Abstract
A subpopulation of calbindin-immunoreactive neurons in lamina VII of the spinal cord has been identified by its location as Renshaw cells, the anatomical substrate for recurrent inhibition. The expression of calbindin (28 kDa) in these calbindin-containing rat ventral horn interneurons was studied with immunocytochemistry after sciatic nerve injuries. One week after axotomy calbindin immunoreactivity was strongly reduced on the lesioned side between levels L4 and L6, while calbindin-containing neurons and fibers were still numerous contralaterally and cranially to the lesioned levels. With the progression of regeneration, calbindin-immunoreactive neurons reappeared, reaching a normal distribution 6-8 weeks after the crush. Similar changes could be mimicked by the intramuscular administration of botulinum toxin. These results suggest that calbindin expression in putative Renshaw cells of the spinal cord might be functionally responsive and that maintenance of calbindin expression may depend on the integrity of motoneurons and neuromuscular transmission.
Full text
PDF
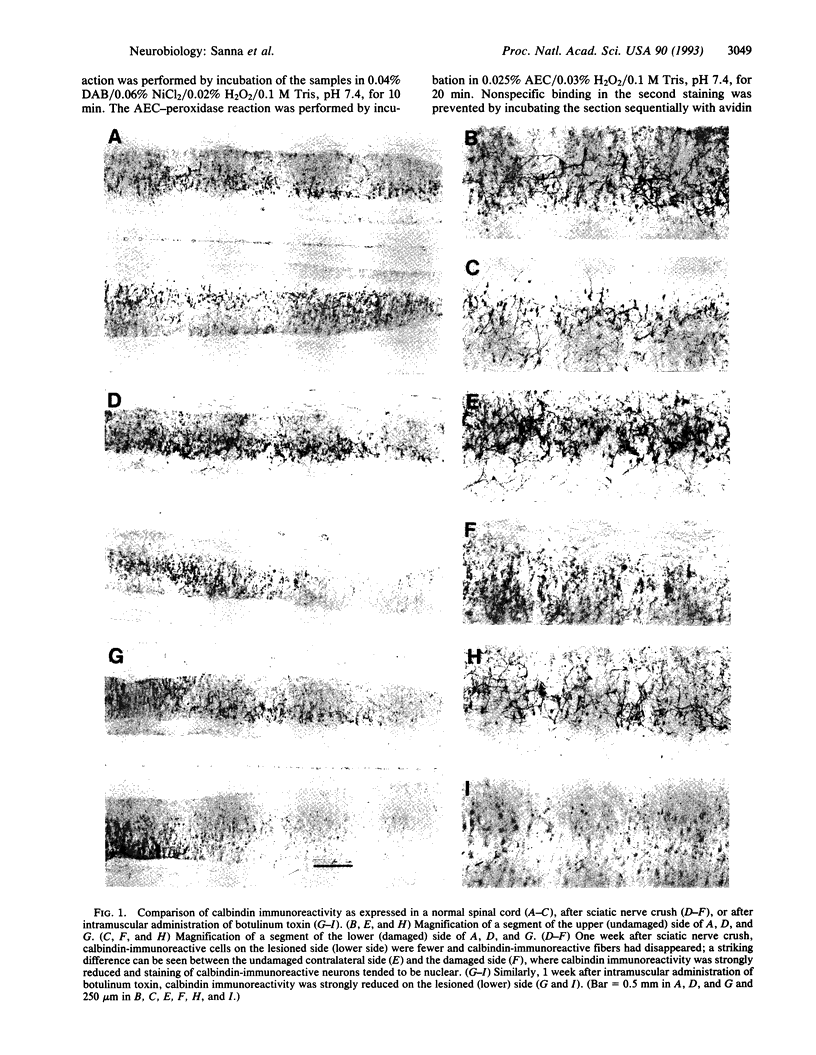



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antal M., Freund T. F., Polgár E. Calcium-binding proteins, parvalbumin- and calbindin-D 28k-immunoreactive neurons in the rat spinal cord and dorsal root ganglia: a light and electron microscopic study. J Comp Neurol. 1990 May 15;295(3):467–484. doi: 10.1002/cne.902950310. [DOI] [PubMed] [Google Scholar]
- Arvidsson U., Ulfhake B., Cullheim S., Ramírez V., Shupliakov O., Hökfelt T. Distribution of calbindin D28k-like immunoreactivity (LI) in the monkey ventral horn: do Renshaw cells contain calbindin D28k-LI? J Neurosci. 1992 Mar;12(3):718–728. doi: 10.1523/JNEUROSCI.12-03-00718.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit P., Changeux J. P. Consequences of blocking the nerve with a local anaesthetic on the evolution of multiinnervation at the regenerating neuromuscular junction of the rat. Brain Res. 1978 Jun 23;149(1):89–96. doi: 10.1016/0006-8993(78)90589-9. [DOI] [PubMed] [Google Scholar]
- Benoit P., Changeux J. P. Consequences of tenotomy on the evolution of multiinnervation in developing rat soleus muscle. Brain Res. 1975 Dec 5;99(2):354–358. doi: 10.1016/0006-8993(75)90036-0. [DOI] [PubMed] [Google Scholar]
- Berchtold M. W., Celio M. R., Heizmann C. W. Parvalbumin in non-muscle tissues of the rat. Quantitation and immunohistochemical localization. J Biol Chem. 1984 Apr 25;259(8):5189–5196. [PubMed] [Google Scholar]
- Celio M. R., Baier W., Schärer L., Gregersen H. J., de Viragh P. A., Norman A. W. Monoclonal antibodies directed against the calcium binding protein Calbindin D-28k. Cell Calcium. 1990 Oct;11(9):599–602. doi: 10.1016/0143-4160(90)90014-l. [DOI] [PubMed] [Google Scholar]
- Celio M. R. Calbindin D-28k and parvalbumin in the rat nervous system. Neuroscience. 1990;35(2):375–475. doi: 10.1016/0306-4522(90)90091-h. [DOI] [PubMed] [Google Scholar]
- Celio M. R., Heizmann C. W. Calcium-binding protein parvalbumin is associated with fast contracting muscle fibres. Nature. 1982 Jun 10;297(5866):504–506. doi: 10.1038/297504a0. [DOI] [PubMed] [Google Scholar]
