Abstract
Many of the most important plant diseases are caused by fungal pathogens that form specialized cell structures to breach the leaf surface as well as to proliferate inside the plant. To initiate pathogenic development, the fungus responds to a set of inductive cues. Some of them are of extracellular nature (environmental signals) while others respond to intracellular conditions (developmental signals). These signals have to be integrated into a single response that has as a major outcome changes in the morphogenesis of the fungus. The cell cycle regulation is pivotal during these cellular differentiations, and we hypothesized that cell cycle regulation would be likely to provide control points for infection development by fungal pathogens. Although efforts have been done in various fungal systems, there is still limited information available regarding the relationship of these processes with the induction of the virulence programs. Hence, the role of fungal cell cycle regulators –which are wide conserved elements– as true virulence factors, has yet to be defined. Here we discuss the recent finding that the formation of the appressorium, a structure required for plant penetration, in the corn smut fungus Ustilago maydis seems to be incompatible with an active cell cycle and, therefore genetic circuits evolved in this fungus to arrest the cell cycle during the growth of this fungus on plant surface, before the appressorium-mediated penetration into the plant tissue.
Keywords: appressorium, cell cycle, corn smut, phytopathogenic fungus, ustilago maydis, virulence
The entry into the host cell is a critical step during pathogenesis of invasive plant parasites. Furthermore, plant antiparasitic treatments are usually preventive because once the infective agent has penetrated the plant tissue, the possibilities to eradicate infection drastically decrease due to the low accessibility of the therapeutic agents within the plant. Because this, the plant cuticle represents a primary barrier in the defense against pathogens. Nevertheless, phytopathogenic fungi overcome this obstacle by using natural openings such as stomata and wounds or more generally, by producing specific infection structures termed appressoria.1 Therefore, these infection structures are likely to provide targets for therapeutic intervention. However, an important caveat at this level is that the morphology of appressoria is highly variable, most likely reflecting distinct genetic programs in different fungi. In some cases it is a clearly defined structure with a thick, multilayered and highly melanized cell wall. In other cases, appressoria are difficult to be distinguished morphologically because they represent only a slight swelling of the germ tube apex.2 Moreover, the way appressorium guides the plant penetration is heterogeneous. For example, some fungi penetrate the plant by using the turgor pressure produced inside the appressorium, whereas in other fungi the appressorium directs the localized secretion of enzymes that weakens the plant cuticule and cell wall.3
However, despite this diversity in form and function, all appressoria share some common features during its formation such as morphological changes, as well as the readjustment of cell cycle to allow the induction of these new morphogenetic programs. Therefore, the understanding of how growth and cell cycle progression are coordinately regulated during this process seems to be an alternative way to cope with plant fungal infections. We have recently showed that the formation of the appressorium in the corn smut fungus Ustilago maydis seems to be incompatible with an active cell cycle and that genetic circuits evolved in this fungus to arrest the cell cycle during the growth of this fungus on plant surface, before the penetration into the plant tissue.4 A few questions emanated from this work that remain to be uncovered, and our current view and ideas about these questions are discussed below.
Plant Penetration and Cell Cycle Progression have to be Coordinated
The virulence program in U. maydis started with the mating of 2 compatible cells on the plant surface that results in the formation of a dikaryotic infective filament.5 In response to some unclear plant signal, a poorly differentiated appressorium, rather small swelling of the hyphal tip, is formed at the tip of the filament. Appressorium formation is mandatory for infection to proceed, and U. maydis mutant strains unable to produce functional appressoria are avirulent. Interestingly, along all this process, the filament is cell cycle arrested at G2 phase6 and only once the filament enters the plant, the cell cycle is reactivated and mitotic divisions take place, concomitant with the development of clamp-like structures that allow the correct sorting of nuclei to maintain the dikaryotic status.7,8 For many years, it was believed that the explanations for this specific cell cycle arrest were related to mechanistic reasons and that the arrest at G2 phase ensured high-speed movement –since there is no requirement for mitosis and de novo generation of cytoplasm– and thereby enables the fungus to explore the plant surface, most likely looking for an appropriate point of entry. However, our recent results indicated that cell cycle arrest in G2 phase was mandatory in order to induce the formation of the appressorium. To understand this incompatibility between appressorium formation and an active cell cycle, it is required to keep in mind that mitosis demands the recruitment of a large quantity of cytoskeletal elements to form the mitotic spindle, and that the morphogenesis of the appressorium also depends on the coordinated use of both actin- and microtubules-based cytoskeletons9. Therefore, it makes sense that cellular controls exist to force these 2 processes to be incompatible, avoiding competition for the same cytoskeletal components. Interestingly, this sort of incompatibility is akin in developmental processes in metazoan. For instance, during the formation of the neural tube in Ciona intestinalis embryos, epidermal cells have to change their morphology to fuse each other, requiring for that a massive cytoskeleton remodeling. During this process mitosis is inhibited, lengthening the G2 phase, being the inhibitory phosphorylation of CDK the cell cycle regulatory target.10
The requirement for a specific cell cycle phase during appressorium formation has been noted in other fungi that produce appressoria markedly different in form and function from the ones found in U. maydis. For instance, in the case of Magnaporthe oryzae appressoria, the use of inhibitors of DNA replication and conditional mutants in cell cycle regulators showed that the regulation point for initiating appressorium development must occur prior to mitosis and depends on a full DNA replication; In other words, most likely it occurs during G2 phase.11 One clear difference between M. oryzae and U. maydis appressoria regarding cell cycle regulation occurs at the maturation step. For M. oryzae the penetration peg development requires the coupling with mitosis, most likely leaving one daughter nucleus at the appressorium and the other one traveling with the penetration peg.12 However, in U. maydis it has been described that cell cycle seems not to be reactivated until the infective dikaryotic hypha penetrates the plant tissue. In this case, the 2 genetically distinct nuclei travel at the tip of the filament.13 This uncoupling between mitosis and penetration in U. maydis probably is a consequence of the peculiarities of the complex cell cycle required to maintain heterokaryosis after cell division. In some basidiomycete, as it is the case of U. maydis and Coprinopsis cinerea, the nuclear division involves the production of a specific structure called clamp-like cell,7,14,15 devoted microtubules structures16 and the activation of a specific checkpoint controlled by the DNA damage response pathway.7,17 Again, it makes sense that during the penetration step –that in U. maydis seems to be not dependent on turgor pressure but a continuous communication between the plant and the fungus that involves dedicated secretion of effector proteins– mitosis has to be delayed.
