Abstract
Corneal transplantation is the primary treatment option to restore vision for patients with corneal endothelial blindness. Although the success rate of treatment is high, limited availability of transplant grade corneas is a major obstacle. Tissue-engineered corneal endothelial grafts constructed using cultivated human corneal endothelial cells (hCENC) isolated from cadaveric corneas may serve as a potential graft source. Currently, tools for the characterization of cultured hCENC and enrichment of hCENC from potential contaminating cells such as stromal fibroblasts are lacking. In this study, we describe the generation and characterization of novel cell surface monoclonal antibodies (mAbs) specific for hCENC. These mAbs could be used for enrichment and characterization of hCENC. Out of a total of 389 hybridomas, TAG-1A3 and TAG-2A12 were found to be specific to the corneal endothelial monolayer by immunostaining of frozen tissue sections. Both mAbs were able to clearly identify hCENC with good ‘cobblestone-like’ morphology from multiple donors. The antigen targets for TAG-1A3 and TAG-2A12 were found to be CD166/ALCAM and Peroxiredoxin-6 (Prdx-6), respectively, both of which have not been previously described as markers of hCENC. Additionally, unlike other Prdx-6 mAbs, TAG-2A12 was found to specifically bind cell surface Prdx-6, which was only expressed on hCENC and not on other cell types screened such as human corneal stromal fibroblasts (hCSF) and human pluripotent stem cells (hPSC). From our studies, we conclude that TAG-1A3 and TAG-2A12 are promising tools to quantitatively assess hCENC quality. It is also noteworthy that the binding specificity of TAG-2A12 could be used for the enrichment of hCENC from cell mixtures of hCSF and hPSC.
Keywords: human corneal endothelial cells, ALCAM/CD166, Peroxiredoxin-6, monoclonal antibodies, characterization, cell enrichment
Abbreviations
- AA
antibiotic/antimycotic
- CM
conditioned medium
- DM
descement membrane
- DMEM
Dulbecco's modified Eagle's medium
- DSAEK
Descement's stripping automated endothelial keratoplasty
- FBS
fetal bovine serum
- FGF-2
fibroblast growth factor-2
- FNC
fibronectin and collagen-based
- FT
flowthrough
- GPC-4
Glypican-4
- hCENC
human corneal endothelial cells
- hCSF
human corneal stromal fibroblasts
- hPSC
human pluripotent stem cells
- HRP
horseradish peroxidase
- ICC
immunocytochemistry
- IP
immunoprecipitation
- LEC
lens epithelial cells
- mAbs
monoclonal antibodies
- MACS
magnetic affinity cell separations
- MFI
mean fluorescence intensity
- MPL
monophosphryl-lipid A
- Na+K+ATPase
sodium potassium ATPase
- nMFI
normalized mean fluorescence intensity
- Prdx-6
Peroxiredoxin-6
- TDM
trehalose dichorynmycolate
- ZO-1
zonula occludins-1
Introduction
The human corneal endothelium is the most important monolayer of cells in the cornea. It plays a critical role in maintaining corneal transparency by regulating the leaky barriers between the aqueous humor and corneal stroma.1 The corneal endothelial cell layer is derived from neural crest cells during the first 16 weeks of gestation.2-4 Human corneal endothelial cells (hCENC), however, have limited proliferative capacity in-vivo5 and the density of hCENC in the cornea shares an inverse relationship with age.6,7 Damage to the corneal endothelial layer by trauma or hereditary diseases such as Fuchs’ dystrophy can result in corneal edema leading to corneal blindness.1 Currently, corneal transplantation is the only treatment option to restore vision for these patients. However, the availability of treatment is limited by the scarcity of transplant grade donor corneas, which is a global issue. In order to reduce the reliance on donor corneas, alternative treatment strategies including the use of suitable replacement graft material in the form of tissue-engineered hCENC constructs have been proposed as potential alternatives.8,9
The conceptual notion of using tissue-engineered hCENC constructs in conjunction with Descement's stripping automated endothelial keratoplasty (DSAEK) was first described in an animal model by Mimura et. al. in 2004.10 In their study, cultured hCENC seeded onto sheets of collagen were transplanted into the anterior chamber of rabbit eyes following removal of the host Descemet's membrane.10 Since then, many groups have described the transplantation of similar tissue-engineered hCENC constructs into animal models and demonstrated their therapeutic efficacy for possible clinical therapy.10-13 Ju et. al. recently described the derivation of corneal endothelial-like cells from rat neural crest cells.2 Their work opens the possibility of deriving hCENC from other cell sources such as human pluripotent stem cells (hPSC). One of the unique features of hPSC is their ability to self-renew and expand indefinitely, which makes hPSC a very attractive surrogate cell source for generating hCENC. Directed differentiation of hPSC is often not an efficient process, hence the ability to enrich for the cells of interest will be necessary.
Currently, the characterization of cultured hCENC is predominately based on their morphology i.e., polygonal ‘cobblestone-like’, contact-inhibited appearance, together with the use of 2 functional associated markers zonula occludins-1 (ZO-1) and sodium potassium ATPase (Na+K+ ATPase).1,14-16 These markers, however, are not hCENC-specific, and are found ubiquitously expressed in many other cell types.17,18 Therefore, both ZO-1 and Na+K+ ATPase are not ideal markers for cell isolation and enrichment. Although the raising of mAbs against hCENC has been previously reported,15,19-21 none of these mAbs were made commercially available and there was minimal characterization of the antigens. Our group recently demonstrated the specificity of 2 commercially-available antibodies, anti-glypican-4 (GPC4) and anti-CD200, to characterize and enrich for hCENC. They were reported to bind specifically to hCENC but not human corneal stromal fibroblasts (hCSF).14 However, both CD200 and GPC4 play a part in neurogenesis and have been reported to be present on neural precursor cells; 22-24 therefore, the use of these mAbs for hCENC enrichment from a heterogeneous population of differentiated hPSC culture may be limited. The current lack of bona fide hCENC specific markers presents a unique opportunity for the discovery of new markers on hCENC via an antibody generation strategy. The availability of mAbs will allow investigators a better opportunity to isolate and characterize hCENCs cultured under different conditions and derived from different cell sources.
In this study, we generated a panel of mAbs using cadaveric hCENC and found 2 mAbs that were specific to human corneal endothelium in frozen tissue sections as well as cultured hCENC. Additionally, these mAbs were able to provide quantitative assessments to the state of the cultured hCENC as opposed to conventional qualitative morphological assessment. Importantly, TAG-2A12 showed specificity only to hCENC and was able to enrich hCENC from cell mixtures of hCSFs and hPSCs.