- Celio M. R. Parvalbumin in most gamma-aminobutyric acid-containing neurons of the rat cerebral cortex. Science. 1986 Feb 28;231(4741):995–997. doi: 10.1126/science.3945815. [DOI] [PubMed] [Google Scholar]
- Clark P., Jones K. J., LaVelle A. Ultrastructural changes in the nucleolus of facial motor neurons following axotomy during an early critical period in development. J Comp Neurol. 1991 Oct 1;312(1):132–144. doi: 10.1002/cne.903120110. [DOI] [PubMed] [Google Scholar]
- Cullheim S., Kellerth J. O. Two kinds of recurrent inhibition of cat spinal alpha-motoneurones as differentiated pharmacologically. J Physiol. 1981 Mar;312:209–224. doi: 10.1113/jphysiol.1981.sp013624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., IGGO A., ITO M. Distribution of recurrent inhibition among motoneurones. J Physiol. 1961 Dec;159:479–499. doi: 10.1113/jphysiol.1961.sp006822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P., Henschen A., Olson L., Persson H. Expression of nerve growth factor receptor mRNA is developmentally regulated and increased after axotomy in rat spinal cord motoneurons. Neuron. 1989 Jun;2(6):1605–1613. doi: 10.1016/0896-6273(89)90049-4. [DOI] [PubMed] [Google Scholar]
- Foehring R. C., Sypert G. W., Munson J. B. Properties of self-reinnervated motor units of medial gastrocnemius of cat. II. Axotomized motoneurons and time course of recovery. J Neurophysiol. 1986 May;55(5):947–965. doi: 10.1152/jn.1986.55.5.947. [DOI] [PubMed] [Google Scholar]
- Fyffe R. E. Evidence for separate morphological classes of Renshaw cells in the cat's spinal cord. Brain Res. 1990 Dec 17;536(1-2):301–304. doi: 10.1016/0006-8993(90)90038-d. [DOI] [PubMed] [Google Scholar]
- Gordon T., Gillespie J., Orozco R., Davis L. Axotomy-induced changes in rabbit hindlimb nerves and the effects of chronic electrical stimulation. J Neurosci. 1991 Jul;11(7):2157–2169. doi: 10.1523/JNEUROSCI.11-07-02157.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B. Changes in motoneurone electrical properties following axotomy. J Physiol. 1979 Aug;293:197–215. doi: 10.1113/jphysiol.1979.sp012885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B., Pinter M. J. Effects of axotomy on the distribution of passive electrical properties of cat motoneurones. J Physiol. 1984 Nov;356:433–442. doi: 10.1113/jphysiol.1984.sp015474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havton L., Kellerth J. O. Elimination of intramedullary axon collaterals of cat spinal alpha-motoneurons following peripheral nerve injury. Exp Brain Res. 1990;79(1):65–74. doi: 10.1007/BF00228873. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Hultborn H. Convergence on interneurones in the reciprocal Ia inhibitory pathway to motoneurones. Acta Physiol Scand Suppl. 1972;375:1–42. doi: 10.1111/j.1748-1716.1972.tb05298.x. [DOI] [PubMed] [Google Scholar]
- Jenkins R., Hunt S. P. Long-term increase in the levels of c-jun mRNA and jun protein-like immunoreactivity in motor and sensory neurons following axon damage. Neurosci Lett. 1991 Aug 5;129(1):107–110. doi: 10.1016/0304-3940(91)90731-8. [DOI] [PubMed] [Google Scholar]
