Abstract
In addition to being a major nuisance biter, the lone star tick, Amblyomma americanum (L.), is increasingly recognized as an important vector of pathogens affecting humans, domestic animals, and wildlife. Despite its notoriety, efforts have been lacking to define the spatial occurrence of A. americanum in the continental United States with precision beyond that conveyed in continental-scale distribution maps. Here we present a county-level distribution map for A. americanum generated by compiling collection records obtained from a search of the published literature and databases managed by the USDA, U.S. National Tick Collection, and Walter Reed Biosystematics Unit. Our decadal and cumulative maps, which visually summarize 18,121 collections made between 1898 and 2012, show that A. americanum is either established (≥six ticks or ≥two life stages) or reported (<six ticks of a single life stage or number of ticks not specified) in 1,300 counties distributed among 39 states and the District of Columbia. Our cumulative map depicts a species with a core distributional area in the southern part of the eastern United States, but that also occurs further north, especially along the Atlantic Coast and into the Midwest. Although our decadal maps suggest a northward shift in the tick’s distribution in recent decades, the lack of systematic tick surveillance makes this difficult to confirm. The data presented herein should aid in identifying areas posing risk for A. americanum-associated illnesses and environmental correlates that define the tick’s distributional limits.
Keywords: Amblyomma americanum, geographic distribution, surveillance, tick, vector
The lone star tick, Amblyomma americanum (L.), was first described in 1758 as Acarus americanus L. but later reassigned to Ixodes americanus (L.) by Fabricius in 1805 and, ultimately, to A. americanum by Koch in 1844 (Cooley and Kohls 1944). It was the first North American tick species to be formally described, and the type locality is Pennsylvania or New Jersey (Cooley and Kohls 1944, Bishopp and Trembley 1945). The current generalized geographic range of A. americanum in the continental United States includes the southeastern states (as far west as eastern Texas) but also stretches north to the southern parts of Nebraska, Iowa, Illinois, Indiana, Ohio, and West Virginia, and along the Atlantic Coast states north to Maine (Childs and Paddock 2003, Yabsley 2010, Cortinas and Spomer 2013). Cervids, particularly the white-tailed deer (Odocoileus virginianus), are favored hosts of A. americanum (Childs and Paddock 2003, Paddock and Yabsley 2007, Yabsley 2010), but all active stages readily bite humans and domestic animals such as cattle, horses, dogs, and cats (Cooley and Kohls 1944, Goddard and McHugh 1990, Felz et al. 1996, Merten and Durden 2000). Because of its aggressive questing behavior and local hyperabundance, A. americanum has long been recognized as a major nuisance biter of humans (Fitch 1870, Cooley and Kohls 1944, Bishopp and Trembley 1945, Hair and Howell 1970, Merten and Durden 2000).
Together with the blacklegged tick (Ixodes scapularis (Say)) and the American dog tick (Dermacentor variabilis (Say)), A. americanum has recently emerged as one of the most important tick vectors in the eastern United States transmitting pathogens to both humans and domestic animals (Childs and Paddock 2003, Mixson et al. 2006, Goddard and Varela-Stokes 2009, Fritzen et al. 2011). In addition to being the primary enzootic and bridge vector to humans of Ehrlichia chaffeensis (causative agent of human monocytic ehrlichiosis; Paddock and Yabsley 2007, Yabsley 2010), evidence suggests that A. americanum has the capacity to transmit Ehrlichia ewingii (ehrlichiosis of humans and dogs; Varela-Stokes 2007), Panola Mountain Ehrlichia sp. (ehrlichiosis of humans; Loftis et al. 2006), Francisella tularensis (tularemia) (Hopla 1953), and Cytauxzoon felis (cytauxzoonosis [bobcat fever] of cats; Reichard et al. 2010). Infection by Rickettsia rickettsii (Rocky Mountain spotted fever; Breitschwerdt et al. 2011) and Rickettsia parkeri (spotted fever rickettsiosis; Goddard 2003) may also be possible, although associated evidence is incomplete or inconclusive. A. americanum has been further implicated as a vector of Borrelia lonestari (Barbour et al. 1996, Stromdahl et al. 2003), which was initially thought to be a causative agent of southern tick-associated rash illness (STARI) or Masters disease (Masters et al. 2008, Stromdahl and Hickling 2012). Recently, field-collected A. americanum have been found infected with the newly described Heartland virus (McMullan et al. 2012).
