Abstract
Background:
Breathlessness is a common problem in chronic heart failure (CHF) patients, and respiratory muscle strength has been proposed to play an important role in causing breathlessness in these patients.
Objectives:
The aim of this study was to investigate the relation between respiratory muscle strength and the severity of CHF, and the influence of respiratory muscle strength on abnormal ventilation during exercise in CHF patients.
Patients and Methods:
In this case series study, we assessed clinically stable CHF outpatients (N = 66, age: 57.7 ± 14.6 years). The peak oxygen consumption (peak VO2), the slope relating minute ventilation to carbon dioxide production (VE/VCO2 slope), and the slope relating tidal volume to respiratory rate (TV/RR slope) were measured during cardiopulmonary exercise testing. Respiratory muscle strength was assessed by measuring the maximal inspiratory pressure (MIP) and maximal expiratory pressure (MEP).
Results:
The MIP and MEP decreased significantly as the New York Heart Association functional class increased (MIP, P = 0.021; MEP, P < 0.01). The MIP correlated with the TV/RR slope (r = 0.57, P < 0.001) and the VE/VCO2 slope (r = -0.44, P < 0.001), and the MEP also correlated with the TV/RR slope (r = 0.53, P < 0.001) and the VE/VCO2 slope (r = -0.25, P < 0.040). Stepwise multiple regression analysis revealed that age and MIP were statistically significant predictors of the TV/RR and VE/VCO2 slopes (both P < 0.05).
Conclusions:
Respiratory muscle strength is related to the severity of CHF, and associated with rapid and shallow ventilation or excessive ventilation during exercise.
Keywords: Heart Failure, Respiratory Muscle, Exercise Test
1. Background
Patients with chronic heart failure (CHF) have reduced exercise tolerance and are limited in performing the activities of daily living (1). The main goal of cardiac rehabilitation programs for CHF patients is to improve their exercise tolerance and quality of life (2). Cardiac rehabilitation programs should play an important role in reducing the symptoms of breathlessness during the performance of activities of daily living or during exercise (3). However, the physical index that is used to clarify the cause of exercise intolerance and breathlessness in CHF patients is not well known.
Breathlessness is a common problem in CHF patients, and respiratory muscle strength has been proposed to play an important role in causing breathlessness in these patients (4). There is a significant correlation between respiratory muscle weakness and the subjective rating of dyspnea during the performance of daily life activities (4). Breathlessness is characterized by rapid and shallow ventilation or excessive ventilation, and these phenomena are assessed objectively in cardiopulmonary exercise testing (CPX) as indices of abnormal ventilation in CHF patients (5, 6). Previous studies have evaluated rapid and shallow ventilation during CPX by using the slope relating the tidal volume to the respiratory rate (TV/RR slope) (7), and excessive ventilation by using the slope relating the minute ventilation to the carbon dioxide production (VE/VCO2 slope) (6). Previous studies also reported that the worsening of both the TV/RR and VE/VCO2 slopes represents symptomatic and severe CHF (5, 6), and this worsening is affected by several respiratory functions such as overactivation of the ergoreflex, chemosensitivity, and decreased lung compliance (8-10). Thus, it is important to clarify the mechanisms underlying the symptoms of dyspnea during CPX in CHF patients.
Ventilatory limitation during exercise in CHF patients is related to weakness of the respiratory muscles (11). Although it is well known that a significant correlation exists between the VE/VCO2 slope and both respiratory muscle strength and clinical background characteristics such as age, sex, and brain natriuretic peptide (BNP) level (12, 13), there is no study on the relation between respiratory muscle strength and excessive ventilation in CHF patients in regard to these clinical characteristics. Understanding the relation of respiratory muscle strength to disease severity and abnormal ventilation during exercise in CHF patients may help guide exercise prescription recommendations.
2. Objectives
The objective of the present study was to clarify both the relation between respiratory muscle strength and the disease severity in CHF, and the association of respiratory muscle strength with abnormal ventilation during exercise in CHF patients in relation to clinical characteristics.
3. Patients and Methods
3.1. Study Design and Subjects
This was a case series study in which the subjects were selected from 84 outpatients who visited St. Marianna university school of medicine hospital for the evaluation of CHF. The inclusion criterion was a left ventricular ejection fraction (LVEF) of < 45% on echocardiography. The exclusion criteria included New York heart association (NYHA) functional class IV or the presence of central neurological, peripheral vascular, orthopedic, or pulmonary disease. Of the 84 outpatients, 66 met the study criteria and were included in this study (57 men, 9 women; mean age 57.7 ± 14.6 years). None of the patients had been hospitalized within the month preceding the start of the study. All patients were clinically stable and were studied while on stable doses of medications. The present study was approved by the university school of medicine institutional committee on human research. Informed consent was obtained from each subject before study entry.
