibronectin (35). In addition, initial adhesive strength increases with increasing ligand density (36, 37). Changes induced by ligand binding can be detected by Abs that recognize conformational changes on integrins (38). Adhesion of unstimulated leukocytes to the subendothelial matrix suggests an alternate pathway to initiate “outside-in”- mediated inflammatory responses. Posttrafficking interactions of leukocytes with subendothelial matrix result in “outside-in” signaling that contribute to the inflammatory response. For example, ligand binding of α4β1 enhanced production of inflammatory mediators such as TNF-α, IL-1, and tissue factor (39–41), and α4β1 binding to VCAM-1 increased β2-mediated cell adhesion (42). α1β1-mediated outside-in signaling on leukocytes also regulates cell proliferation, adhesion, migration, and activation, which contributes to the inflammatory response (reviewed in Ref. 43). Blockade of α1β1 in animal models of immune-mediated disorders including graft-ver-sus-host disease (44), arthritis (45), colitis (46),and glomerulonephritis (27) markedly decreased the inflammatory response. In addition, α1−/− mice demonstrate a decreased inflammatory response in several models of immune-mediated inflammation (43). Thus, posttrafficking leukocyte signaling is an important contributor to the inflammatory response, and ligand-induced adhesion may play an important role in this process.
We previously reported that leukocyte adhesion to staurospo-rine-treated EC was mainly α4β1 integrin-dependent (9). Since staurosporine-induced EC retraction is an early stage of EC apoptosis, it now appears likely that the induced adhesion was due to exposure of the EC matrix rather than to apoptotic EC per se. Two lines of evidence support this: treatment of EC with agents that induced retraction but did not induce apoptosis increased adhesion of unstimulated cells and agents that induced apoptosis without causing EC retraction did not enhance adhesion.
The major defined substrate for Cdk4 is Rb. However, our data suggest that Rb phosphorylation is not required for ligand-induced adhesion. Thus, it is likely that a novel substrate for Cdk is involved. We also show that integrin expression and conformation is not affected by Cdk blockade. Although our data support a role for Cdk4 in ligand-induced adhesion in leukocytes, additional Cdk family members may participate in other cell types.
Interestingly, Cdk4−/− mice are viable, although the mice are infertile and small. Some of the mice have abnormalities in the hypothalamic-pituitary axis (47, 48) and develop diabetes due to abnormal pancreatic islet cell formation (17). Heterozygote mice do not display any overt phenotype. Cdk4−/− mouse embryo fibroblasts proliferate normally under conditions that promote continuous growth, but have impaired proliferative capacity following induction of a short quiescent period (17). In addition, keratinocytes from Cdk4−/− mice display normal proliferative capabilities in response to wounding (49). We now show that Cdk4−/− mice have a selective block in recruitment of lymphocytes to the lung following injury, complementing our in vitro data demonstrating that Cdk4 blockade inhibits leukocyte migration across the suben-dothelial matrix.
In summary, we report a new pathway that allows leukocytes to adhere to and migrate through the exposed endothelial matrix in the absence of exogenous cytokine or chemokine stimulation, termed ligand-induced adhesion. Furthermore, we provide evidence for a novel role of Cdk4 in regulating this adhesion. Primary monocytes and lymphocytes are capable of ligand-induced adhesion using several ligands including fibronectin and EC-derived matrices. We demonstrate a role of Cdk4-mediated ligand-induced adhesion in leukocyte trafficking by regulating migration. Furthermore, we show that the absence of Cdk4 in mice decreases lymphocyte recruitment in the BALF following lung injury. Thus, Cdk4 may be a novel therapeutic target for regulating lymphocyte recruitment during injury and inflammation. Further characterization of this novel pathway should provide new insights into post-trafficking events in leukocyte emigration.
Footnotes
This work was supported by National Institutes of Health Grants HL18645 (to J.M.H. and E.W.R.), HL067267 (to E.W.R.), and HL HL083481 and American Lung Association Career Investigator Award (to L.M.S.).