Results
Generation of hCENC specific mAbs
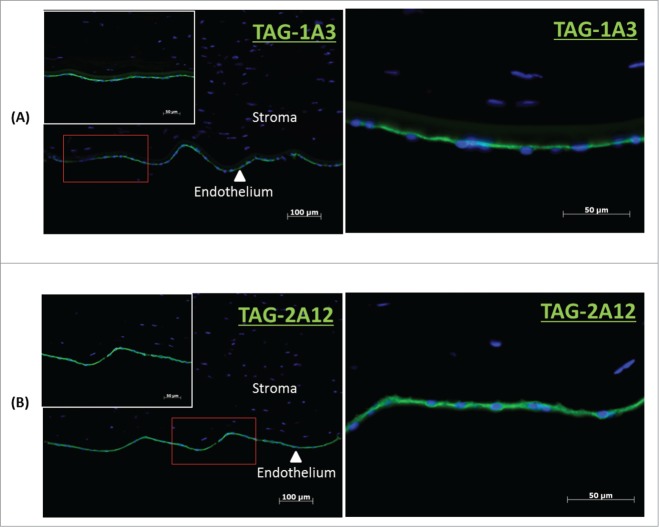
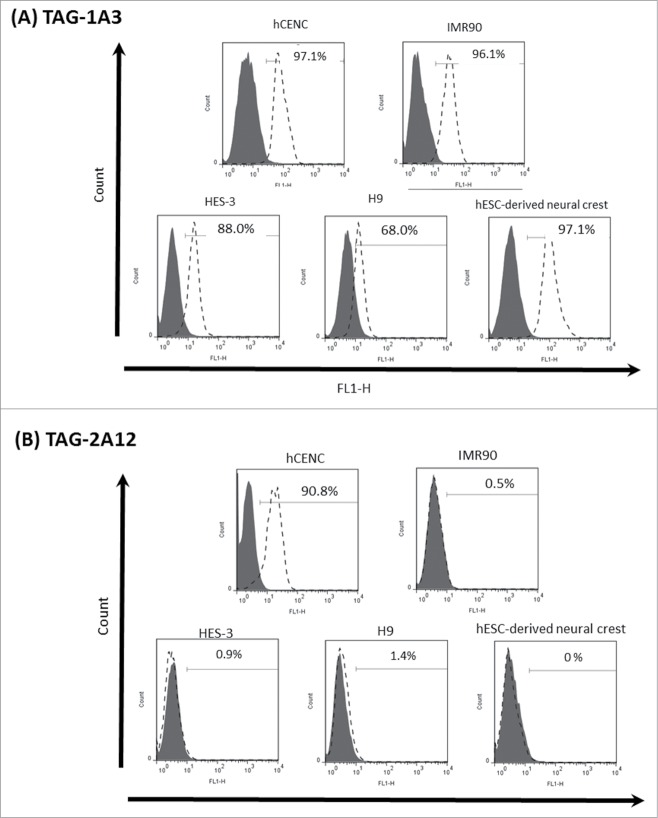
Using cadaveric hCENC, a total of 389 hybridoma clones were generated from the immunization. Supernatants from these clones were used to screen cultured hCENC for positive binding using flow cytometry. Only 18 mAbs were found to be binding to at least 20% of hCENC (Table S1). Binding specificity of these mAbs was further determined by tissue immunostaining with frozen human cornea sections. Our data indicated that only 2 out of the 18 mAbs, TAG-1A3 and TAG-2A12, bound specifically to the corneal endothelial monolayer of the tissue section (Fig. 1 and Table S2) and no staining was observed on the epithelial or stromal layers. To further assess the specificity of these mAbs, screening was also conducted on a panel of other cell types such as lung fibroblasts (IMR90), human embryonic stem cell lines (HES-3 and H9) and H9-derived neural crest cells. Interestingly, only TAG-2A12 demonstrated high binding specificity to hCENC, (>90% TAG-2A12 +ve) while no binding was observed for all the other cells types screened (Fig. 2 and Table S1). In contrast, even though TAG-1A3 bound to more than 90% of hCENC, the antigen it targets is also expressed on other cell types however with varying degrees (based on its normalized mean fluorescence intensity, nMFI) (Fig. S1). For example, TAG-1A3 had higher nMFI for hCENC compared to both hESC lines and IMR90, suggesting a higher expression level of the antigen target on hCENC. However, the nMFI expression of TAG-1A3 was highest for hESC-derived neural crest cells. It was also observed that both TAG-1A3 and TAG-2A12 do not bind to the cultured stromal fibroblast cells (Fig. S2).
Figure 1.
Immunocytochemistry of frozen cornea tissue sections. (A) TAG-1A3 and (B) TAG 2A12. Primary antibodies were detected with Alexa Flour 488 secondary antibody and nuclei of cells were stained with DAPI. Both TAG-1A3 and TAG-2A12 had shown specific of staining for the endothelium layer in corneal tissue section.
Figure 2.
Histogram analysis of TAG-1A3 and TAG-2A12 on various cell lines. Solid line (filled) represents the isotype control and dotted line (open) represents antibody staining. (A) TAG-1A3 was binding to different cells lines with varying normalized mean fluorescence intensity (nMFI) and higher nMFI values were observed with both hCENC and hESC-derived neural crest cells. (B) TAG-2A12 was found to be binding specifically to hCENC only.
Characterization of antigen targets
In order to identify the antigen target of TAG-1A3 and TAG-2A12 on hCENCs, characterization studies involved estimating the molecular weight of the cell surface protein by Western blotting, immunoprecipitation (IP), silver-staining, mass spectrometry, and finally validation.
By Western blotting, TAG-1A3 and TAG-2A12 were found to bind to protein bands corresponding to ∼80kDa and 28kDa respectively (Fig. 3A and 3C, Panel 2). IP using TAG-1A3 and TAG-2A12 successfully enriched for the antigen targets (Fig. 3A and 3C panel 2). The IP products of TAG-1A3 and TAG-2A12 were excised from a duplicate silver stained gel and sent for identification using LC/MS-MS. After database search using the MS data, the putative antigen target that TAG-1A3 enriched for was ALCAM/CD166, while peroxiredoxin-6 (Prdx-6) was detected by TAG-2A12. To validate the antigen targets, commercial antibodies against ALCAM/CD166 and Prdx-6 were used.
Figure 3.
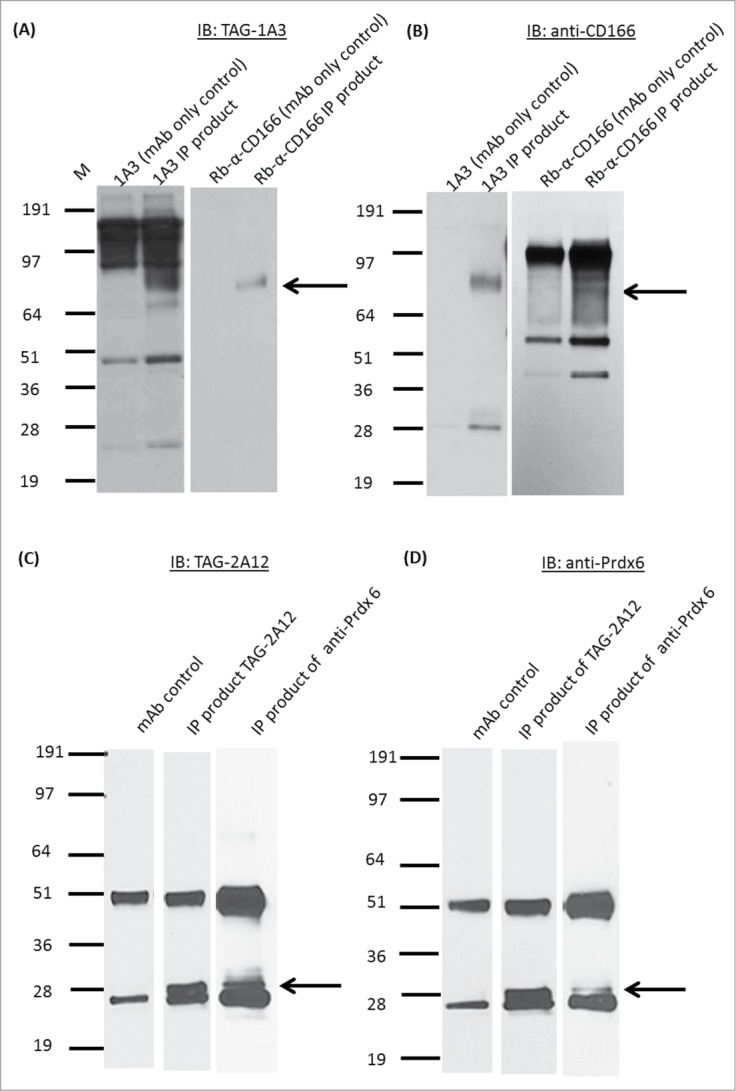
Antigen target identification of mAb TAG-1A3 and mAb TAG-2A12. Immunoprecipitation (IP) using TAG-1A3 and commercial CD166 and, immunoblotted (IB) with (A) TAG-1A3 and (B) anti-CD 166, respectively. Results show both forward and reverse IP using the TAG-1A3 and CD166 are enriching for the same antigen bands between 64kDa and 97kDa suggesting that TAG-1A3 and CD166 are targeting at the same antigen. IP using TAG-2A12 and commercial Prdx6, IB (C) TAG-2A12, and (D) Prdx6, respectively. Results shows both forward and reverse IP using the TAG-2A12 and Prdx6 are enriching for the same antigen bands between 28kDa and 36kDa suggesting that the mAb may be targeting at the same antigen.