3.2. Clinical Characteristics
Age, sex, etiology of CHF (dilated cardiomyopathy, ischemic heart disease, and others), and medications were assessed from medical records. The LVEF was calculated by using M-mode echocardiography and served as the index of left ventricular systolic function, and patients were classified into three groups: those with mild (40 ≤ LVEF < 45), moderate (35 ≤ LVEF < 40), and severe (LVEF <35) left ventricular dysfunction. The NYHA functional class and BNP level were evaluated as indices of the severity of CHF. The NYHA functional class was determined in all patients by an independent investigator, and the BNP level was measured by means of blood sampling at rest before CPX. After centrifugation at 3000 rpm at 4°C for 10 min, the separated plasma was stored at -70°C until analyzed.
3.3. CPX
All 66 patients underwent symptom-limited CPX on a cycle ergometer (CORIVAL 400; Lode Co., Groningen, the Netherlands) with a ramp protocol. After patients had rested on the ergometer for 3-min, exercise was performed with the load increased linearly by 10 or 20 W/min following a 3-min warm-up period at 0-W load. A 12-lead electrocardiogram and the heart rate were continuously monitored on an ML-500 stress test system (Fukuda Denshi Co., Tokyo, Japan). Expired gas was sampled by using a breath-by-breath method, and the peak oxygen uptake (VO2), carbon dioxide output, minute ventilation, respiratory rate, and tidal volume were measured simultaneously with a respirometer (AE-300S aero monitor; Minato Ikagaku Co., Tokyo, Japan) and a gas analyzer (MG-360; Minato Ikagaku Co., Tokyo, Japan). The respiratory exchange ratio was also calculated.
To assess exercise tolerance, peak VO2 was calculated from the average of the VO2 values measured during the 30 seconds before peak exercise was reached, and VO2 at the anaerobic threshold (AT) was determined by using the V-slope method (14). The VE/VCO2 slope was calculated with linear regression analysis by using the minute ventilation and carbon dioxide production values obtained during exercise (15). The TV/RR slope was also calculated with linear regression analysis by using the tidal volume and respiratory rate values obtained during exercise (16). Each value was determined as an index of the ventilatory response to graded exercise below the respiratory compensation point, to avoid the influence of the end point of CPX. Ratings of perceived exertion as measured by using the Borg scale were obtained at the end point of CPX (17).
3.4. Respiratory Muscle Strength
Measurements of maximal respiratory muscle strength were performed with Vitalopower KH-101 (Chest M. I. Inc., Tokyo, Japan), and followed a method similar to that described by Black and Hyatt (18). By using a nose clip and mouthpiece, the patients maintained maximal inspiratory and expiratory effort against a closed valve for at least 1 s to measure the maximal inspiratory pressure (MIP) at residual volume or the maximal expiratory pressure (MEP) at total lung capacity, respectively. Each measurement was repeated three times, and the highest values of the MIP and MEP were used as the indices of maximal inspiratory and expiratory muscle strength, respectively. Because the MIP and MEP negatively correlate with age, the percent (%) predicted MIP and MEP were calculated by using the linear regression models reported by Black and Hyatt (18):
| (1) |
According to the Equations 2
| (2) |
| (3) |
3.5. Statistical Analysis
Results are expressed as means ± SE. The Kolmogorov-Smirnov test was performed to assess normality, and logarithmic transformation of the variables was performed as needed to improve normality. One-way analysis of variance (ANOVA) and the χ2 test were used to analyze the differences in clinical characteristics. ANOVA or analysis of covariance (ANCOVA) was performed to determine the differences among the NYHA class groups and among the left ventricular dysfunction groups in the results from CPX and respiratory muscle strength testing. ANCOVA was used if age was determined as the covariate with the results of CPX and respiratory muscle strength. If the F ratio was significant in ANOVA or ANCOVA, Tukey’s multiple range test was applied to determine the significant differences between the two NYHA class groups. Pearson’s correlation coefficient was used in a regression analysis to determine the correlations between the respiratory muscle strength parameters and CPX or BNP. Spearman rank correlation coefficient was used to evaluate the relations between Borg scale values and muscle strength parameters, CPX, or BNP. Age, sex, body mass index, LVEF, BNP, MIP, and MEP were entered stepwise in the multiple regression analysis for the prediction of the VE/VCO2 and TV/RR slopes. A P value of <0.05 was considered statistically significant. The statistical analyses were performed with SPSS 12.0J statistical software program (SPSS Japan, Tokyo, Japan), and statistical power analysis was performed for multiple regression analysis with the G*Power 3 program (Heinrich-Heine-Universität, Düsseldorf, Germany) in the post hoc test.
4. Results
The patients’ clinical characteristics, including age, sex, body mass index, BNP level, LVEF, etiology of CHF, and medications, are shown in Table 1. There were no significant differences between the NYHA class groups in sex, body mass index, LVEF, etiology of CHF, and medications. Carvedilol was prescribed as the β-blocker in 57 patients. Age was significantly higher in NYHA class III patients than in NYHA class I/II patients (P = 0.003 and P = 0.014, respectively). The log BNP level was significantly higher in NYHA class II/III patients than in NYHA class I patients (P = 0.040 and P = 0.005, respectively).