- Kuno M., Miyata Y., Muñoz-Martinez E. J. Differential reaction of fast and slow alpha-motoneurones to axotomy. J Physiol. 1974 Aug;240(3):725–739. doi: 10.1113/jphysiol.1974.sp010631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Miyata Y., Muñoz-Martinez E. J. Properties of fast and slow alpha motoneurones following motor reinnervation. J Physiol. 1974 Oct;242(1):273–288. doi: 10.1113/jphysiol.1974.sp010706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer R., Changeux J. P. Activity-dependent regulation of gene expression in muscle and neuronal cells. Mol Neurobiol. 1989 Spring-Summer;3(1-2):1–53. doi: 10.1007/BF02935587. [DOI] [PubMed] [Google Scholar]
- Leberer E., Pette D. Neural regulation of parvalbumin expression in mammalian skeletal muscle. Biochem J. 1986 Apr 1;235(1):67–73. doi: 10.1042/bj2350067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström S., Schomburg E. D. Recurrent inhibition from motor axon collaterals of ventral spinocerebellar tract neurones. Acta Physiol Scand. 1973 Aug;88(4):505–515. doi: 10.1111/j.1748-1716.1973.tb05479.x. [DOI] [PubMed] [Google Scholar]
- Miller F. D., Tetzlaff W., Bisby M. A., Fawcett J. W., Milner R. J. Rapid induction of the major embryonic alpha-tubulin mRNA, T alpha 1, during nerve regeneration in adult rats. J Neurosci. 1989 Apr;9(4):1452–1463. doi: 10.1523/JNEUROSCI.09-04-01452.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter M. J., Vanden Noven S., Muccio D., Wallace N. Axotomy-like changes in cat motoneuron electrical properties elicited by botulinum toxin depend on the complete elimination of neuromuscular transmission. J Neurosci. 1991 Mar;11(3):657–666. doi: 10.1523/JNEUROSCI.11-03-00657.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rende M., Hagg T., Manthorpe M., Varon S. Nerve growth factor receptor immunoreactivity in neurons of the normal adult rat spinal cord and its modulation after peripheral nerve lesions. J Comp Neurol. 1992 May 8;319(2):285–298. doi: 10.1002/cne.903190208. [DOI] [PubMed] [Google Scholar]
- Ryall R. W. Renshaw cell mediated inhibition of Renshaw cells: patterns of excitation and inhibition from impulses in motor axon collaterals. J Neurophysiol. 1970 Mar;33(2):257–270. doi: 10.1152/jn.1970.33.2.257. [DOI] [PubMed] [Google Scholar]
- Sanna P. P., Keyser K. T., Battenberg E., Bloom F. E. Parvalbumin immunoreactivity in the rat retina. Neurosci Lett. 1990 Oct 2;118(1):136–139. doi: 10.1016/0304-3940(90)90267-d. [DOI] [PubMed] [Google Scholar]
- Scott J. N., Parhad I. M., Clark A. W. Beta-amyloid precursor protein gene is differentially expressed in axotomized sensory and motor systems. Brain Res Mol Brain Res. 1991 Jul;10(4):315–325. doi: 10.1016/0169-328x(91)90090-k. [DOI] [PubMed] [Google Scholar]
- Sutter A., Riopelle R. J., Harris-Warrick R. M., Shooter E. M. Nerve growth factor receptors. Characterization of two distinct classes of binding sites on chick embryo sensory ganglia cells. J Biol Chem. 1979 Jul 10;254(13):5972–5982. [PubMed] [Google Scholar]
- Vanden Noven S., Pinter M. J. Effects of preventing reinnervation on axotomized spinal motoneurons in the cat. II. Changes in group Ia synaptic function. J Neurophysiol. 1989 Aug;62(2):325–333. doi: 10.1152/jn.1989.62.2.325. [DOI] [PubMed] [Google Scholar]
- Wasserman R. H., Taylor A. N. Vitamin d3-induced calcium-binding protein in chick intestinal mucosa. Science. 1966 May 6;152(3723):791–793. doi: 10.1126/science.152.3723.791. [DOI] [PubMed] [Google Scholar]