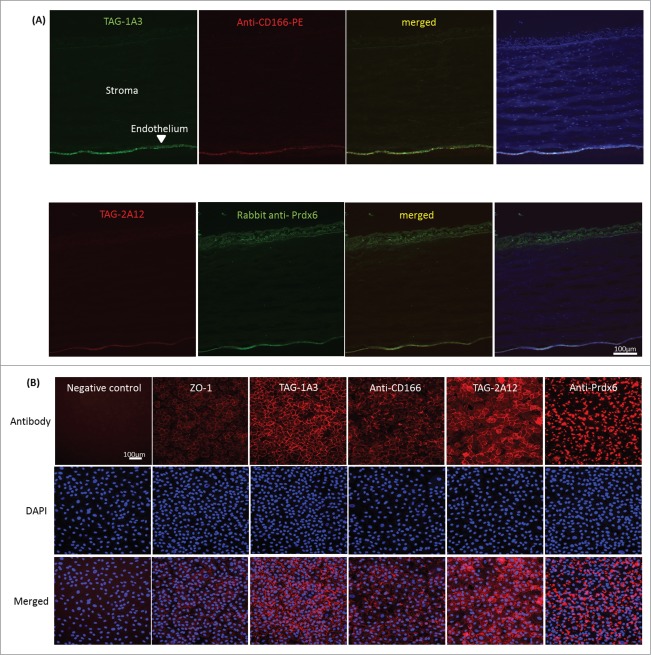
The samples from IP using TAG-1A3 and TAG-2A12 were immunoblotted against their respective commercial antibodies. Protein bands of similar size compared to those detected by our mAbs were observed (Fig. 3A and 3C panel 3). The procedure was then reversed by using the commercial antibodies to IP the antigen targets and immunoblotted with TAG-1A3 and TAG-2A12 respectively. Similar results were obtained (Fig. 3B and 3D) confirming the antigen targets of mAbs TAG-1A3 and TAG-2A12 to be ALCAM/CD166 and Prdx-6 respectively. Next, frozen cornea tissue sections were immunostained with anti-CD166 and anti-Prdx-6 commercial antibodies. Similar to our mAbs, staining to the corneal endothelial cells was observed (Fig. 4A), thus validating the expression of the identified antigen targets. However, unlike TAG-2A12 that stained specifically to hCENC, the commercial anti-Prdx6 cross-reacted with corneal epithelial cells.
Figure 4.
Comparing staining of commercial antibodies vs. TAG-1A3 and TAG-2A12 on both corneal tissue sections and cultured hCENCs. Primary antibodies were detected with Alexa Flour (AF) 488 or AF 594 secondary antibody and nuclei of cells were stained with DAPI. (A) Frozen corneal tissue staining. Both commercial antibodies and in-house antibodies showed specific staining of the endothelium layer. (B) Staining of cultured hCENCs. Cells were stained with an isotype antibody as negative control, and ZO-1 staining was used a positive control. Staining pattern for TAG-1A3 appeared more membrane-like similar to commercial anti-CD166. Membrane localization of TAG-2A12 was shown as compared to commercial anti-Prdx-6 antibody where more intracellular staining was observed.
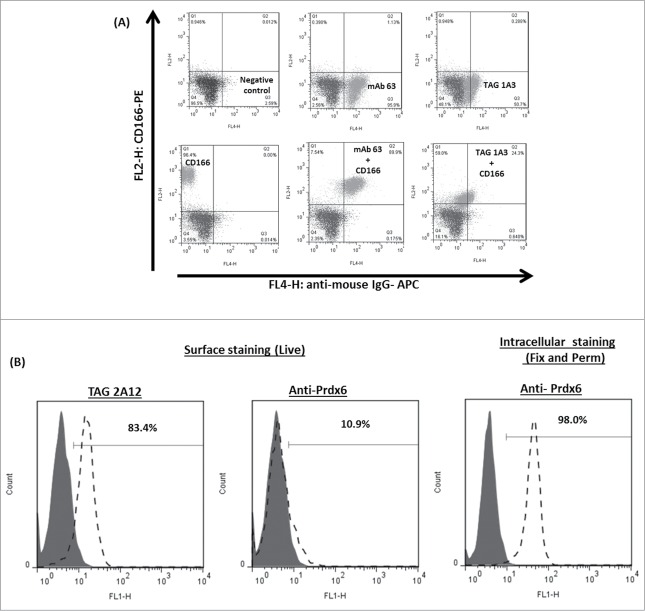
Next, cultured hCENC were stained using immunocytochemistry (ICC) and flow cytometry to determine if the commercial and our antibodies were co-localizing and recognizing the same epitopes. For cultured hCENCs, TAG-1A3 and anti-CD166 antibody staining localized predominantly on the cell membrane or cell-to-cell junctions (Fig. 4B). By FACS, a reduction in anti-CD166 antibody binding was observed following pre-incubation of hCENCs with TAG-1A3 but not the isotype control, mAb 63 (Fig. 5A; Fig. S3). This suggests that anti-CD166 and TAG-1A3 were competitively binding either to a common ALCAM/CD166 epitope or in close proximity to sterically hinder binding. To investigate if TAG-1A3 and anti-CD166 may be recognizing the same epitope, several assays were performed. First, to determine if the epitope was conformational or linear, samples were resolved on SDS-PAGE gels under reducing and non-reducing conditions followed by Western blotting. It was observed that binding of TAG-1A3 was sensitive to reducing agent (ie conformational epitope), but anti-CD166 was not (ie linear epitope) (Fig S4A-B; Lanes 1-2). Next, to determine if the mAbs were binding to a glycan epitope, periodate treatment and PNGase-F treatment were performed. From the assays, it was demonstrated that TAG-1A3 and anti-CD166 both bound to glycan epitopes that were N-linked dependent (Fig. S4A-D).
Figure 5.
Comparing surface binding of TAG-1A3 and TAG-2A12 with its commercial antibodies. (A) Double staining of TAG-1A3 and CD166. MAb 63 is an in-house antibody that binds universally to human cell lines, and was used as positive control (upper left panel). An isotype antibody was used as a negative control (dark gray). TAG-1A3 and CD166, co-incubated with the positive control, did not alter the binding of CD166 (upper right panel and lower middle panel, respectively) compared to CD166 alone (lower left panel). A reduction in anti-CD166 antibody binding was observed following pre-incubation of hCENCs with TAG-1A3 (lower right panel) but not the isotype control, mAb 63. This suggests that the antibodies were competitively binding to common epitopes on hCENC. (B) Results on flow cytometry staining of cultured hCENCs with TAG-2A12 and commercial anti-Prdx-6 antibody. TAG-2A12 showed binding to cultured hCENCs compared to anti-Prdx-6 where no binding was observed when cells were stained live for surface antigen target binding. Anti-Prdx-6 binding was observed when cells are fixed and permeabilized, thus suggesting anti-Prdx-6 is binding to the intracellular pool of Prdx-6 target in the cells.
TAG-2A12 showed strong cell membrane staining of hCENC with some extent of intracellular staining. This could be due to the mild fixation of cells prior to staining. In contrast, the anti-Prdx-6 antibody only stained clusters of intracellular proteins in hCENC (Fig. 4B). Furthermore, cross-reactivity with cultured stromal fibroblast was observed (Fig. S2). By flow cytometry, only TAG-2A12 but not anti-Prdx6 antibody, bound to the cell surface of cultured hCENC (Fig. 5B). Taken together, this highlights the specificity of TAG-2A12 compared to anti-Prdx-6 in distinguishing hCENCs from other cell types within the eye. It also suggests that TAG-2A12 is recognizing an epitope specific to cell surface-expressed Prdx-6 on hCENC, whereas the commercial mAb only recognizes intracellular Prdx-6.
