Abstract
The plant root system is important for plant anchorage and nutrition. Among the different characteristics of the root system, root branching is a major factor of plasticity and adaptation to changing environments. Indeed, many biotic and abiotic stresses, such as drought or symbiotic interactions, influence root branching. Many studies concerning root development and root branching were performed on the model plant Arabidopsis thaliana, but this model plant has a very simplified root structure and is not able to establish any symbiotic interactions. We have recently described 7 stages for lateral root development in the model legume Medicago truncatula and found significant differences in the tissular contribution of root cell layers to the formation of new lateral roots (LR). We have also described 2 transgenic lines expressing the DR5:GUS and DR5:VENUS-N7 reporter genes that are useful to follow LR formation at early developmental stages. Here, we describe the use of these transgenic lines to monitor LR developmental responses of M. truncatula to the phytohormone abscisic acid (ABA) which is a major actor of stress and symbiotic interactions. We show that ABA promotes the formation of new lateral root primordia and their development, mostly at the late, pre-emergence stage.
Keywords: abscisic acid (ABA), DR5: GUS and DR5:VENUS transgenic lines, lateral root development, Medicago truncatula
Introduction
Plant root system development is a major ecological and agronomical issue, as it is the major site of plant anchorage and nutrition but is also a place for biotic and abiotic interactions. Among the different parameters of the root system development is root branching, i.e the formation of new lateral roots (LR) that is an extraordinary source of plasticity to increase the root surface. Thus, many biotic and abiotic interactions are known to interfere with LR development, among which drought and symbiotic interactions.1 Abscisic acid (ABA) is a phytohormone involved in both abiotic and biotic interactions, such as drought tolerance and pathogen or symbiosis interactions.2-4 Although ABA is involved in these different types of interaction, its action on LR development is different in legume species where it stimulates LR formation (LRF), in contrast to non legumes, such as Arabidopsis thaliana, where it inhibits LRF.5 For instance, ABA at a concentration of 1 μM reduces LR density in Arabidopsis whereas it increases LR density in M. truncatula.5Although the action of ABA on LRF has been quite well described in Arabidopsis 6, little is known about its stimulatory effect on LRF in legumes. Liang and co-workers have shown that ABA can rescue the elongation of emerged LR in the latd mutant of Medicago truncatula 7, thus acting on post-emergence growth of LR but nothing is described on ABA action during LRF, i.e prior to emergence. Especially, it is not known on which developmental stage of LRF ABA is active. We have recently described 7 stages for LRF in Medicago truncatula and shown that it involved different root tissues contributions than in Arabidopsis thaliana. We have also developed DR5:GUS and DR5:VENUS-N7 M. truncatula transgenic lines to monitor stages of LRF in planta.8 Here, we have taken advantage of these lines to monitor the effect of ABA on LRF in M. truncatula, and to address at which developmental stage ABA was the most active.
Results and Discussion
In order to test the effect of ABA on the development of LR in M. truncatula, we grew DR5:VENUS-N7 M. truncatula seedlings for 7 days (d) on a M medium containing 0.1 μM ABA, as this was in our system the optimum concentration to stimulate the formation of LR (data not shown). As shown on Figure 1A, we could see a significant increase in emerged LR at 6 and 7 d in the presence of ABA (with a mean of 3.61 in the ABA condition versus 2.41 in control condition at 6 d and 5.64 vs 3.69 at 7 d of treatment), as previously described in other studies.5 However, we did not see any significant difference in the primary root length of the plants grown for 3 or 7 d in the presence of 0.1 μM ABA compared to control conditions (Fig. 1B). In order to further investigate if the action of ABA could take place at early stages of LR development, we used the VENUS fluorescence to look for developing lateral root primordia (LRP) (Fig. 1C). As shown in Fig. 1A, we could detect an increase in LR primordia (LRP) visible at 6 and 7 d (with 8.36 LRP in the presence of 0.1 μM of ABA vs 6.59 in control condition at 6 d, and 9.19 LRP in the presence of ABA vs 6.73 in control conditions at 7 d, Fig. 1A). The number of LRP observed through the VENUS fluorescence was less different at 3 d post treatment (4.1 in ABA treated vs 3.64 in control, Fig. 1A).
Figure 1.