Abbreviations used in this paper: Cdk, cyclin-dependent kinase; BAEC, bovine aortic endothelial cell; EC, endothelial cell; siRNA, small interfering RNA; BALF, bronchoalveolar lavage fluid; Rb, retinoblastoma; DN, dominant negative; PDBu, phorbol dibutyrate.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Knockaert M, Greengard P, Meijer L. Pharmacological inhibitors of cyclin-dependent kinases. Trends Pharmacol. Sci. 2002;23:417–425. doi: 10.1016/s0165-6147(02)02071-0. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro GI. Cyclin-dependent kinase pathways as targets for cancer treatment. J. Clin. Oncol. 2006;24:1770–1783. doi: 10.1200/JCO.2005.03.7689. [DOI] [PubMed] [Google Scholar]
- 3.Besson A, Assoian RK, Roberts JM. Regulation of the cytoskel-eton: an oncogenic function for CDK inhibitors? Nat. Rev. Cancer. 2004;4:948–955. doi: 10.1038/nrc1501. [DOI] [PubMed] [Google Scholar]
- 4.Strock CJ, Park JI, Nakakura EK, Bova GS, Isaacs JT, Ball DW, Nelkin BD. Cyclin-dependent kinase 5 activity controls cell motility and metastatic potential of prostate cancer cells. Cancer Res. 2006;66:7509–7515. doi: 10.1158/0008-5472.CAN-05-3048. [DOI] [PubMed] [Google Scholar]
- 5.Park DS, Morris EJ, Padmanabhan J, Shelanski ML, Geller HM, Greene LA. Cyclin-dependent kinases participate in death of neurons evoked by DNA-damaging agents. J. Cell Biol. 1998;143:457–467. doi: 10.1083/jcb.143.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li BS, Zhang L, Gu J, Amin ND, Pant HC. Integrin α1β1-mediated activation of cyclin-dependent kinase 5 activity is involved in neurite outgrowth and human neurofilament protein H Lys-Ser-Pro tail domain phosphorylation. J. Neurosci. 2000;20:6055–6062. doi: 10.1523/JNEUROSCI.20-16-06055.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steeber DA, Venturi GM, Tedder TF. A new twist to the leukocyte adhesion cascade: intimate cooperation is key. Trends Immunol. 2005;26:9–12. doi: 10.1016/j.it.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Liu L, Schwartz BR, Tupper J, Lin N, Winn RK, Harlan JM. The GTPase Rap1 regulates phorbol 12-myristate 13-acetate-stimulated but not ligand-induced β1 integrin-dependent leukocyte adhesion. J. Biol. Chem. 2002;277:40893–40900. doi: 10.1074/jbc.M206208200. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz BR, Karsan A, Bombeli T, Harlan JM. A novel β1 integrin-dependent mechanism of leukocyte adherence to apoptotic cells. J. Immunol. 1999;162:4842–4848. [PubMed] [Google Scholar]
- 10.Kato Y, Gospodarowicz D. Effect of exogenous extracellular matrices on proteoglycan synthesis by cultured rabbit costal chondrocytes. J. Cell Biol. 1985;100:486–495. doi: 10.1083/jcb.100.2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van den Heuvel S, Harlow E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science. 1993;262:2050–2054. doi: 10.1126/science.8266103. [DOI] [PubMed] [Google Scholar]
- 12.Negash S, Wang HS, Gao C, Ledee D, Zelenka P. Cdk5 regulates cell-matrix and cell-cell adhesion in lens epithelial cells. J. Cell Sci. 2002;115:2109–2117. doi: 10.1242/jcs.115.10.2109. [DOI] [PubMed] [Google Scholar]
- 13.Lukas J, Herzinger T, Hansen K, Moroni MC, Resnitzky D, Helin K, Reed SI, Bartek J. Cyclin E-induced S phase without activation of the pRb/E2F pathway. Genes Dev. 1997;11:1479–1492. doi: 10.1101/gad.11.11.1479. [DOI] [PubMed] [Google Scholar]
- 14.Lukas J, Sorensen CS, Lukas C, Santoni-Rugiu E, Bartek J. p16INK4a, but not constitutively active pRb, can impose a sustained G1 arrest: molecular mechanisms and implications for oncogenesis. Oncogene. 1999;18:3930–3935. doi: 10.1038/sj.onc.1202777. [DOI] [PubMed] [Google Scholar]