Quantitative assessment of cultured hCENC
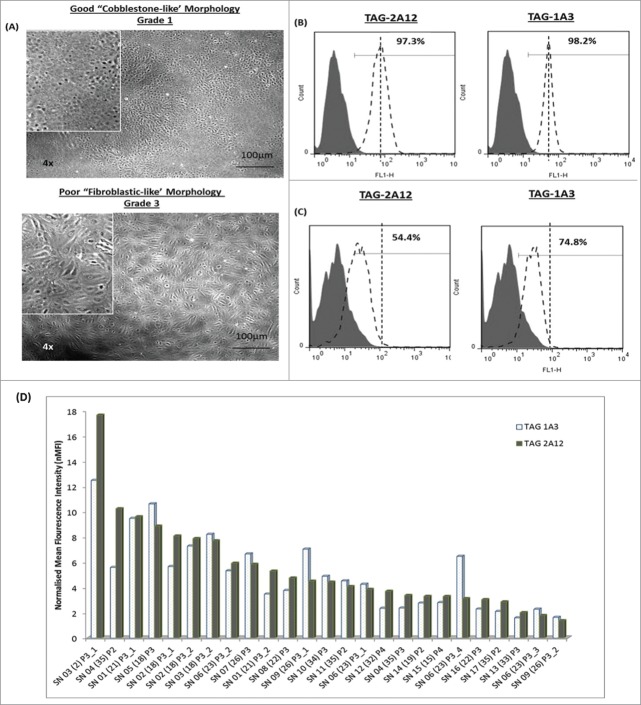
Currently, cultured hCENC are typically characterized by their “cobblestone-like” morphology and its ZO-1 and Na+K+ ATPase markers expression. Samples from different donor-derived hCENC were plated and graded based on their cellular morphology; hCENCs with “cobblestone-like” morphology were given a grade 1 and cells that became “fibroblastic-like” were given a grade 3 (Fig. 6A). Expression levels of the cell surface antigens that TAG-1A3 and TAG-2A12 recognized were examined. It was found that both mAbs were binding to cultured hCENC derived from different donors however with varying MFI. More detailed analysis of the data revealed a close correlation between antigen expression of these mAbs and morphological grading (Fig. 6B-C), i.e., grade 1 had higher expression of TAG-1A3 and TAG-2A12 antigens. Similar trends were observed when we extended this data to a larger panel of donor-derived hCENC (Fig. 6D, Table S3). These results demonstrated differential trends in expression of both cell surface ALCAM/CD166 and Prdx-6 from cultured hCENC derived from various donors. Both TAG-1A3 and TAG-2A12 were highly specific for hCENC with good “cobblestone-like” morphology, and hence can be a useful tool to provide both qualitative and quantitative assessment on the state of the cultured hCENC for both research and clinical application.
Figure 6.
Characterization of cultured hCENCs with TAG-1A3 and TAG-2A12. (A) Top panel: Cells with ‘cobblestone-like’ morphology and bottom panel: cells with ‘fibroblastic-like’ morphology. (B-C) Expression of TAG-1A3 and TAG-2A12 on the cell surface via flow cytometry analysis. (B) Cells with ‘cobblestone-like’ morphology have high expression of TAG-1A3 and TAG-2A12 than cells with (C) ‘fibroblastic-like’ morphology (D) nMFI levels of TAG-1A3 and TAG-2A12 on various donor samples.
Enrichment of hCENC from complex cell mixture
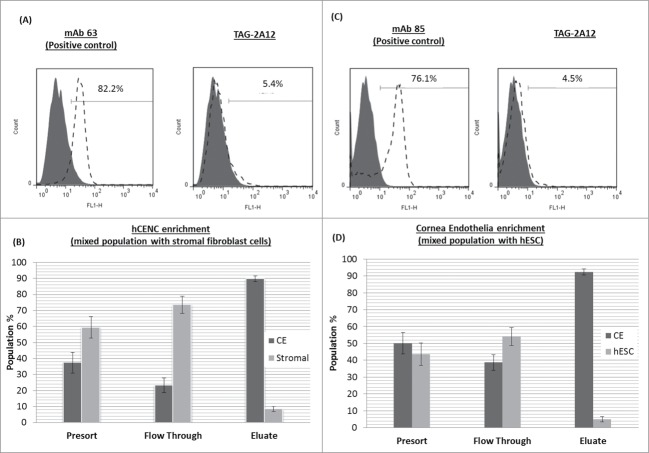
TAG-2A12 was found to bind specifically to cell surface Prdx-6 expressed on hCENC, whereas TAG-1A3 was found to recognize surface antigens of CD166 on multiple cell types (Table S1). Hence, the ability of TAG-2A12 to enrich for hCENC non-invasively within a mixed population of cells was investigated. Results showed that using a one-step immunoaffinity separation, the purity of hCENC can be enriched from a starting population of ∼50% to a purity of ∼90% of hCENC for both SF and hESC mixtures (Fig. 7). Therefore, TAG-2A12 can potentially be used for the enrichment of hCENC in a complex cell mixture.
Figure 7.
Enrichment of hCENCs from mix cell population containing hCENCs. Enrichment of mix cell population of hCENCs with stromal fibroblast cells. Enrichment of mix cell population of hCENCs with stromal fibroblast cells. (A) Top panel: Flow cytometry binding data of TAG-2A12 on stromal fibroblast cells. mAb 63 was used as a positive control for stromal fibroblast cells. (B) Bottom panel: hCENC enrichment in 50:50 mix population with stromal fibroblast. Enrichment of hCENCs with approximately 90% purity was achieved using MACS sorting. Experiment was conducted in triplicates. Enrichment of mix cell population of hCENCs with hESC (HES-3). (C) Top panel: Flow cytometry binding data of mAb TAG-2A12 on hESC. MAb 85 was used as a positive control for hESC binding. (D) Bottom panel: hCENC enrichment in 50:50 mix population with hESC. Enrichment of hCENCs with approximately 92% purity was achieved using MACS sorting. Experiment was conducted in triplicates.
Discussion
Irreversible corneal blindness secondary to corneal endothelial dysfunction can be treated successfully with a corneal transplantation. However, global shortage of available donor graft material severely limits the numbers of transplantation performed worldwide. Studies have shown that using tissue-engineered hCENC constructs together with the DSAEK technique can be a plausible route to circumvent the reliance on donor tissue.8,10-13 Currently, cultured hCENC are poorly characterized, primarily by their ‘cobblestone-like’ morphology and their expression of ZO-1 and Na+K+ ATPase markers via immunostaining, indicative of the corneal endothelium's ability to regulate corneal hydration through its ‘leaky’ cellular barrier and metabolic active pumps. Previous studies had suggested that differences in both the proliferative response and the protein expression have been observed from different donors.10-13 The establishment of a reliable panel of mAbs against hCENC is critical for quality assessment of these cells, especially to address issues of donor-to-donor variations. However, these characterization tools are at best, qualitative, and do not present a quantitative measure on the entire culture population. Flow cytometry has been routinely used to characterize cell populations via their specific markers expression. Although several mAbs had been previously reported to have specificity to hCENC,15,19-21 information on these antibodies is severely lacking and often limited to their original publications. It is unclear if these antibodies are suitable for use in flow cytometry since they are not commercially available. In this study, new mAbs were generated via whole-cell immunization using fresh cadaveric corneal endothelial cells. This strategy allows presentation of the native cell surface antigens to the mouse immune system. Antibodies generated using this approach was more likely to recognize antigen targets that are post-translationally modified (such as glycosylation) and unique only to the immunogen cells. Two candidates, TAG-1A3 and TAG-2A12, were shortlisted from a panel of 389 mAbs based on their ability to quantitatively distinguish between populations of hCENC isolated from different donors with desirable morphology. Hence, these mAbs will be useful as a quality assurance biomarker for characterizing cultured hCENC during the expansion phase and prior to transplantation in tissue-engineered corneal endothelial constructs. Characterization of TAG-1A3 and TAG-2A12 identified the antigen targets of the mAbs to be CD166 and Prdx-6 respectively. To date, this is the first report suggesting the presence of these antigens on the cell surface of hCENCs.
CD166 (also known as ALCAM), belongs to the immunoglobulin superfamily. CD166 has also been detected in a wide variety of tissues, however, it is limited to only specific subsets of proliferative cells.25 It forms part of the adhesive complex at the intercellular junction which helps to maintain tissue architecture.25 More recently, CD166 had been implicated in many different types of cancers including lung, breast, ovarian, prostate, and colon cancers.26-28 Its role in promoting cell-cell adhesion, which leads to the formation of cell aggregates, aids in the survival of the inner cells, particularly during migration and dissemination, as the outer cells protect them from immune-mediated cell death. This is believed to be one of the mechanisms involved in metastatic pathways and cancer progression. Binding for TAG-1A3 was found to be higher for hCENC compared to both hESC lines tested but lower compared to hESC-derived neural crest cells. This upregulation of CD166 (antigen target of TAG-1A3) is transient during neural lineage differentiation.25
We have compared the commercial anti-CD166 antibody against our mAb, TAG-1A3, and found that both mAbs competitively bind to N-linked glycans on CD166 despite recognizing different epitope conformations. Functionally, both mAbs were comparable based on their immunostaining results using flow cytometry, ICC, and tissue distribution. Although the function of CD166 in hCENC remains unknown, the availability of mAbs can potentially help researchers elucidate it role and better characterize hCENC during large-scale expansion.