Table 1. Clinical Characteristics Stratified by NYHA Functional Class a,b.
| Characteristics | Total (N = 66) | NYHA I (n = 23) | NYHA II (n = 32) | NYHA III (n = 11) | P Valuec |
|---|---|---|---|---|---|
| Age, y | 57.7 ± 1.8 | 53.1 ± 3.0 | 56.6 ± 2.4 | 70.5 ± 3.3 d,e | 0.003 |
| Sex, male/female | 57/9 | 21/2 | 27/5 | 9/2 | 0.678 |
| BMI, kg/m 2 | 23.4 ± 0.5 | 24.3 ± 0.9 | 22.7 ± 0.8 | 23.4 ± 0.6 | 0.387 |
| BNP, pg/mL | 253.2 ± 29.4 | 124.4 ± 16.2 | 279.5 ± 47.8 d | 399.3 ± 70.3 d | 0.005 |
| Log BNP | 5.12 ± 0.12 | 4.57 ± 0.17 | 5.21 ± 0.17 d | 5.83 ± 0.18 d | 0.001 |
| LVEF, % | 28.1 ± 1.1 | 27.9 ± 1.8 | 28.9 ± 1.8 | 26.0 ± 2.2 | 0.673 |
| Etiology | 0.418 | ||||
| DCM | 31 (47.0) | 8 (34.8) | 17 (53.1) | 6 (54.5) | |
| IHD | 16 (24.2) | 5 (19.4) | 8 (25.0) | 3 (27.3) | |
| Others (HHD, AF, HCM) | 19 (28.8) | 10 (43.5) | 7 (21.9) | 2 (18.2) | |
| Medication | |||||
| β-Blocker | 57 (86.4) | 22 (95.7) | 27 (84.4) | 8 (72.7) | 0.171 |
| ACE-I or ARB | 61 (92.4) | 19 (73.9) | 31 (93.8) | 11 (100) | 0.192 |
| Digoxin | 14 (21.2) | 7 (30.4) | 4 (12.5) | 3 (27.2) | 0.239 |
| Diuretics | 63 (95.5) | 20 (87.0) | 32 (100) | 11 (100) | 0.053 |
a Abbreviations: ACE-I, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin-receptor blocker; BMI, body mass index; BNP, brain natriuretic peptide; DCM, dilated cardiomyopathy; HCM, hypertrophied cardiomyopathy; HHD, hypertensive heart disease; IHD, ischemic heart disease; LVEF, left ventricular ejection fraction; NS, not significant; NYHA, New York Heart Association functional class.
b Data are presented as No. (%) or mean ± SE.
c P values in the analysis of variance or χ2 test.
d P < 0.05 vs. NYHA I in the post hoc test.
e P < 0.05 vs. NYHA II in the post hoc test.
The results obtained from CPX and respiratory muscle strength testing are shown in Table 2. No patient showed ischemic ST change, or experienced chest pain or serious arrhythmia during CPX. ANCOVA revealed that age was the only covariate in the MIP (F = 5.61, P = 0.021), whereas age was not determined to be a significant covariate in CPX and the MEP.
Table 2. Parameters of Cardiopulmonary Exercise Testing and Respiratory Muscle Strength Stratified by NYHA Functional Classa,b.
| Variables | Total (N = 66) | NYHA I (n = 23) | NYHA II (n = 32) | NYHA III (n = 11) | P Value c |
|---|---|---|---|---|---|
| VO 2 at AT, mLkg -1 min -1 | 14.9 ± 0.5 | 15.8 ± 0.8 | 14.1 ± 0.5 | 9.3 ± 0.5 d,e | <0.001 |
| Peak VO 2 , mLkg -1 min -1 | 18.9 ± 0.7 | 22.3 ± 0.9 | 18.7 ± 0.7 d | 12.2 ± 0.7 d,e | <0.001 |
| Peak RER | 1.22 ± 0.01 | 1.19 ± 0.01 | 1.22 ± 0.01 | 1.22 ± 0.01 | 0.387 |
| VE/VCO 2 slope | 32.6 ± 0.9 | 28.5 ± 1.0 | 32.8 ± 1.3 | 40.5 ± 2.3 d,e | <0.001 |
| TV/RR slope | 66.2 ± 5.0 | 82.4 ± 8.7 | 64.3 ± 7.1 | 37.9 ± 5.4 d | 0.009 |
| MIP, cm H 2 O | 79.7 ± 4.1 | 96.4 ± 6.9 | 76.1 ± 5.6 d | 55.3 ± 5.2 d | 0.024 f |
| Predicted MIP, % | 74.2 ± 3.3 | 86.3 ± 5.2 | 71.0 ± 4.6 d | 58.1 ± 6.5 d | 0.008 |
| MEP, cm H 2 O | 108.7 ± 5.5 | 129.4 ± 11.0 | 102.6 ± 6.5 | 83.3 ± 9.3 d | 0.009 |
| Predicted MEP, % | 54.4 ± 2.4 | 61.9 ± 4.6 | 51.5 ± 2.9 | 47.0 ± 6.5 | 0.061 |
| Ramp exercise time, min | 8.83 ± 0.25 | 9.87 ± 0.45 | 8.72 ± 0.25 | 7.00 ± 0.57 | <0.001 |
a Abbreviations: ANOVA, analysis of variance; AT, anaerobic threshold; MEP, maximal expiratory pressure; MIP, maximal inspiratory pressure; NYHA, New York Heart Association functional class; RER, respiratory exchange ratio; RR, respiratory rate; TV, tidal volume; VCO2, carbon dioxide output; VE, minute ventilation; VO2, oxygen uptake.