Down-Regulation of the Hsl1 Hinase Serves Distinct Purposes
Cell cycle arrest during the formation of the infective filament relies on the down-regulation of the expression of hsl1, encoding a kinase that negatively regulates the mitotic inhibitory kinase Wee1. Hsl1 belongs to the Nim1 family of protein kinases, having roles in cell cycle as well as in morphogenesis control. In Saccharomyces cerevisiae, for instance, Hsl1 negatively regulate the Wee1-like kinase Swe1, and it also controls the septin ring responsible of the bud neck morphology.18 Interestingly, we believe that the downregulation of hsl1 during the formation of the infective filament serves 2 distinct purposes. One is the reported role establishing the G2 cell cycle arrest. However, we think there is a second reason to keep down the levels of Hsl1 during the formation of the infective filament. This second reason seems to be related to the distinct morphology of the neck depending whether the mother cell is producing a bud or an infective filament. When forming a bud, the neck showed a constriction between adjacent cellular compartments. In this constriction, cell separation eventually will take place. However the neck connecting the infective filament and the mother cell lacks this constriction and shows the characteristic hyphal shape of a long tube-like structure with parallel sides along its entire length. Interestingly, in infective filaments from strains that do not down-regulate the expression of hsl1, the neck between the mother cell and the filaments shows a constriction that reminds a bud neck (Fig. 1). Although this morphological defect has no influence on the functionality of the infective filament, we think it reflects distinct programs of cellular construction that most likely are controlled by the Hsl1 kinase. Since in other organisms, Hsl1 is involved in the control of septins, and we also observed this morphological defect in infective filaments from septin mutants,19 we believe that septins should be differentially regulated during the formation of the infective filament in comparison to the formation of a bud. These predictions are being tested currently in our laboratory.
Figure 1.
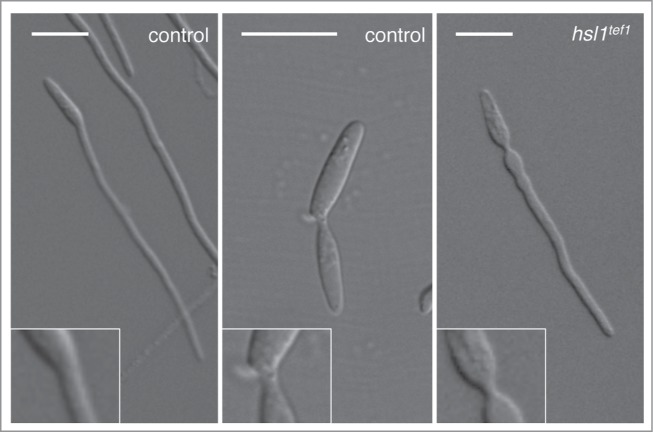
Neck morphology in a wild-type (control) cell forming either an infective filament (left panel) or a bud (middle panel). Note the constriction observed in the bud neck (middle inset) in comparison with the absence of constrictions in the filament neck (left inset). In a strain that is not able to down-regulate the hsl1 expression during the formation of the infective filament (hsl1tef1) strikingly, the neck of the filament shows a constriction (right inset). Bar: 15 μm.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Kolattukudy PE, Rogers LM, Li D, Hwang CS, Flaishman MA. Surface signaling in pathogenesis. Proc Natl Acad Sci U S A 1995; 92:4080-7; PMID:7753774; http://dx.doi.org/ 10.1073/pnas.92.10.4080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mendgen K, Deising H. Infection structures of fungal plant pathogens - a cytological and physiological evaluation. New Phytol 1993; 124:193-213; http://dx.doi.org/ 10.1111/j.1469-8137.1993.tb03809.x [DOI] [PubMed] [Google Scholar]
- 3.Deising HB, Werner S, Wernitz M. The role of fungal appressoria in plant infection. Microbes Infect 2000; 2:1631-41; PMID:11113382; http://dx.doi.org/ 10.1016/S1286-4579(00)01319-8 [DOI] [PubMed] [Google Scholar]
- 4.Castanheira S, Mielnichuk N, Perez-Martin J. Programmed cell cycle arrest is required for infection of corn plants by the fungus Ustilago maydis. Development 2014; 141:4817-26; PMID:25411209; http://dx.doi.org/ 10.1242/dev.113415 [DOI] [PubMed] [Google Scholar]
- 5.Perez-Martin J. Cell cycle and morphogenesis connections during the formation of the infective filament in Ustilago maydis In: Perez-Martin J, di Pietro A, eds. Morphogenesis and Pathogenicity in Fungi. Berlin Heidelberg: Taylor & Francis, 2012: 97-114 [Google Scholar]