For TAG-2A12, we determined that the mAb binds to Prdx-6. Prdx-6 is the sixth member of the Prdx family. However, Prdx-6 is the only 1-cysteine member of the Prdx family and it uses glutathione as its physiological reductant as opposed to thioredoxin. Prdx-6 is a bifunctional protein providing both antioxidant defense and phospholipid homeostasis.29 Overexpression of Prdx-6 offers increased resistance to oxidative stress whereby it helps to reduce pre-oxidized membrane phospholipids.29 Prdx-6 also helps to maintain phospholipid homeostasis by regenerating its lysophospholipids substrate.29 Prdx-6 was first discovered in the lung, and subsequently found to have wide spread expression in many tissues of the human body.29 Expression of Prdx-6 has been previously described in the eye, more specifically on lens epithelia cells (LECs).30-36 Deficiency of Prdx-6 in LECs induces ER stress response and apoptosis.37 The increase of Prdx-6 levels in LECs helps to protect against cell death and delays lens opacity.31 Prdx-6 expression has also found been to be negatively correlated with age.35,36 A study by Kubo et al. found that the expression of Prdx-6 in mice gradually increased in the lens from 4th week of gestation until 6 months old after birth and declined thereafter.35 Interestingly, topical administration of Prdx-6 on the cornea helped to suppress inflammation and neovascularization induced by UV radiation.30
TAG-2A12 binds to Prdx-6 expressed on the surface of hCENC. In our study, we noted the expression levels of cell surface Prdx-6 differed from donor to donor, but the variations correlated very well with the morphological grading of the cells. Similar to CD166, this is the first report of cell surface expression Prdx-6 in hCENC. Although Prdx-6 had been reported to be mainly cytosolic, a recent publication has suggested that Prdx-6 can be translocated to the plasma membrane via its interaction with p67-phox (Neutrophil cytosol factor 2).38 When we compared the functionality between the commercial anti-Prdx-6 and TAG-2A12, it was found that the commercial mAb not only bound to intracellular Prdx-6 on hCENC, it also cross-reacted with both corneal epithelial cells and stromal fibroblasts. Hence, this would limit its application. In contrast, TAG-2A12 specifically binds to cell surface Prdx-6 on hCENC, making it an ideal marker for hCENC characterization and facilitate enrichment of hCENC from complex cell mixtures.
Surrogate cells such as hPSC have unlimited expansion and offer an alternative cell source to alleviate issues with the lack of donor corneas. However, differentiation of hPSC is often plagued with low differentiation efficiencies and heterogeneous cell populations.39-41 The ability to use specific markers to enrich for hCENC in a differentiated cell population will be necessary. TAG-2A12 was found to bind specifically to the cell surface of hCENC but not hESC nor hESC-derived neural crest cells. In addition, TAG-2A12 did not bind to B4G12, an immortalized hCENC line (data not shown). This suggests that the antigen target of TAG-2A12 may only be present in primary hCENC. This difference between the primary hCENC and B4G12 further highlights the limited utility of immortalized hCENC lines as a surrogate for corneal endothelial cell research.
In a proof-of-concept experiment, enrichment of viable hCENC using MACS was demonstrated from heterogeneous cell populations comprising of hCENC mixed either with hCSFs cells or hESC. In a recent study, our group demonstrated that it was possible to enrich hCENC from a mixture including hCSF cells using FACS sorting using commercial antibodies (anti-GPC-4 and anti-CD200).14 However, it is known that both CD200 and GPC-4 are widely expressed on multiple cell/tissues especially in the neural lineage.22,23 Therefore, these antibodies will not be suitable for enrichment of hCENC from heterogenous cell cultures (e.g., hPSC-derived cells). The proof-of-concept experiment clearly demonstrated the utility of TAG-2A12 in enrichment from mixture cell populations. It is possible that TAG-2A12 alone may not be comprehensive enough for isolation of hCENCs from a heterogeneous cell culture. However, TAG-2A12 can be use in combination or tandem with other mAbs that delineates the different stages of neural differentiation to increase the specificity of TAG-2A12 such as p75 and HNK1 to first enrich for neural crest cells. The enriched neural crest cells can then be further differentiated toward hCENCs, where TAG-2A12 can be exploited as an affinity marker. To further increase the selectivity and specificity for hCENCs, other markers such as TAG-1A3, CD200, GPC4, ZO-1 and NA+K+ATPase can also be employed during hCENC enrichment.Surface proteins have been targets for isolation of specific cell types. It is important that changes in expression of these proteins correlate with measurable characteristic that reflects the state of the cells. The expression of cell surface CD166 and Prdx-6 were monitored using TAG-1A3 and TAG-2A12 respectively and our data clearly demonstrates good correlation with the current standard of morphological grading of hCENC. The binding specificity of TAG-2A12 to only hCENC was exploited to help enrichment of hCENC from complex cell mixtures of either hESC or hCSF. From our study, we conclude that TAG-2A12 is a promising tool to quantitatively assess the quality of hCENC since expression of Prdx-6 correlated with morphological assessment and enrichment of viable hCENC from mixed cell populations for cell transplantation.
Materials and Methods
Cell culture
Human corneal endothelial cells and human corneal stromal fibroblasts were isolated from corneas of cadaveric donors as previously described.14,42,43 Briefly, the Descement's membrane (DM) and corneal endothelial cells were carefully peeled off from the stroma under a dissecting stereomicroscope. The DM-corneal endothelial cells were dislodged from the DM into small clumps using collagenase followed by further dissociation using TrypLE Express (Life Technologies, #17100-017). Isolated hCENC were cultured on tissue culture plates coated with a fibronectin and collagen-based (FNC) coating mix (United States Biologicals, # C2605), and propagated in F99 medium (1:1 Ham's F12 and M199)44 during the expansion phase, supplemented with 5% fetal bovine serum (FBS)(Life Technologies, #10437-028), 20 ng/ml ascorbic acid (Sigma-Aldrich, A5960), 1× Insulin-Transferrin-Selenium (Life Technologies, #41400-45), 1× antibiotic/antimycotic (AA)(Life Technologies,#15140-122/#15290-018)and 10 ng/ml of fibroblast growth factor-2 (FGF-2,R&D Systems, 233-FB-025/CF). The cells received fresh culture medium every other day. When hCENC reached approximately 90% confluency, the culture was replaced with a maintenance medium which comprised of endothelium, serum free medium (SFM) supplemented with 5% FBS and 1× AA for 5 days before further sub-culturing.45
The stromal button was obtained by trephination after peeling off the DM-endothelial layer as previously described.14 These stromal cells were isolated via collagenase digestion of the stroma button over-night, and cultured in serum-containing medium that was changed every 2 days. Both stromal fibroblasts and lung fibroblast cells (IMR90) were cultured in Dulbecco's modified Eagle's medium ((DMEM) (#11960-044) basal media width 10% FBS (#16140-071) and supplemented with 2 mM L-glutamine (#10378-016) and 1% penicillin and streptomycin (#5070-063)(all from Life Technologies). The human embryonic stem cell line (hESC), HES-3, was obtained from ES Cell International (ESI BIO, Alameda, CA, USA) and cultured on Matrigel (Becton, Dickinson and Company, #354234) with conditioned medium (CM) containing FGF-2 (#PHG0024, Life Technologies) as described previously.46 Cultures of H9-hESC and H9-derived neural crest cells were kindly provided by Dr Alan Colman and Dr Chng Zhenzhi from the Institute of Medical Biology, A*STAR Singapore.