b Data are presented as means ± SE.
c P values in the analysis of variance or χ2 test.
d P < 0.05 vs. NYHA I in the post hoc test.
e P < 0.05 vs. NYHA II in the post hoc test.
f F and P value in the analysis of covariance.
The VO2 at AT was significantly lower in NYHA class III patients than in NYHA class I/II patients (P < 0.001 and P < 0.001, respectively). Although there was no significant difference in the peak respiratory exchange ratios between the NYHA class groups, the peak VO2 was significantly lower in NYHA class III patients than in NYHA class I/II patients (P < 0.001 and P < 0.001, respectively), and was significantly lower in NYHA class II patients than in NYHA class I patients (P < 0.004). The VE/VCO2 slope was significantly higher in NYHA class III patients than in NYHA class I/II patients (P < 0.001 and P = 0.004, respectively). The TV/RR slope was significantly lower in NYHA class III patients than in NYHA class I patients (P = 0.004); however, there was no significant difference in the TV/RR slope between the NYHA class II and III patients.
The MIP was significantly lower in NYHA class II and III patients than in NYHA class I patients (P = 0.029 and P = 0.014, respectively), and the % predicted MIP was significantly lower in NYHA class III patients than in NYHA class I patients (P = 0.009). The MEP was significantly lower in NYHA class III patients than in NYHA class I patients (P = 0.012), whereas there was no significant difference among NYHA class patients in % predicted MEP.
There was no significant difference between the mild, moderate, and severe left ventricular dysfunction groups in respiratory muscle strength and the results in CPX.
The MIP and MEP positively correlated with the peak VO2 (r = 0.35, P = 0.004 and r = 0.31, P = 0.011, respectively). There was a positive but poor correlation between the MIP and VO2 at AT (r = 0.26, P = 0.038), whereas there was no statistical significance in the relation between the MEP and VO2 at AT (r = 0.24, P = 0.054).
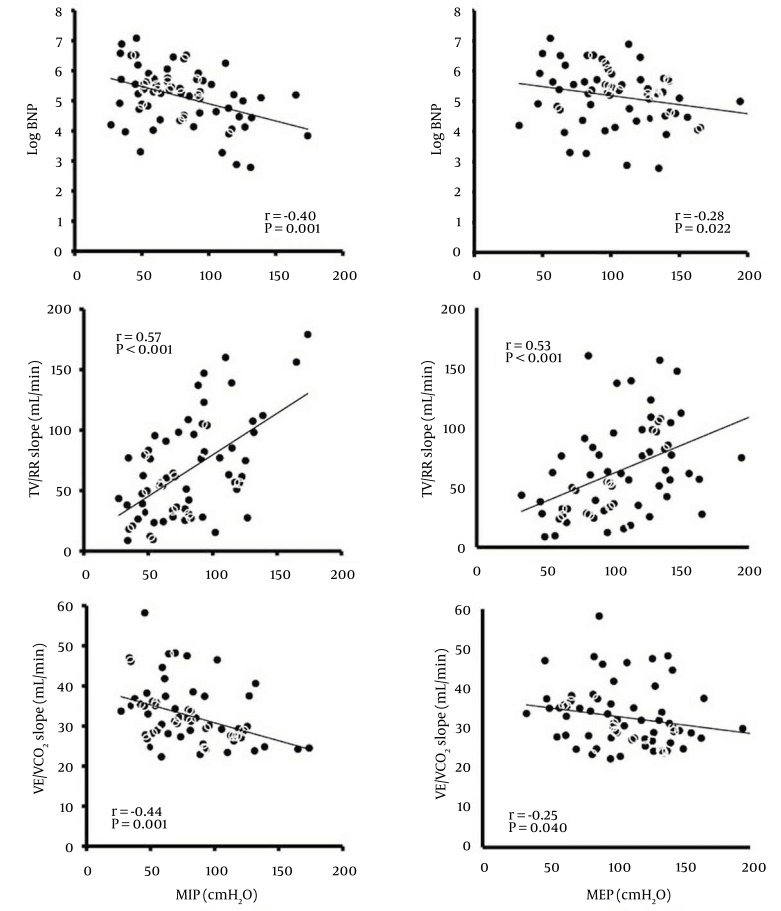
The correlations between the MIP or MEP and the log BNP level are shown in Figure 1. There were negative correlations between the MIP or MEP and the log BNP level (r = -0.40, P = 0.001 and r = -0.28, P = 0.022, respectively).