Despite the prominence of A. americanum as a nuisance biter and vector of human pathogens in the eastern United States, efforts have been lacking to define the species’ geographic range in the continental United States beyond the presentation of a generalized distribution map (Bishopp and Trembley 1945, Childs and Paddock 2003). This is in striking contrast to I. scapularis, the primary vector of Borrelia burgdorferi and other human pathogens in the eastern United States (Piesman and Eisen 2008), for which a complete national distribution map based on historical records at the county scale has been developed (Dennis et al. 1998) and systematic field sampling recently was conducted in locations throughout the eastern United States (Diuk-Wasser et al. 2006, 2010). Similar efforts to define the current spatial distribution of A. americanum at the county scale would not only aid in identifying human populations at risk for exposure to A. americanum and its associated pathogens but also in determining the landscape, host assemblage, and climatic conditions that are favorable for establishment and proliferation of this tick species. Importantly, neither direct assessment of spatiotemporal changes in the geographic distribution of A. americanum (based on collection records), nor predictive modeling of future changes in biogeography (e.g., driven by changes in climate, landscape, and deer abundance), are possible without finer scale knowledge of the species’ current geographic distribution.
The aims of this study were to first review collection records of A. americanum in the continental United States, gathered from the published literature as well as databases maintained by the United States National Tick Collection (USNTC), Veterinary Services within the United States Department of Agriculture, and the Walter Reed Biosystematics Unit VectorMap initiative, and thereafter to define the spatial distribution of established and reported A. americanum populations by county following the methodology used by Dennis et al. (1998) in an analogous study involving I. scapularis.
Materials and Methods
Development of a Database for Collection Records of A. americanum
We gathered collection records from the published literature using a search of the Web of Science database (Thompson Reuters, NY) conducted on 4 April 2013. The search spanned the years 1898 to present and used the search string “Amblyomma americanum” (in title) or “Amblyomma americanum (in topic) or “Lone star tick” (in title) or “Lone star tick” (in topic). The snowball technique, which identifies additional records based on referenced materials, was used to increase the total number of collection records examined. The database that we developed for collection records of A. americanum includes all records from the continental United States that allow for the county of collection to be identified and also report the date(s) of collection and tick count (and by life stage when this information was provided). Following the approach of Dennis et al. (1998) for I. scapularis, we used the life stage-specific count data for A. americanum to make inferences about establishment of this tick species at the county scale. For each individual collection record, A. americanum was classified, by county, as: 1) established if six or more ticks, or two or more life stages, were recorded during a specified time period, or 2) reported if fewer than six ticks of a single life stage were recorded over the sampling period, or if the number of ticks collected was not specified. If the collections reported in a record spanned multiple years within a single county but the number of ticks collected per year was not provided, we used the total tick count across years and the last year of collection. If no collection year was reported, we used the reference’s year of publication. This applied to 12% (276 of 2,388) of the collection records deemed usable from the published literature database. In some cases a single collection record spanned multiple counties or was reported at a site spanning multiple counties. When these counties formed a contiguous area, we split the records into single-county records but classified A. americanum as reported in each county irrespective of the total number of ticks collected. As with single-county records, we used the last year of collection if multiyear collection counts were reported in aggregate and the year of publication if the collection year was not reported. If the counties in a multicounty record did not form a contiguous area, we flagged the record and noted the associated counties. If counties in any of these flagged records shared a border with one or more counties associated with other records in the full database, the flagged records (and associated counties) were included in the database. Through this overall evaluation process, each collection record was associated with a classification of the status for A. americanum (established or reported) by single year and county of collection.