- 15.Lenter M, Uhlig H, Hamann A, Jeno P, Imhof B, Vestweber D. A monoclonal antibody against an activation epitope on mouse integrin chain β1 blocks adhesion of lymphocytes to the endothelial integrin α6β1 . Proc. Natl. Acad. Sci. USA. 1993;90:9051–9055. doi: 10.1073/pnas.90.19.9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kovach NL, Carlos TM, Yee E, Harlan JM. A monoclonal antibody to β1 integrin (CD29) stimulates VLA-dependent adherence of leukocytes to human umbilical vein endothelial cells and matrix components. J. Cell Biol. 1992;116:499–509. doi: 10.1083/jcb.116.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsutsui T, Hesabi B, Moons DS, Pandolfi PP, Hansel KS, Koff A, Kiyokawa H. Targeted disruption of CDK4 delays cell cycle entry with enhanced p27Kip1 activity. Mol. Cell. Biol. 1999;19:7011–7019. doi: 10.1128/mcb.19.10.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matute-Bello G, Wurfel MM, Lee JS, Park DR, Frevert CW, Madtes DK, Shapiro SD, Martin TR. Essential role of MMP-12 in Fas-induced lung fibrosis. Am. J. Respir. Cell Mol. Biol. 2007;37:210–221. doi: 10.1165/rcmb.2006-0471OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis GE, Senger DR. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ. Res. 2005;97:1093–1107. doi: 10.1161/01.RES.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]
- 20.Mittnacht S. Control of pRB phosphorylation. Curr. Opin. Genet. Dev. 1998;8:21–27. doi: 10.1016/s0959-437x(98)80057-9. [DOI] [PubMed] [Google Scholar]
- 21.Knockaert M, Lenormand P, Gray N, Schultz P, Pouyssegur J, Meijer L. p42/p44 MAPKs are intracellular targets of the CDK inhibitor purvalanol. Oncogene. 2002;21:6413–6424. doi: 10.1038/sj.onc.1205908. [DOI] [PubMed] [Google Scholar]
- 22.Harbour JW, Dean DC. The Rb/E2F pathway: expanding roles and emerging paradigms. Genes Dev. 2000;14:2393–2409. doi: 10.1101/gad.813200. [DOI] [PubMed] [Google Scholar]
- 23.Chandler DB, Hyde DM, Giri SN. Morphometric estimates of infiltrative cellular changes during the development of bleomycin-induced pulmonary fibrosis in hamsters. Am. J. Pathol. 1983;112:170–177. [PMC free article] [PubMed] [Google Scholar]
- 24.Namgung U, Choi BH, Park S, Lee JU, Seo HS, Suh BC, Kim KT. Activation of cyclin-dependent kinase 5 is involved in axonal regeneration. Mol. Cell. Neurosci. 2004;25:422–432. doi: 10.1016/j.mcn.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Gao C, Negash S, Guo HT, Ledee D, Wang HS, Zelenka P. CDK5 regulates cell adhesion and migration in corneal epithelial cells. Mol. Cancer Res. 2002;1:12–24. [PubMed] [Google Scholar]
- 26.Manes T, Zheng DQ, Tognin S, Woodard AS, Marchisio PC, Languino LR. αvβ3 integrin expression up-regulates cdc2, which modulates cell migration. J. Cell Biol. 2003;161:817–826. doi: 10.1083/jcb.200212172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cook HT, Khan SB, Allen A, Bhangal G, Smith J, Lobb RR, Pusey CD. Treatment with an antibody to VLA-1 integrin reduces glomerular and tubulointerstitial scarring in a rat model of crescentic glomerulonephritis. Am. J. Pathol. 2002;161:1265–1272. doi: 10.1016/S0002-9440(10)64403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gherardi D, D’Agati V, Chu TH, Barnett A, Gianella-Borradori A, Gelman IH, Nelson PJ. Reversal of collapsing glomerulopathy in mice with the cyclin-dependent kinase inhibitor CYC202. J. Am. Soc. Nephrol. 2004;15:1212–1222. doi: 10.1097/01.asn.0000124672.41036.f4. [DOI] [PubMed] [Google Scholar]
- 29.Bukanov NO, Smith LA, Klinger KW, Ledbetter SR, Ibraghimov-Beskrovnaya O. Long-lasting arrest of murine polycystic kidney disease with CDK inhibitor roscovitine. Nature. 2006;444:949–952. doi: 10.1038/nature05348. [DOI] [PubMed] [Google Scholar]
- 30.Rossi AG, Sawatzky DA, Walker A, Ward C, Sheldrake TA, Riley NA, Caldicott A, Martinez-Losa M, Walker TR, Duffin R, et al. Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nat. Med. 2006;12:1056–1064. doi: 10.1038/nm1468. [DOI] [PubMed] [Google Scholar]
- 31.Chiba T, Ohtani H, Mizoi T, Naito Y, Sato E, Nagura H, Ohuchi A, Ohuchi K, Shiiba K, Kurokawa Y, Satomi S. Intraepithelial CD8 + T-cell-count becomes a prognostic factor after a longer follow-up period in human colorectal carcinoma: possible association with suppression of micrometas-tasis. Br. J. Cancer. 2004;91:1711–1717. doi: 10.1038/sj.bjc.6602201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl. Acad. Sci. USA. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma P, Shen Y, Wen S, Yamada S, Jungbluth AA, Gnjatic S, Bajorin DF, Reuter VE, Herr H, Old LJ, Sato E. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc. Natl. Acad. Sci. USA. 2007;104:3967–3972. doi: 10.1073/pnas.0611618104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bombeli T, Schwartz BR, Harlan JM. Endothelial cells undergoing apoptosis become proadhesive for nonactivated platelets. Blood. 1999;93:3831–3838. [PubMed] [Google Scholar]