Figure 1. Correlation between Respiratory Muscle Strength and the BNP Level, TV/RR Slope, or VE/VCO2 Slope.
MEP, maximal expiratory pressure; MIP, maximal inspiratory pressure; BNP, brain natriuretic peptide; TV/RR slope, slope relating tidal volume to respiratory rate; VE/VCO2 slope, slope relating minute ventilation to carbon dioxide production.
The correlations between the MIP or MEP and the TV/RR slope, and between the MIP or MEP and the VE/VCO2 slope in all 66 patients are shown in Figure 1. There were positive correlations between the MIP or MEP and the TV/RR slope (r = 0.57, P < 0.001 and r = 0.53, P < 0.001, respectively), and negative correlations between the MIP or MEP and the VE/VCO2 slope (r = -0.44, P < 0.001 and r = -0.25, P = 0.040, respectively). In addition, there was a negative correlation between the TV/RR slope and the VE/VCO2 slope (r = -0.54, P < 0.001).
The results of multiple regression analysis are shown in Table 3. Stepwise multiple regression analysis revealed that age and the MIP were statistically significant predictors of the TV/RR and VE/VCO2 slopes (both P < 0.05). The estimated r2 values were 0.25 for the VE/VCO2 slope and 0.37 for the TV/RR slope. The statistical power was calculated to be 0.99 in the regression model for the VE/VCO2 slope (effect size = 0.33, sample size = 66, tested predictors = 2, total predictor = 7), and to be 1.0 in the regression model for TV/RR (effect size = 0.59, sample size = 66, tested predictors = 2, total predictor = 7).
Table 3. Results of Stepwise Multiple Regression Analysis for the Prediction of the VE/VCO2 and TV/RR Slopes from Age, Sex, BMI, LVEF, BNP, MIP, and MEP a.
| Independent variables | Dependent Variable: VE/VCO2 Slope (R2 = 0.25, F = 10.54, P < 0.001) | Dependent Variable: TV/RR Slope (R2 = 0.37, F = 18.7, P < 0.001) | ||||
|---|---|---|---|---|---|---|
| Regression Coefficient B | Standard Error | P Value | Regression Coefficient B | Standard Error | P Value | |
| MIP, cm H 2 O | -0.06 | 0.03 | 0.035 | 0.57 | 0.13 | < 0.001 |
| Age, y | 0.18 | 0.06 | 0.006 | -0.66 | 0.30 | 0.031 |
| Constant | 27.10 | 4.91 | < 0.001 | 57.91 | 23.65 | 0.017 |
a Abbreviations: BMI, body mass index; BNP, brain natriuretic peptide; CI, confidence interval; LVEF, left ventricular ejection fraction; MIP, maximal inspiratory pressure; RR, respiratory rate; TV, tidal volume; VCO2, carbon dioxide output; VE, minute ventilation.
The correlations between the Borg scale and physiological parameters are shown in Table 4. There were negative correlations between the Borg scale values and the MIP or the TV/RR slope, and there was a positive correlation between the Borg scale values and the VE/VCO2 slope in all 66 patients.
Table 4. Correlations between Borg Scale at Peak Exercise and Physiological Measures a,b.
| All Patients (N = 66) | MIP, cm H2O | MEP, cm H2O | VO2 at AT, mLkg-1min-1 | Peak VO2, mLkg-1min-1 | VE/VCO2, Slope | TV/RR, Slope |
|---|---|---|---|---|---|---|
| Borg scale | -0.28 c | -0.23 | -0.25 | -0.23 | 0.26 c | -0.47 c |
a Abbreviations: AT, anaerobic threshold; MEP, maximal expiratory pressure; MIP, maximal inspiratory pressure; RER, respiratory exchange ratio; RR, respiratory rate; TV, tidal volume; VCO2, carbon dioxide output; VE, minute ventilation; VO2, oxygen uptake.
b Data are Rho values in Spearman’s correlation.
c P < 0.05.
5. Discussion
5.1. Respiratory Muscle Dysfunction and CHF Severity
It is well known that respiratory muscle strength is decreased in CHF patients compared with normal persons (19). However, there are few reports on the relation between respiratory muscle strength and the severity of CHF. One previous study reported that inspiratory muscle strength is significantly decreased in NYHA class III patients compared with that in NYHA class I and II patients (19). Although there are significant correlations between inspiratory muscle strength and age, no study, to our knowledge, has investigated the difference among NYHA classes in relation to age. In the present study, differences in respiratory muscle strength were assessed with ANCOVA by using age as the covariant.
When age was determined as the covariant, the inspiratory muscle strength was significantly lower in NYHA class II/III patients than in NYHA class I patients. Furthermore, the % predicted inspiratory muscle strength was significantly lower in NYHA class III than in NYHA class I patients. NYHA classification is determined according to the symptoms of dyspnea occurring during the activities of daily living, such as walking, stair climbing, and self-care in bed. In CHF patients, inspiratory and expiratory muscle weakness contributed significantly to the dyspnea occurring during exercise testing or daily activities (4, 20). Thus, as our findings indicate, respiratory muscle strength appears to reflect the severity of CHF through the symptoms manifested during exercise or the performance of the activities of daily living.