We further expanded our database gathering additional A. americanum collection records from databases maintained by the United States National Tick Collection (USNTC), which is maintained at Georgia Southern University, Statesboro, GA, the National Veterinary Services Laboratories (NVSL) within the United States Department of Agriculture, and the VectorMap database curated by the Walter Reed Bio-systematics Unit (www.vectormap.org). Like the Web of Science search, all three of these databases were queried on 4 April 2013. The USNTC is an acarological archive containing 125,000 individually accessioned tick lots and >1 million specimens deposited over a period spanning 1806 to present time. Although not complete, the USNTC electronic database contains records for ≈70% of these holdings. Records from the USNTC database were recovered using a query for any specimens associated with the search string “Amblyomma americanum,” “americanum,” “americanum*.” The NVSL tick identification dataset is associated with a passive surveillance system for ticks of importance to animal health. A tick geodatabase, maintained at the Center for Epidemiology and Animal Health (CEAH, Fort Collins, CO), includes NVSL’s tick identification data along with other tick collection records to map distributions of ticks of veterinary importance. Entries in the database span 1989 to present time. This NVSL database was queried using the search terms “Amblyomma americanum” and “lone star tick(s).” The VectorMap database was queried for records associated with genus Amblyomma, species americanum, and date after 1 January 1801 through the online data portal (http://www.tickmap.org/). Collection records for A. americanum recovered from these three databases were processed to identify usable records using methods similar to those used for published references obtained through the Web of Science search. When multiple collections associated with the same collector(s) occurred during a single year in a county, we merged those into a single record by summing the number of ticks collected in each intraannual sampling period.
Mapping of Collection Records of A. americanum
To generate maps displaying counties in the continental United States in which A. americanum could be classified as established or reported in a particular year, records from our database were plotted using ArcMap 10.1 (ESRI, Redlands, CA) by associating a Federal Information Processing Standard (FIPS) code, a unique county identifier, with the county of collection of each database record. A. americanum was considered established in a county in the first year with an established record or six or more reported records. Spatial patterns were characterized at the decadal and regional scales, the latter based on Standard Federal Regions established by the U.S. government’s Office of Management and Budget (United States Office of Management and Budget 1974, see map insert in Fig. 2). The classification status for A. americanum (established or reported) for individual counties was assessed in two different ways: 1) by decade and 2) cumulatively across decades; once A. americanum was classified as reported during a particular decade, it was considered reported in that county for all subsequent decades or until the first record as established, at which point the county assumed the status of established for all subsequent decades.
Fig. 2.

Number of counties (cumulative by decade) where A. americanum is known to be established or reported, by decade of collection and region (based exclusively on records from the published literature). Map depicts Standard Federal Regions within the continental United States.
Results
Spatial Distribution of Counties in Which A. americanum is Established or Reported
Our final database, made up of data from each of the four sources described above, contains 18,121 usable collection records for A. americanum in the continental United States (Tables 1 and 2). These records span collections made over the time period from 1898 to 2012, spread across 39 states (and the District of Columbia) and 1,300 counties, and represent 4,882 unique county–collection year combinations. Only one county (Unicoi County, TN) was not included in our database because associated collection records involved multiple nonadjacent counties and it was not clear from which counties samples were derived. The number and source(s) of collection records for each state and county combination in the database are provided in the electronic appendix (Supp Table 1 [online only]).
Table 1.
Summary of A. americanum collection records
| Source | No. original records or publications examined | No. usable records | No. states | No. counties | Span of collection years |
|---|---|---|---|---|---|
| USNTC | 1,264 | 492 | 32a | 236 | 1898–2006 |
| NVSL | 10,410 | 6,004 | 22 | 596 | 1985–2008 |
| VectorMap | 9,345 | 9,237 | 31a | 296 | 1945–2010 |
| Web of Science | 1,384 (420b) | 2,388c | 31a | 908 | 1898–2012 |
| All sources | 22,403 | 18,121 | 40a | 1,300 | 1898–2012 |
Total includes the District of Columbia.
A Web of Science literature search conducted on 4 April 2013 returned 1,226 publications for the topic or title words “Amblyomma americanum” or “Lone Star tick.” When combined with publications identified using the snowball technique, a total of 1,384 publications were screened for information on field collection(s) of A. americanum: 379 of those identified in the original Web of Science search included reports of field collection(s), as did an additional 106 references identified via the snowball technique, but only 326 and 94 of these, respectively, yielded usable records.
Of these 1,712 were from publications identified through the original Web of Science search and 676 were from publications identified via the snowball technique.
Table 2.