- 35.Holub A, Byrnes J, Anderson S, Dzaidzio L, Hogg N, Huttenlocher A. Ligand density modulates eosinophil signaling and migration. J. Leukocyte Biol. 2003;73:657–664. doi: 10.1189/jlb.0502264. [DOI] [PubMed] [Google Scholar]
- 36.Garcia AJ, Takagi J, Boettiger D. Two-stage activation for α5β1 integrin binding to surface-adsorbed fibronectin. J. Biol. Chem. 1998;273:34710–34715. doi: 10.1074/jbc.273.52.34710. [DOI] [PubMed] [Google Scholar]
- 37.Garcia AJ, Huber F, Boettiger D. Force required to break α5β1 integrin-fibronectin bonds in intact adherent cells is sensitive to integrin activation state. J. Biol. Chem. 1998;273:10988–10993. doi: 10.1074/jbc.273.18.10988. [DOI] [PubMed] [Google Scholar]
- 38.Newham P, Craig SE, Clark K, Mould AP, Humphries MJ. Analysis of ligand-induced and ligand-attenuated epitopes on the leukocyte in-tegrin α4β1: VCAM-1, mucosal addressin cell adhesion molecule-1, and fi-bronectin induce distinct conformational changes. J. Immunol. 1998;160:4508–4517. [PubMed] [Google Scholar]
- 39.Kanda E, Jin Z-H, Mizuchi D, Arai A, Miura O. Activation of Rac and tyrosine phosphorylation of cytokine receptors induced by cross-linking of integrin α4β1 and cell adhesion in hematopoietic cells. Biochem. Biophys. Res. Commun. 2003;301:934–940. doi: 10.1016/s0006-291x(03)00075-5. [DOI] [PubMed] [Google Scholar]
- 40.Lin TH, Rosales C, Mondal K, Bolen JB, Haskill S, Juliano RL. Integrin-mediated tyrosine phosphorylation and cytokine message induction in monocytic cells: a possible signaling role for the Syk tyrosine kinase. J. Biol. Chem. 1995;270:16189–16197. doi: 10.1074/jbc.270.27.16189. [DOI] [PubMed] [Google Scholar]
- 41.McGilvray ID, Lu Z, Bitar R, Dackiw AP, Davreux CJ, Rotstein OD. VLA-4 integrin cross-linking on human monocytic THP-1 cells induces tissue factor expression by a mechanism involving mitogen-acti-vated protein kinase. J. Biol. Chem. 1997;272:10287–10294. doi: 10.1074/jbc.272.15.10287. [DOI] [PubMed] [Google Scholar]
- 42.Chan JR, Hyduk SJ, Cybulsky MI. α4β1 integrin/VCAM-1 interaction activates αLβ2 integrin-mediated adhesion to ICAM-1 in human T cells. J. Immunol. 2000;164:746–753. doi: 10.4049/jimmunol.164.2.746. [DOI] [PubMed] [Google Scholar]
- 43.Ben-Horin S, Bank I. The role of very late antigen-1 in immune-mediated inflammation. Clin. Immunol. 2004;113:119–129. doi: 10.1016/j.clim.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 44.Tanaka T, Ohtsuka Y, Yagita H, Shiratori Y, Omata M, Okumura K. Involvement of α1 and α4 integrins in gut mucosal injury of graft-versus-host disease. Int. Immunol. 1995;7:1183–1189. doi: 10.1093/intimm/7.8.1183. [DOI] [PubMed] [Google Scholar]
- 45.De Fougerolles AR, Sprague AG, Nickerson-Nutter CL, Chi-Rosso G, Rennert PD, Gardner H, Gotwals PJ, Lobb RR, Koteliansky VE. Regulation of inflammation by collagen-binding integrins α1β1 and α2β1 in models of hypersensitivity and arthritis. J. Clin. Invest. 2000;105:721–729. doi: 10.1172/JCI7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fiorucci S, Mencarelli A, Palazzetti B, Sprague AG, Distrutti E, Morelli A, Novobrantseva TI, Cirino G, Koteliansky VE, de Fougerolles AR. Importance of innate immunity and collagen binding integrin α1β1 in TNBS-induced colitis. Immunity. 2002;17:769–780. doi: 10.1016/s1074-7613(02)00476-4. [DOI] [PubMed] [Google Scholar]
- 47.Rane SG, Dubus P, Mettus RV, Galbreath EJ, Boden G, Reddy EP, Barbacid M. Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in β-islet cell hyperplasia. Nat. Genet. 1999;22:44–52. doi: 10.1038/8751. [DOI] [PubMed] [Google Scholar]
- 48.Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18:2699–2711. doi: 10.1101/gad.1256504. [DOI] [PubMed] [Google Scholar]
- 49.Rodriguez-Puebla ML, Miliani de Marval PL, LaCava M, Moons DS, Kiyokawa H, Conti CJ. Cdk4 deficiency inhibits skin tumor development but does not affect normal keratinocyte proliferation. Am. J. Pathol. 2002;161:405–411. doi: 10.1016/S0002-9440(10)64196-X. [DOI] [PMC free article] [PubMed] [Google Scholar]