The relation between respiratory muscle strength and BNP level and exercise capacity as indicators of disease severity in CHF patients was also examined. The BNP level increases as CHF becomes more severe, and the BNP level is an independent predictor of mortality in CHF (21). Similarly, exercise capacity is a powerful predictor of mortality and an index of the severity of CHF. The present study showed that both inspiratory and expiratory muscle strength correlated positively with the anaerobic threshold and peak VO2, and these results seem to support previous studies showing a relation between inspiratory muscle strength and peak VO2 in CHF patients (11).
In the present study, inspiratory and expiratory muscle strength correlated negatively with the BNP level. The BNP level is elevated in patients with left ventricular dysfunction (22), and a previous study demonstrated that BNP is related to histological skeletal muscle abnormalities in CHF patients (23). Biopsies of respiratory muscles show a variety of histological abnormalities in CHF (24), including atrophy of type 1 fibers in the diaphragm in experimental CHF in animals (25). The diaphragm consists predominantly of type 1 fibers, whereas the limb muscles consist of type 2A and 2B fibers; thus, the diaphragm may be more dependent on adequate blood flow than are the limb muscles. Therefore, a greater decrease in respiratory muscle strength occurs in patients with severe CHF because of chronically insufficient blood flow to the skeletal muscles and depressed oxidative capacity (1), including the respiratory muscles.
On the other hand, there is no significant difference between the left ventricular dysfunction groups in respiratory muscle strength and the results in CPX in the present study. These results support previous studies in which there was no significant correlation between LVEF and respiratory muscle strength, and there was a poor relation between LVEF and exercise capacity (26, 27).
5.2. Relations between Respiratory Muscles and Excessive Ventilation during Exercise
Respiratory muscle weakness has also been proposed to cause symptoms of breathlessness in CHF patients (4), and rapid and shallow ventilation and excessive ventilation are often observed in CHF patients during incremental exercise testing (5, 20). The present study demonstrated a relation between the ratings of perceived exertion and respiratory muscle strength or parameters of abnormal ventilation. The Borg scale value at the end point of CPX correlated significantly with the MIP. The results of the present study seem to support those of a previous study reporting a correlation between inspiratory muscle strength and ratings of perceived exertion during the performance of daily activities (4). Furthermore, there were significant correlations between Borg scale values and the TV/RR slope or the VE/VCO2 slope, and thus it seems that objective parameters of abnormal ventilation assessed during CPX may reflect the subjective parameter of dyspnea during exercise.
Anatomical dead space ventilation was reported to be higher in CHF patients than in control subjects during exercise, and the ratio of anatomical dead space ventilation for VE is higher in CHF patients than in control subjects at peak exercise (28). The results of the present study indicated that respiratory muscle weakness and rapid and shallow ventilation occurred in patients with severe CHF, and such ventilation may result from inspiratory muscle weakness because the MIP was significant in predicting the TV/RR slope in multiple regression analysis. The results of the present study imply that inspiratory muscle strength weakness leads to rapid and shallow ventilation, which may result in an increase of anatomical dead space during exercise.
The present study also showed that the TV/RR slope correlated with the VE/VCO2 slope. This result seems to be related to an increase of anatomical dead space, and indicates that rapid and shallow ventilation may be an important cause of excessive ventilation as shown by the VE/VCO2 slope (20, 28). Several factors result in excessive ventilation, such as overactivity of the ergoreflex (10), hypersensitized peripheral and/or central chemoreceptors (29), and reduced lung compliance (9). However, it is not clear whether respiratory muscle strength affects excessive ventilation during exercise. The present study showed that MIP was significant in predicting the VE/VCO2 slope in multiple regression analysis. Thus, it appears that inspiratory muscle weakness, rather than clinical characteristics such as age, sex, body mass index, BNP, or LVEF, may cause an increase in the VE/VCO2 slope through the influence of an inefficient ventilation pattern.
5.3. Limitations
The present study has several limitations. First, we enrolled CHF patients who were predominantly men. Therefore, a future study should validate the possible effects of sex differences. Second, although several studies have reported on the effect of respiratory muscle training (30), we did not examine the effect of improvement in respiratory muscle strength on excessive ventilation during exercise in this study. Therefore, the influence of respiratory muscle strength training on breathlessness or excessive ventilation during exercise should be investigated in the future.
5.4. Conclusions
In the present study, we found decreased respiratory muscle strength in patients with severe CHF and showed that the decrease in respiratory muscle strength correlates with the decreased exercise tolerance of patients with CHF.
5.5. Clinical Implication
Inspiratory muscle strength relates to rapid and shallow ventilation or excessive ventilation during exercise. Therefore, it may be useful to assess the respiratory muscle strength of CHF patients with symptoms of breathlessness, to predict patients who may experience abnormal ventilation during exercise.