Monoclonal antibodies to human corneal endothelial cells
Monoclonal antibodies were raised using whole cell immunization strategy. Approximately 1 × 106 fresh cadaveric hCENC were harvested and immunized with monophosphoryl-lipid A trehalose dicorynomycolate (MPL+TDM) adjuvant (Sigma Adjuvant System, #S6322; Sigma Aldrich Inc.) into Balb/C mice for 6 consecutive weeks followed by fusion of B cells with SP 2/O myeloma cells. Hybridomas were clonally picked after 10-14 days and expanded in fresh culture medium (Medium E, Stemcell Technologies, Inc., #03805). Cell-free supernatant was collected after 7-10 days and used as primary antibodies for screening against hCENC.
Flow cytometry
Antibodies binding to the surface of the cells were identified using flow cytometry as previously described.47 Normalized mean fluorescent intensity (MFI) was calculated based on the MFI of the sample divided by the MFI of the isotype control.48 As a control for positive binding to human cell lines, an in-house derived antibody, mAb 63 was used.47 Biontinylated mAb 63 was also used as the positive control in the double staining of hCENC with TAG-1A3. Strepavidin-FITC conjugated secondary antibody was used for detection of the biotinylated mAb. Biotinylation was perform as per described in the product insert (EZ-Link Sulfo-NHS-Biotin, #21217, Thermo Fisher Scientific Inc.).
Immunostaining
Cornea tissue sections were prepared as previously described.14 The frozen sections were thawed at room temperature followed by PBS wash before blocking with 10% goat serum (Dako, #X0907) for 30 min. Staining was carried out with primary antibody (supernatant) incubation for 1 h at room temperature. Alexafluor 488 (#A-11059) and 594 (#A-11062) conjugated rabbit anti-mouse Ig (1:500) (Life Technologies) were used as secondary antibodies. Nucleus was counter-stained with Vectorshield containing DAPI (Vector Laboratories, #H-1200). The staining of cultured hCENC was performed with cells seeded on glass slides. The cells were fixed with Reagent A (Caltag Fix and Perm Kit; An Der Grub Bio Research GmbH, #GAS001) for 15 min at room temperature. Staining was carried out as described above with either supernatant or commercial antibodies. The commercial primary antibodies used in this study were mouse IgG1 anti-ZO-1 (1:50; BD Biosciences PharMingen, #610967), mouse IgG anti-CD166 (1:1000; Abcam, #ab109215), and mouse IgG anti-Prdx6 (1:1000; Abcam, #ab16946). Images were taken using the Olympus light microscope IX71 fitted with a digital monochrome camera XM10 (Olympus Corporation, Tokyo, Japan).
Characterization of antigen target
Isotype of TAG-1A3 and TAG-2A12
Isotyping was performed with Mouse Monoclonal Antibody Isotyping kit from Roche (Roche, #11493027001). The protocol was carried out according to manufacturer's instructions. Briefly, the pellet in the tube was reconstituted with 150 μl of hybridoma culture supernatant from either clones TAG-1A3 and TAG-2A12. The solution was thoroughly mixed by vortexing before adding the isostrip. The results were analyzed after 10 min of incubation.
Immunoprecipitation
Total cell lysate of hCENC was prepared with 2% Triton in PBS. Immunoprecipitation (IP) was carried out using the Phynexus instrument (Phynexus Inc., California, USA), loaded with Protein G tips (Phynexus Inc., #PTR 92-05-02). The automated program allowed sequential incubation with hybridoma culture supernatant, cell lysate and washing buffer. Low pH elution was performed at the final step and the eluted sample was neutralized before use.
The samples were boiled at 95°C after adding 5× sample loading dye and subjected to SDS-PAGE using 4-12% gradient NuPAGE Bis-Tris gel (#NP0335 Box) with 1× MOPS buffer (#NP001) and ran along with SeeBlu Plus2 marker (#LC5925) (all from Life Technologies). The proteins were separated at 100-120 V for 1-2 h. The samples were prepared in duplicates, one set used for Western blot transfer onto PVDF membrane and the other for silver staining. The membrane blot was blocked with 5% low fat milk for 30 min before incubating overnight at 4°C with diluted culture supernatant from the primary antibody (1:3) with blocking buffer. Blots were washed with 0.1% Tween in PBS, and incubated with horseradish peroxidase (HRP) conjugated anti-mouse Ig (1:10000, DAKO, #P0260) at room temperature for 1 h. Finally, the blots were developed using chemiluminescence, ECL prime Western blotting detection reagent (GE Healthcare, #RPN2232). The protein band on the silver stained gel that corresponded to the Western blot was excised and digested with trypsin prior to antigen target identification using mass spectrometry (LC/MS-MS). For target validation, commercial antibodies against CD166 (Abcam, #ab109215) and Prdx-6 were diluted 1:100 for IP and 1:1000 for Western blotting.
Periodate treatment
Periodate treatment was used to determine if the antigen detected by the immunoblots were binding to glycoproteins. Meta-periodate is used to oxidize carbohydates by opening up the saccharide rings between vincial diols hence leaving 2 aldehyde groups. Binding of antibodies that recognizes glycan based targets will be affected. The transferred protein blots were first blocked with 5% milk for 15 min at room temperature. After blocking, blots were washed twice with 100 mM sodium acetate (pH 4.5)(Merck Millipore, 1.06268.1000), and followed by 2 incubations with 100 mM sodium metaperiodate (Sigma, #S1878) (freshly prepared in 100 mM sodium acetate) for 30 min at room temperature in the dark for each incubation. The blots were then washed 4 times with sodium acetate. The treatment was then quenched with 0.5 M sodium borohydride (Sigma, #21346-2) (prepared freshly in PBS) for 30 min at room temperature and followed by washing with 5 ml of PBS twice. Finally, the blot is then re-blocked with 5% milk for another 30 min prior to addition for the primary antibodies. For the control blot, all incubation was performed as described as before but without the addition of sodium metaperiodate.
PNGase F treatment
PNGase F is an enzyme that had been optimized for efficient release of N-linked glycans on proteins. The PNGase F treatment was performed according to the manufactures’ protocol (New England Biolabs, #P0704L). Briefly, cell lysates of 10 μg of total protein was first denatured using 1 μl of 10× glycoprotein denaturing buffer (5% SDS without DTT) and water to make up a 10 μl reaction volume. The glycoproteins were denatured by heating the reaction to 100°C for 5 mins. Next, a total reaction volume of 20 μl was prepared with the denatured glycoproteins by adding 2 μl of 10× G7 reaction buffer, 2 μl of 10% NP40, and 1 μl of PNGase F, and 5μl of water. The samples were then incubated for 1 h at 37°C. Finally, samples were then boiled in 5× loading dye and ran in 4-12% gradient gel followed by Western Blotting as described above.
Cell enrichment
TAG-2A12 was used to enrich for hCENC from cell mixtures containing a 50:50 mix of either hESC or hCSF. The proof-of-concept study was performed using a positive-cell selection method with anti-mouse IgG beads from magnetic affinity cell separation (MACS) (Miltenyi Biotech, # (#130-047-102). Enrichment of hCENC was done following the manufacturers’ protocol. Briefly, cells that constitute the negative population in the mixture (hESC or hCSFs) were first labeled with a red fluorescent cell linker PKH26 that binds to the cell membrane (Sigma Aldrich Inc.). The labeled cells were then added to unlabeled hCENC to obtain the starting cell mixture., After which, the cells were incubated with TAG-2A12 for 30 min at 4°C. The cells were spun down to remove unbound mAbs and washed with 1% BSA in PBS. The cell pellet was re-suspended in MACS buffer and incubated with anti-mouse IgG beads for 10 min at 4°C. Cells were washed and re-suspended in MACS buffer before loading onto the magnetic column. The column was subjected to several washes with MACS buffer to remove the unbound cells. Finally, bound cells were eluted in MACS buffer by removing the column from the magnetic holder and plunging the cells through the column. Flow cytometry was used to analyze the purity of the population of cells in the flowthrough (FT) which contained mostly the unbound cells while the retentate contained enriched hCENC. In-house positive controls mAb 63 and mAb 84 was used for fibroblast cells and hESC respectively.47
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Ethical statements
This study was approved by the institutional review board of the Singapore Eye Research Institute/Singapore National Eye Centre according to the tenets of the Declaration of Helsinki, and written consent was acquired from the next of kin of all deceased donors regarding eye donation for research.