- 6.Mielnichuk N, Sgarlata C, Perez-Martin J. A role for the DNA-damage checkpoint kinase Chk1 in the virulence program of the fungus Ustilago maydis. J Cell Sci 2009; 122:4130-40; PMID:19861497; http://dx.doi.org/ 10.1242/jcs.052233 [DOI] [PubMed] [Google Scholar]
- 7.de Sena-Tomas C, Fernandez-Alvarez A, Holloman WK, Perez-Martin J. The DNA damage response signaling cascade regulates proliferation of the phytopathogenic fungus Ustilago maydis in planta. Plant Cell 2011; 23:1654-65; PMID:21478441; http://dx.doi.org/ 10.1105/tpc.110.082552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez-Martin J, de Sena-Tomas C. Dikaryotic cell cycle in the phytopathogenic fungus Ustilago maydis is controlled by the DNA damage response cascade. Plant Signal Behav 2011; 6:1574-7; PMID:21918381; http://dx.doi.org/ 10.4161/psb.6.10.17055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinberg G, Perez-Martin J. Ustilago maydis, a new fungal model system for cell biology. Trends Cell Biol 2008; 18:61-7; PMID:18243705; http://dx.doi.org/ 10.1016/j.tcb.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 10.Ogura Y, Sakaue-Sawano A, Nakagawa M, Satoh N, Miyawaki A, Sasakura Y. Coordination of mitosis and morphogenesis: role of a prolonged G2 phase during chordate neurulation. Development 2011; 138:577-87; PMID:21205801; http://dx.doi.org/ 10.1242/dev.053132 [DOI] [PubMed] [Google Scholar]
- 11.Saunders DG, Aves SJ, Talbot NJ. Cell cycle-mediated regulation of plant infection by the rice blast fungus. Plant Cell 2010; 22:497-507; PMID:20190078; http://dx.doi.org/ 10.1105/tpc.109.072447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saunders DG, Dagdas YF, Talbot NJ. Spatial uncoupling of mitosis and cytokinesis during appressorium-mediated plant infection by the rice blast fungus Magnaporthe oryzae. Plant Cell 2010; 22:2417-28; PMID:20639448; http://dx.doi.org/ 10.1105/tpc.110.074492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snetselaar K, Mims CW. Infection of maize stigmas by Ustilago maydis: light and electron microscopy. Phytopathology 1993; 83:843-50; http://dx.doi.org/ 10.1094/Phyto-83-843 [DOI] [Google Scholar]
- 14.Inada K, Morimoto Y, Arima T, Murata Y, Kamada T. The clp1 gene of the mushroom Coprinus cinereus is essential for A-regulated sexual development. Genetics 2001; 157:133-40; PMID:11139497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scherer M, Heimel K, Starke V, Kamper J. The Clp1 protein is required for clamp formation and pathogenic development of Ustilago maydis. Plant Cell 2006; 18:2388-401; PMID:16920779; http://dx.doi.org/ 10.1105/tpc.106.043521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanabe S, Kamada T. The role of Astral microtubules in conjugate division in the dikaryon of Coprinus cinereus. Exp Mycol 1994; 18:338-48; http://dx.doi.org/ 10.1016/S0147-5975(06)80007-1 [DOI] [Google Scholar]
- 17.de Sena-Tomas C, Navarro-Gonzalez M, Kues U, Perez-Martin J. A DNA damage checkpoint pathway coordinates the division of dikaryotic cells in the ink cap mushroom Coprinopsis cinerea. Genetics 2013; 195:47-57; PMID:23792951; http://dx.doi.org/ 10.1534/genetics.113.152231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barral Y, Parra M, Bidlingmaier S, Snyder M. Nim1-related kinases coordinate cell cycle progression with the organization of the peripheral cytoskeleton in yeast. Genes Dev 1999; 13:176-87; PMID:9925642; http://dx.doi.org/ 10.1101/gad.13.2.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alvarez-Tabares I, Perez-Martin J. Septins from the phytopathogenic fungus Ustilago maydis are required for proper morphogenesis but dispensable for virulence. PLoS One 2010; 5:e12933; PMID:20885997; http://dx.doi.org/ 10.1371/journal.pone.0012933 [DOI] [PMC free article] [PubMed] [Google Scholar]