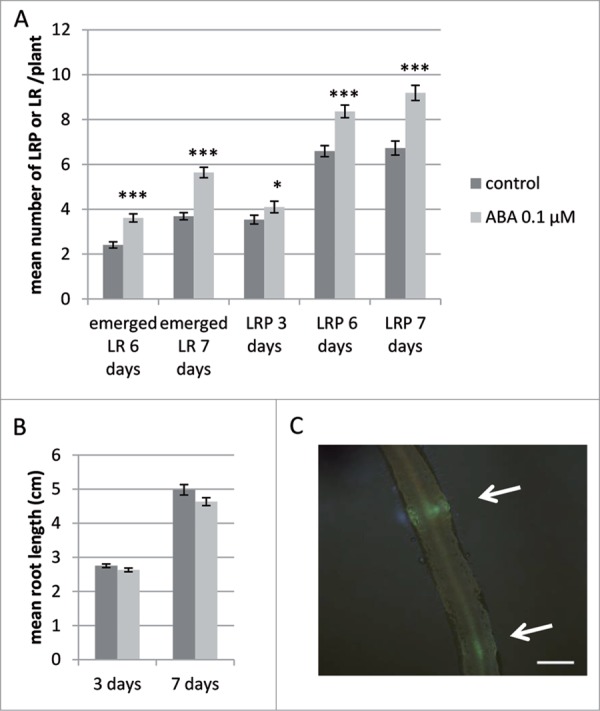
Effect of abscisic acid (ABA) on emerged lateral roots, lateral root primordia and primary root length followed using the DR5:VENUS-N7 reporter line. (A) Number of Lateral Roots (LR) and Lateral Root Primordia (LRP) observed using the DR5:VENUS-N7 marker line. Plants were grown in the absence (control, dark gray bars) or presence of 0.1 μM ABA (light gray bars) for 3, 6 or 7 d. Data is from 81 plants in 2 independent experiments. (B) Mean root length of plants grown for 3 or 7 d in the presence (light gray) or absence (dark gray) of 0.1 μM ABA, data is from 30 to 50 plants. Bars = standard error of the mean (sem). Statistically different means following a standard pair wise t-test is shown (*, P-value < 0.05; ***, P-value < 0.001). (C) Developing LRP (white arrows) detected in the DR5:VENUS-N7 transgenic roots by fluorescence. Scale bar is 500 μm.
Thus, we decided to focus on 6 d of treatment to further investigate the effect of ABA on specific LR developmental stages. To do this, we used the DR5:GUS expression patterns to roughly discriminate between different developmental stages. DR5:GUS expression patterns were separated in 3 different stages (Fig. 2A, B, and C). These stages were not as well defined as the ones described at the cellular level in Herrbach et al.,8 but were grouping LRF stages as follows: stage A (Fig. 2A) encompassed Stages I to III from our former description,8 stage B (Fig. 2B) corresponded to both stages IV and V, finally stage C (Fig. 2C) corresponded to stage VI, just prior emergence. As shown on Fig. 2D, we observed after 6 d LRP mostly at developmental stages B and C in the control condition, with stage C being the most represented with a mean number of 1.66/plant (Fig. 2D). We observed 0.375/plant stage B primordia (Fig. 2D) and very few stage A primordia (3 primordia/56 observed roots) in control condition. In contrast, we mostly observed LRP at stage C on ABA containing medium (4.39/plants, Fig. 2B) and one single stage A LRP in this condition.
Figure 2.
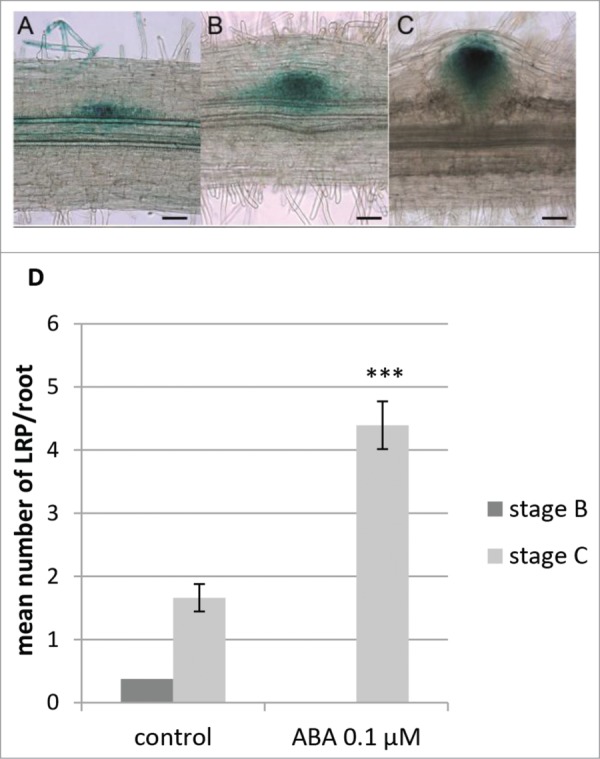
Effect of abscisic acid (ABA) on lateral root primordia (LRP) development in M. truncatula followed using the DR5:GUS reporter line. (A) Stage A LRP DR5:GUS expression pattern. (B) Stage B LRP DR5:GUS expression pattern. (C) Stage C LRP DR5:GUS expression pattern. Scale bar = 50 μm. (D) Developmental stages of LRP observed using the DR5:GUS marker line on 6 day-old plants grown with or without ABA. Developmental stages (stage B, dark gray, and stage C, light gray) are as described in Figure 2B and C. Data is from 56 plants in 2 independent experiments. Bars = standard error of the mean (sem). Statistically different means following a standard pair wise t-test is shown (***, P-value < 0.001).
Taken together, our data indicate that the effect of ABA on LRF in Medicago truncatula is an increased number of LRP with a stronger effect at the later stages of LRF, namely from stage B to C (corresponding to V to VI in the description by Herrbach et al., 2014). We can notice that fewer LRP were detected after GUS staining of the DR5:GUS lines than through observation of the fluorescence of VENUS (DR5:VENUS-N7 lines) at 6 d. We can speculate that the fluorescence is more efficient to detect early events of LR initiation than GUS staining. Unfortunately, fine description of LRF stages was not possible on DR5:VENUS-N7 lines without sections.