Acknowledgments
We would like to thank Dr Alan Colman and Dr Chng Zhen Zhi from the Institute of Medical Biology for providing the H9 and H9-derived neural crest cells.
Funding
This study is supported by Agency for Science, Technology and Research TCRP Grant (TCR0101673). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1.Peh GS, Toh KP, Wu FY, Tan DT, Mehta JS. Cultivation of human corneal endothelial cells isolated from paired donor corneas. PLoS One 2011; 0:e28310; http://dx.doi.org/ 10.1371/journal.pone.0028310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ju C, Zhang K, Wu X. Derivation of corneal endothelial cell-like cells from rat neural crest cells in vitro. PLoS One 2012; 7:e42378; PMID:22860120; http://dx.doi.org/ 10.1371/journal.pone.0042378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delfino-Machin M, Chipperfield TR, Rodrigues FS, Kelsh RN. The proliferating field of neural crest stem cells. Dev Dyn 2007; 236:3242-54; PMID:17823935; http://dx.doi.org/ 10.1002/dvdy.21314 [DOI] [PubMed] [Google Scholar]
- 4.Wulle KG. Electron microscopy of the fetal development of the corneal endothelium and Descemet's membrane of the human eye. Invest Ophthalmol 1972; 11:897-904; PMID:4634956 [PubMed] [Google Scholar]
- 5.Joyce NC. Proliferative capacity of the corneal endothelium. Prog Retin Eye Res 2003; 22:359-89; PMID:12852491; http://dx.doi.org/ 10.1016/S1350-9462(02)00065-4 [DOI] [PubMed] [Google Scholar]
- 6.Senoo T, Joyce NC. Cell cycle kinetics in corneal endothelium from old and young donors. Invest Ophthalmol Vis Sci 2000; 41:660-7; PMID:10711678 [PubMed] [Google Scholar]
- 7.Zhu C, Joyce NC. Proliferative response of corneal endothelial cells from young and older donors. Invest Ophthalmol Vis Sci 2004; 45:1743-51; PMID:15161835; http://dx.doi.org/ 10.1167/iovs.03-0814 [DOI] [PubMed] [Google Scholar]
- 8.Levis HJ, Peh GS, Toh KP, Poh R, Shortt AJ, Drake RA, Mehta JS, Daniels JT. Plastic compressed collagen as a novel carrier for expanded human corneal endothelial cells for transplantation. PLoS One 2012; 7:e50993; PMID:23226443; http://dx.doi.org/ 10.1371/journal.pone.0050993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoeruek E, Bayyoud T, Maurus C, Hofmann J, Spitzer MS, Bartz-Schmidt KU, Szurman P. Decellularization of porcine corneas and repopulation with human corneal cells for tissue-engineered xenografts. Acta Ophthalmol 2012; 90:e125-31; PMID:22136333; http://dx.doi.org/ 10.1111/j.1755-3768.2011.02261.x [DOI] [PubMed] [Google Scholar]
- 10.Mimura T, Yamagami S, Yokoo S, Usui T, Tanaka K, Hattori S, Irie S, Miyata K, Araie M, Amano S. Cultured human corneal endothelial cell transplantation with a collagen sheet in a rabbit model. Invest Ophthalmol Vis Sci 2004; 45:2992-7; PMID:15326112; http://dx.doi.org/ 10.1167/iovs.03-1174 [DOI] [PubMed] [Google Scholar]
- 11.Fan TJ, Zhao J, Hu XZ, Ma XY, Zhang WB, Yang CZ. Therapeutic efficiency of tissue-engineered human corneal endothelium transplants on rabbit primary corneal endotheliopathy. J Zhejiang Univ Sci B 2011; 12:492-8; PMID:21634043; http://dx.doi.org/ 10.1631/jzus.B1000199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honda N, Mimura T, Usui T, Amano S. Descemet stripping automated endothelial keratoplasty using cultured corneal endothelial cells in a rabbit model. Arch Ophthalmol 2009; 127:1321-6; PMID:19822849; http://dx.doi.org/ 10.1001/archophthalmol.2009.253 [DOI] [PubMed] [Google Scholar]
- 13.Koizumi N, Sakamoto Y, Okumura N, Tsuchiya H, Torii R, Cooper LJ, Ban Y, Tanioka H, Kinoshita S. Cultivated corneal endothelial transplantation in a primate: possible future clinical application in corneal endothelial regenerative medicine. Cornea 2008; 27 Suppl 1:S48-55; PMID:18813075; http://dx.doi.org/ 10.1097/ICO.0b013e31817f2298 [DOI] [PubMed] [Google Scholar]
- 14.Cheong YK, Ngoh ZX, Peh GS, Ang HP, Seah XY, Chng Z, Colman A, Mehta JS, Sun W. Identification of cell surface markers glypican-4 and CD200 that differentiate human corneal endothelium from stromal fibroblasts. Invest Ophthalmol Vis Sci 2013; 54:4538-47; PMID:23744997; http://dx.doi.org/ 10.1167/iovs.13-11754 [DOI] [PubMed] [Google Scholar]
- 15.Engelmann K, Bednarz J, Schafer HJ, Friedl P. Isolation and characterization of a mouse monoclonal antibody against human corneal endothelial cells. Exp Eye Res 2001; 73:9-16; PMID:11428858; http://dx.doi.org/ 10.1006/exer.2001.0993 [DOI] [PubMed] [Google Scholar]
- 16.Engelmann K, Bohnke M, Friedl P. Isolation and long-term cultivation of human corneal endothelial cells. Invest Ophthalmol Vis Sci 1988; 29:1656-62; PMID:3182201 [PubMed] [Google Scholar]
- 17.Vinciguerra M, Deschenes G, Hasler U, Mordasini D, Rousselot M, Doucet A, Vandewalle A, Martin PY, Feraille E. Intracellular Na+ controls cell surface expression of Na,K-ATPase via a cAMP-independent PKA pathway in mammalian kidney collecting duct cells. Mol Biol Cell 2003; 14:2677-88; PMID:12857856; http://dx.doi.org/ 10.1091/mbc.E02-11-0720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li CX, Poznansky MJ. Characterization of the ZO-1 protein in endothelial and other cell lines. J Cell Sci 1990; 97 (Pt 2):231-7; PMID:2277090 [DOI] [PubMed] [Google Scholar]
- 19.Howell DN, Burchette JL Jr, Paolini JF, Geier SS, Fuller JA, Sanfilippo F. Characterization of a novel human corneal endothelial antigen. Invest Ophthalmol Vis Sci 1991; 32:2473-82; PMID:1714428 [PubMed] [Google Scholar]
- 20.Sakamoto T, Nakashima Y, Maeda K, Sueishi K. Monoclonal antibody to the corneal endothelium: partial characterization of the antigen and its expression in fetal and adult rabbits. Graefes Arch Clin Exp Ophthalmol 1991; 229:587-92; PMID:1722477; http://dx.doi.org/ 10.1007/BF00203327 [DOI] [PubMed] [Google Scholar]
- 21.Srivastava OP, Srivastava K, Shukla SD. Characterization of a 66-kilodalton surface glycoprotein of the human corneal endothelium. Invest Ophthalmol Vis Sci 1990; 31:1982-93; PMID:2210994 [PubMed] [Google Scholar]
- 22.Walker DG, Lue LF. Understanding the neurobiology of CD200 and the CD200 receptor: a therapeutic target for controlling inflammation in human brains? Future Neurol 2013; 8:321–332; PMID:2419871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagihara K, Watanabe K, Chun J, Yamaguchi Y. Glypican-4 is an FGF2-binding heparan sulfate proteoglycan expressed in neural precursor cells. Dev Dyn 2000; 219:353-67; PMID:11066092; http://dx.doi.org/ 10.1002/1097-0177(2000)9999:9999%3c::AID-DVDY1059%3e3.0.CO;2- [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Hjorth E, Zhu M, Calzarossa C, Samuelsson EB, Schultzberg M, Akesson E. Interplay between human microglia and neural stemprogenitor cells in an allogeneic co-culture model. J Cell Mol Med 2013; 17:1434-43; PMID:24034597; http://dx.doi.org/ 10.1111/jcmm.12123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swart GW. Activated leukocyte cell adhesion molecule (CD166ALCAM): developmental and mechanistic aspects of cell clustering and cell migration. Eur J Cell Biol 2002; 81:313-21; PMID:12113472; http://dx.doi.org/ 10.1078/0171-9335-00256 [DOI] [PubMed] [Google Scholar]