Acknowledgments
The authors are grateful to the staff of the department of rehabilitation medicine and the department of cardiology of St. Marianna school of medicine for their assistance in collection of the data.
Footnotes
Authors’ Contributions:Study concept and design: Yusuke Kasahara and Kazuhiro P. Izawa. Analysis and interpretation of data: Yusuke Kasahara and Kazuhiro P. Izawa. Drafting of the manuscript: Yusuke Kasahara. Critical revision of the manuscript for important intellectual content: Kazuhiro P. Izawa, Satoshi Watanabe, Naohiko Osada and Kazuto Omiya. Statistical analysis: Yusuke Kasahara and Kazuhiro P. Izawa.
References
- 1.Drexler H, Riede U, Munzel T, Konig H, Funke E, Just H. Alterations of skeletal muscle in chronic heart failure. Circulation. 1992;85(5):1751–9. doi: 10.1161/01.cir.85.5.1751. [DOI] [PubMed] [Google Scholar]
- 2.Izawa KP, Watanabe S, Yokoyama H, Hiraki K, Morio Y, Oka K, et al. Muscle strength in relation to disease severity in patients with congestive heart failure. Am J Phys Med Rehabil. 2007;86(11):893–900. doi: 10.1097/PHM.0b013e318154b592. [DOI] [PubMed] [Google Scholar]
- 3.Pina IL. Exercise and Heart Failure: A Statement From the American Heart Association Committee on Exercise, Rehabilitation, and Prevention. Circulation. 2003;107(8):1210–25. doi: 10.1161/01.cir.0000055013.92097.40. [DOI] [PubMed] [Google Scholar]
- 4.McParland C, Krishnan B, Wang Y, Gallagher CG. Inspiratory muscle weakness and dyspnea in chronic heart failure. Am Rev Respir Dis. 1992;146(2):467–72. doi: 10.1164/ajrccm/146.2.467. [DOI] [PubMed] [Google Scholar]
- 5.Yokoyama H, Sato H, Hori M, Takeda H, Kamada T. A characteristic change in ventilation mode during exertional dyspnea in patients with chronic heart failure. Chest. 1994;106(4):1007–13. doi: 10.1378/chest.106.4.1007. [DOI] [PubMed] [Google Scholar]
- 6.Witte KK, Thackray SD, Nikitin NP, Cleland JG, Clark AL. Pattern of ventilation during exercise in chronic heart failure. Heart. 2003;89(6):610–4. doi: 10.1136/heart.89.6.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Metra M, Cas LD, Panina G, Visioli O. Exercise hyperventilation chronic congestive heart failure, and its relation to functional capacity and hemodynamics. Am J Cardiol. 1992;70(6):622–8. doi: 10.1016/0002-9149(92)90202-a. [DOI] [PubMed] [Google Scholar]
- 8.Ponikowski PP, Chua TP, Francis DP, Capucci A, Coats AJ, Piepoli MF. Muscle ergoreceptor overactivity reflects deterioration in clinical status and cardiorespiratory reflex control in chronic heart failure. Circulation. 2001;104(19):2324–30. doi: 10.1161/hc4401.098491. [DOI] [PubMed] [Google Scholar]
- 9.Agostoni P, Pellegrino R, Conca C, Rodarte JR, Brusasco V. Exercise hyperpnea in chronic heart failure: relationships to lung stiffness and expiratory flow limitation. J Appl Physiol (1985). 2002;92(4):1409–16. doi: 10.1152/japplphysiol.00724.2001. [DOI] [PubMed] [Google Scholar]
- 10.Piepoli MF, Dimopoulos K, Concu A, Crisafulli A. Cardiovascular and ventilatory control during exercise in chronic heart failure: role of muscle reflexes. Int J Cardiol. 2008;130(1):3–10. doi: 10.1016/j.ijcard.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 11.Meyer FJ, Zugck C, Haass M, Otterspoor L, Strasser RH, Kubler W, et al. Inefficient ventilation and reduced respiratory muscle capacity in congestive heart failure. Basic Res Cardiol. 2000;95(4):333–42. doi: 10.1007/s003950070053. [DOI] [PubMed] [Google Scholar]
- 12.Habedank D, Reindl I, Vietzke G, Bauer U, Sperfeld A, Glaser S, et al. Ventilatory efficiency and exercise tolerance in 101 healthy volunteers. Eur J Appl Physiol Occup Physiol. 1998;77(5):421–6. doi: 10.1007/s004210050354. [DOI] [PubMed] [Google Scholar]
- 13.Trojnarska O, Gwizdala A, Katarzynski S, Katarzynska A, Szyszka A, Lanocha M, et al. Evaluation of exercise capacity with cardiopulmonary exercise test and B-type natriuretic peptide in adults with congenital heart disease. Cardiol J. 2009;16(2):133–41. [PubMed] [Google Scholar]