Interestingly, the effect of ABA on LR emergence in M. truncatula is dose dependent with high concentrations of ABA (> = 50 μM) having an inhibitory effect and lower doses a stimulatory effect.5,9 Ariel et al. have shown that high concentrations of ABA inhibit the auxin induction of LDB1, a transcription factor possibly involved in LRF in M. truncatula.9 Thus ABA could crosstalk with auxin responsive pathways. Moreover, the higher number of total LRP we observed suggests also an early action of ABA on the stimulation on LRP formation in M. truncatula, namely on stage A primordia that were too rare to address in our experimental set-up. In Arabidopsis, it was shown that ABA signaling could interfere with auxin transport or sensitivity, notably for LR formation.6,10 Thus, ABA stimulatory effect in M. truncatula could be related to a difference in the level of auxin sensitivity required for LRF in legumes. In that case, low doses of ABA may lower the auxin concentration threshold required for LR initiation in M. truncatula.
Materials and Methods
Plants were grown is square petri dishes on a M medium.11 ABA powder (+/- enantiomers mix, Sigma) was diluted in methanol for 10−2 M stock solution. ABA was used at 10−7 M and the corresponding amount of solvent was used for control conditions. GUS staining and microscopic observations were as described.8 Statistical analysis was performed in Excel software using the standard t-test comparison. Root length was measured using the ImageJ software.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the French Laboratory of Excellence project “TULIP” (ANR-10-LABX-41; ANR-11-IDEX-0002–02). Violaine Herrbach was supported by CNRS (INEE) and Novozymes company.
References
- 1. Jung JKH, McCouch SR. Getting to the roots of it: Genetic and hormonal control of root architecture. Front Plant Sci 2013; 4:186; PMID:23785372; http://dx.doi.org/ 10.3389/fpls.2013.00186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ding Y, Oldroyd GE. Positioning the nodule, the hormone dictum. Plant Signal Behav 2009; 4:89-93; PMID:19649179; http://dx.doi.org/ 10.4161/psb.4.2.7693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ton J, Flors V, Mauch-Mani B. The multifaceted role of ABA in disease resistance. Trends Plant Sci 2009; 14:310-7; PMID:19443266; http://dx.doi.org/ 10.1016/j.tplants.2009.03.006 [DOI] [PubMed] [Google Scholar]
- 4. Nakashima K, Yamaguchi-Shinozaki K, Shinozaki K. The transcriptional regulatory network in the drought response and its crosstalk in abiotic stress responses including drought, cold and heat. Front Plant Sci 2014; 5:170; PMID:24904597; http://dx.doi.org/ 10.3389/fpls.2014.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liang Y, Harris JM. Response of root branching to abscisic acid is correlated with nodule formation both in legumes and non legumes. Am J Bot 2005; 92:1675-83; PMID:21646084; http://dx.doi.org/ 10.3732/ajb.92.10.1675 [DOI] [PubMed] [Google Scholar]
- 6. De Smet I, Zhang H, Inzé D, Beeckman T. A novel role for abscisic acid emerges from underground. Trends Plant Sci 2006; 11:4349. [DOI] [PubMed] [Google Scholar]
- 7. Liang Y, Mitchell DM, Harris JM. Abscisic acid rescues the root meristem defects of the Medicago truncatula latd mutant. Dev Biol 2007; 304:297307; http://dx.doi.org/ 10.1016/j.ydbio.2006.12.037 [DOI] [PubMed] [Google Scholar]
- 8. Herrbach V, Remblière C, Gough C, Bensmihen S. Lateral root formation and patterning in Medicago truncatula. J Plant Physiol 2014; 171:301-10; PMID:24148318; http://dx.doi.org/ 10.1016/j.jplph.2013.09.006 [DOI] [PubMed] [Google Scholar]
- 9. Ariel F, Diet A, Verdenaud M, Gruber V, Frugier F, Chan R, Crespi M. Environmental regulation of lateral root emergence in Medicago truncatula requires the HD-Zip I transcription factor HB1. Plant Cell 2010; 22:2171-83; PMID:20675575; http://dx.doi.org/ 10.1105/tpc.110.074823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brady SM, Sarkar SF, Bonetta D, McCourt P. The ABSCISIC ACID INSENSITIVE 3 (ABI3) gene is modulated by farnesylation and is involved in auxin signaling and lateral root development in Arabidopsis. Plant J 2003; 34:67-75; PMID:12662310; http://dx.doi.org/ 10.1046/j.1365-313X.2003.01707.x [DOI] [PubMed] [Google Scholar]
- 11. Bécard G, Fortin JA. Early events of vesicular arbuscular mycorrhiza formation on Ri T-DNA transformed roots. New Phytologist 1988; 108:211-8; http://dx.doi.org/ 10.1111/j.1469-8137.1988.tb03698.x [DOI] [PubMed] [Google Scholar]