- 26.Teicher BA. Targets in small cell lung cancer. Biochem Pharmacol 2014; 87:211–219; PMID:24091017 [DOI] [PubMed] [Google Scholar]
- 27.Wai Wong C, Dye DE, Coombe DR. The role of immunoglobulin superfamily cell adhesion molecules in cancer metastasis. Int J Cell Biol 2012; 2012:340296; PMID:22272201; http://dx.doi.org/ 10.1155/2012/340296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ofori-Acquah SF, King JA. Activated leukocyte cell adhesion molecule: a new paradox in cancer. Transl Res 2008; 151:122-8; PMID:18279810; http://dx.doi.org/ 10.1016/j.trsl.2007.09.006 [DOI] [PubMed] [Google Scholar]
- 29.Fisher AB. Peroxiredoxin 6: a bifunctional enzyme with glutathione peroxidase and phospholipase A(2) activities. Antioxid Redox Signal 2011; 15:831-44; PMID:20919932; http://dx.doi.org/ 10.1089/ars.2010.3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi H, Yu HJ, Wang HY, Wang WT, Jin SH, Zhu P, Li SJ, Rong CT, Li JY. Topical administration of peroxiredoxin-6 on the cornea suppresses inflammation and neovascularization induced by ultraviolet radiation. Invest Ophthalmol Vis Sci 2012; 53:8016-28; PMID:23139277; http://dx.doi.org/ 10.1167/iovs.12-10064 [DOI] [PubMed] [Google Scholar]
- 31.Kubo E, Fatma N, Akagi Y, Beier DR, Singh SP, Singh DP. TAT-mediated PRDX6 protein transduction protects against eye lens epithelial cell death and delays lens opacity. Am J Physiol Cell Physiol 2008; 294:C842-55; PMID:18184874; http://dx.doi.org/ 10.1152/ajpcell.00540.2007 [DOI] [PubMed] [Google Scholar]
- 32.Chhunchha B, Fatma N, Bhargavan B, Kubo E, Kumar A, Singh DP. Specificity protein, Sp1-mediated increased expression of Prdx6 as a curcumin-induced antioxidant defense in lens epithelial cells against oxidative stress. Cell Death Dis 2011; 2:e234; PMID:22113199; http://dx.doi.org/ 10.1038/cddis.2011.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kubo E, Hasanova N, Tanaka Y, Fatma N, Takamura Y, Singh DP, Akagi Y. Protein expression profiling of lens epithelial cells from Prdx6-depleted mice and their vulnerability to UV radiation exposure. Am J Physiol Cell Physiol 2010; 298:C342-54; PMID:19889963; http://dx.doi.org/ 10.1152/ajpcell.00336.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fatma N, Kubo E, Sharma P, Beier DR, Singh DP. Impaired homeostasis and phenotypic abnormalities in Prdx6-mice lens epithelial cells by reactive oxygen species: increased expression and activation of TGFbeta. Cell Death Differ 2005; 12:734-50; PMID:15818411; http://dx.doi.org/ 10.1038/sj.cdd.4401597 [DOI] [PubMed] [Google Scholar]
- 35.Kubo E, Miyazawa T, Fatma N, Akagi Y, Singh DP. Development- and age-associated expression pattern of peroxiredoxin 6, and its regulation in murine ocular lens. Mech Ageing Dev 2006; 127:249-56; PMID:16321424; http://dx.doi.org/ 10.1016/j.mad.2005.10.003 [DOI] [PubMed] [Google Scholar]
- 36.Hasanova N, Kubo E, Kumamoto Y, Takamura Y, Akagi Y. Age-related cataracts and Prdx6: correlation between severity of lens opacity, age and the level of Prdx 6 expression. Br J Ophthalmol 2009; 93:1081-4; PMID:19429582; http://dx.doi.org/ 10.1136/bjo.2008.152272 [DOI] [PubMed] [Google Scholar]
- 37.Fatma N, Singh P, Chhunchha B, Kubo E, Shinohara T, Bhargavan B, Singh DP. Deficiency of Prdx6 in lens epithelial cells induces ER stress response-mediated impaired homeostasis and apoptosis. Am J Physiol Cell Physiol 2011; 301:C954-67; PMID:21677259; http://dx.doi.org/ 10.1152/ajpcell.00061.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ambruso DR, Ellison MA, Thurman GW, Leto TL. Peroxiredoxin 6 translocates to the plasma membrane during neutrophil activation and is required for optimal NADPH oxidase activity. Biochim Biophys Acta 2012; 1823:306-15; PMID:22178385; http://dx.doi.org/ 10.1016/j.bbamcr.2011.11.014 [DOI] [PubMed] [Google Scholar]
- 39.Mummery CL, Zhang J, Ng ES, Elliott DA, Elefanty AG, Kamp TJ. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circ Res 2012; 111:344-58; PMID:22821908; http://dx.doi.org/ 10.1161/CIRCRESAHA.110.227512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moon SH, Kim JM, Hong KS, Shin JM, Kim J, Chung HM. Differentiation of hESCs into Mesodermal Subtypes: Vascular-, Hematopoietic- and Mesenchymal-lineage Cells. Int J Stem Cells 2011; 4:24-34; PMID:24298331; http://dx.doi.org/ 10.15283/ijsc.2011.4.1.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salimi A, Nadri S, Ghollasi M, Khajeh K, Soleimani M. Comparison of different protocols for neural differentiation of human induced pluripotent stem cells. Mol Biol Rep 2014; 41:1713-21; PMID:24469709; http://dx.doi.org/ 10.1007/s11033-014-3020-1 [DOI] [PubMed] [Google Scholar]
- 42.Chng Z, Peh GS, Herath WB, Cheng TY, Ang HP, Toh KP, Robson P, Mehta JS, Colman A. High throughput gene expression analysis identifies reliable expression markers of human corneal endothelial cells. PLoS One 2013; 8:e67546; PMID:23844023; http://dx.doi.org/ 10.1371/journal.pone.0067546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peh GS, Toh KP, Ang HP, Seah XY, George BL, Mehta JS. Optimization of human corneal endothelial cell culture: density dependency of successful cultures in vitro. BMC Res Notes 2013; 6:176; PMID:23641909; http://dx.doi.org/ 10.1186/1756-0500-6-176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Engelmann K, Friedl P. Growth of human corneal endothelial cells in a serum-reduced medium. Cornea 1995; 14:62-70; PMID:7712739; http://dx.doi.org/ 10.1097/00003226-199501000-00011 [DOI] [PubMed] [Google Scholar]
- 45.Peh GS, Chng Z, Ang HP, Cheng TY, Adnan K, Seah XY, George BL, Toh KP, Tan DT, Yam GH, et al. Propagation of human corneal endothelial cells ? A novel dual media approach. Cell Transplant 2013; PMID:24268186 [DOI] [PubMed] [Google Scholar]
- 46.Ding VM, Ling L, Natarajan S, Yap MG, Cool SM, Choo AB. FGF-2 modulates Wnt signaling in undifferentiated hESC and iPS cells through activated PI3-KGSK3beta signaling. J Cell Physiol 2010; 225:417-28; PMID:20506199; http://dx.doi.org/ 10.1002/jcp.22214 [DOI] [PubMed] [Google Scholar]
- 47.Choo AB, Tan HL, Ang SN, Fong WJ, Chin A, Lo J, Zheng L, Hentze H, Philp RJ, Oh SK, et al. Selection against undifferentiated human embryonic stem cells by a cytotoxic antibody recognizing podocalyxin-like protein-1. Stem Cells 2008; 26:1454-63; PMID:18356574; http://dx.doi.org/ 10.1634/stemcells.2007-0576 [DOI] [PubMed] [Google Scholar]
- 48.Chan LY, Yim EK, Choo AB. Normalized median fluorescence: an alternative flow cytometry analysis method for tracking human embryonic stem cell states during differentiation. Tissue Eng Part C Methods 2013; 19:156-65; PMID:22838642; http://dx.doi.org/ 10.1089/ten.tec.2012.0150 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.