- 14.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol (1985). 1986;60(6):2020–7. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- 15.Arena R, Humphrey R, Peberdy MA. Prognostic ability of VE/VCO2 slope calculations using different exercise test time intervals in subjects with heart failure. Eur J Cardiovasc Prev Rehabil. 2003;10(6):463–8. doi: 10.1097/01.hjr.0000102817.74402.5b. [DOI] [PubMed] [Google Scholar]
- 16.Akaishi S, Adachi H, Oshima S, Taniguchi K, Hasegawa A, Kurabayashi M. Relationship between exercise tolerance and TV vs. RR relationship in patients with heart disease. J Cardiol. 2008;52(3):195–201. doi: 10.1016/j.jjcc.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Borg G. Subjective effort and physical activities. Scand J Rehab. 1978;6:108–13. [PubMed] [Google Scholar]
- 18.Black LF, Hyatt RE. Maximal respiratory pressures: normal values and relationship to age and sex. Am Rev Respir Dis. 1969;99(5):696–702. doi: 10.1164/arrd.1969.99.5.696. [DOI] [PubMed] [Google Scholar]
- 19.Meyer FJ, Borst MM, Zugck C, Kirschke A, Schellberg D, Kubler W, et al. Respiratory muscle dysfunction in congestive heart failure: clinical correlation and prognostic significance. Circulation. 2001;103(17):2153–8. doi: 10.1161/01.cir.103.17.2153. [DOI] [PubMed] [Google Scholar]
- 20.Mancini DM, Henson D, LaManca J, Levine S. Respiratory muscle function and dyspnea in patients with chronic congestive heart failure. Circulation. 1992;86(3):909–18. doi: 10.1161/01.cir.86.3.909. [DOI] [PubMed] [Google Scholar]
- 21.Stanek B, Frey B, Hülsmann M, Berger R, Sturm B, Strametz-Juranek J, et al. Prognostic evaluation of neurohumoral plasma levels before and during beta-blocker therapy in advanced left ventricular dysfunction. J American College Cardiol. 2001;38(2):436–42. doi: 10.1016/s0735-1097(01)01383-3. [DOI] [PubMed] [Google Scholar]
- 22.Maisel AS, Kazanegra R, McCullough PA, McCord J, Nowak RM, Hollander JE, et al. Beside B-type natriuretic peptide in the emergency diagnosis of systolic and nonsystolic heart failure: Results from the breathing not properly (BNP) multinational study. J Am College Cardiol. 2003;41(6):143. doi: 10.1016/s0735-1097(03)80833-1. [DOI] [Google Scholar]
- 23.Larsen AI, Skadberg O, Aarsland T, Kvaloy JT, Lindal S, Omland T, et al. B-type natriuretic peptide is related to histological skeletal muscle abnormalities in patients with chronic heart failure. Int J Cardiol. 2009;136(3):358–62. doi: 10.1016/j.ijcard.2008.04.085. [DOI] [PubMed] [Google Scholar]
- 24.Lindsay DC, Lovegrove CA, Dunn MJ, Bennett JG, Pepper JR, Yacoub MH, et al. Histological abnormalities of muscle from limb, thorax and diaphragm in chronic heart failure. Eur Heart J. 1996;17(8):1239–50. doi: 10.1093/oxfordjournals.eurheartj.a015042. [DOI] [PubMed] [Google Scholar]
- 25.Howell S, Maarek JM, Fournier M, Sullivan K, Zhan WZ, Sieck GC. Congestive heart failure: differential adaptation of the diaphragm and latissimus dorsi. J Appl Physiol (1985). 1995;79(2):389–97. doi: 10.1152/jappl.1995.79.2.389. [DOI] [PubMed] [Google Scholar]
- 26.Ambrosino N, Opasich C, Crotti P, Cobelli F, Tavazzi L, Rampulla C. Breathing pattern, ventilatory drive and respiratory muscle strength in patients with chronic heart failure. Eur Respir J. 1994;7(1):17–22. doi: 10.1183/09031936.94.07010017. [DOI] [PubMed] [Google Scholar]
- 27.Witte KK, Nikitin NP, De Silva R, Cleland JG, Clark AL. Exercise capacity and cardiac function assessed by tissue Doppler imaging in chronic heart failure. Heart. 2004;90(10):1144–50. doi: 10.1136/hrt.2003.025684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woods PR, Olson TP, Frantz RP, Johnson BD. Causes of breathing inefficiency during exercise in heart failure. J Card Fail. 2010;16(10):835–42. doi: 10.1016/j.cardfail.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chua TP, Clark AL, Amadi AA, Coats AJ. Relation between chemosensitivity and the ventilatory response to exercise in chronic heart failure. J Am Coll Cardiol. 1996;27(3):650–7. doi: 10.1016/0735-1097(95)00523-4. [DOI] [PubMed] [Google Scholar]
- 30.Dall'Ago P, Chiappa GR, Guths H, Stein R, Ribeiro JP. Inspiratory muscle training in patients with heart failure and inspiratory muscle weakness: a randomized trial. J Am Coll Cardiol. 2006;47(4):757–63. doi: 10.1016/j.jacc.2005.09.052. [DOI] [PubMed] [Google Scholar]