Number of collection records for A. americanum by decade across states and regions
| State | Decade of collection (no. of collection records)
|
Total | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1890s | 1900s | 1910s | 1920s | 1930s | 1940s | 1950s | 1960s | 1970s | 1980s | 1990s | 2000s | 2010s | ||
| Alabama | 2 | 13 | 13 | 16 | 184 | 26 | 7 | 261 | ||||||
| Arkansas | 1 | 1 | 12 | 25 | 102 | 21 | 12 | 124 | 103 | 3 | 404 | |||
| Californiaa | 26 | 26 | ||||||||||||
| Colorado | 1 | 1 | ||||||||||||
| Connecticut | 3 | 2 | 7 | 3 | 1 | 16 | ||||||||
| Delaware | 14 | 32 | 2 | 48 | ||||||||||
| District of Columbia | 1 | 1 | 1 | 1 | 6 | 2 | 12 | |||||||
| Florida | 1 | 2 | 13 | 41 | 6 | 20 | 13 | 20 | 246 | 379 | 6 | 747 | ||
| Georgia | 6 | 6 | 3 | 8 | 9 | 49 | 288 | 208 | 6 | 583 | ||||
| Illinois | 1 | 1 | 5 | 3 | 1 | 9 | 20 | |||||||
| Indiana | 4 | 9 | 4 | 27 | 28 | 30 | 102 | |||||||
| Iowa | 1 | 2 | 3 | 62 | 68 | |||||||||
| Kansas | 1 | 1 | 116 | 126 | 7 | 251 | ||||||||
| Kentucky | 2 | 1 | 1 | 2 | 15 | 25 | 88 | 453 | 47 | 634 | ||||
| Louisiana | 3 | 1 | 1 | 4 | 2 | 16 | 3 | 14 | 15 | 59 | ||||
| Maine | 17 | 17 | ||||||||||||
| Maryland | 1 | 1 | 1 | 10 | 306 | 1157 | 216 | 1,692 | ||||||
| Massachusetts | 4 | 4 | ||||||||||||
| Michigan | 1 | 1 | 40 | 42 | ||||||||||
| Minnesota | 1 | 9 | 3 | 2 | 15 | |||||||||
| Mississippi | 3 | 2 | 6 | 18 | 13 | 33 | 290 | 62 | 427 | |||||
| Missouri | 3 | 3 | 9 | 8 | 1 | 2 | 51 | 105 | 82 | 264 | ||||
| Montanab | 1 | 1 | ||||||||||||
| Nebraska | 6 | 22 | 3 | 32 | 63 | |||||||||
| New Hampshirec | 1 | 1 | 2 | |||||||||||
| New Jersey | 2 | 1 | 7 | 596 | 963 | 95 | 1,664 | |||||||
| New Mexico | 1 | 1 | ||||||||||||
| New York | 1 | 3 | 6 | 69 | 19 | 2 | 100 | |||||||
| North Carolina | 2 | 1 | 3 | 2 | 2 | 2 | 16 | 44 | 293 | 405 | 277 | 1,047 | ||
| Ohio | 1 | 1 | 1 | 1 | 18 | 22 | ||||||||
| Oklahoma | 2 | 1 | 5 | 26 | 2 | 17 | 69 | 74 | 40 | 42 | 5 | 283 | ||
| Pennsylvania | 4 | 30 | 1 | 35 | ||||||||||
| Rhode Island | 2 | 2 | 2 | 7 | 1 | 14 | ||||||||
| South Carolina | 3 | 16 | 5 | 12 | 9 | 321 | 352 | 39 | 757 | |||||
| South Dakotad | 1 | 1 | ||||||||||||
| Tennessee | 5 | 8 | 28 | 275 | 295 | 32 | 643 | |||||||
| Texas | 24 | 21 | 9 | 56 | 77 | 19 | 17 | 6 | 25 | 2625 | 1025 | 3,904 | ||
| Virginia | 2 | 2 | 5 | 1 | 14 | 6 | 6 | 40 | 44 | 14 | 709 | 2703 | 335 | 3,881 |
| Wisconsin | 1 | 3 | 3 | 2 | 9 | |||||||||
| Wyoming | 1 | 1 | ||||||||||||
| Region | ||||||||||||||
| Upper Northeast | 3 | 2 | 3 | 2 | 26 | 15 | 2 | 53 | ||||||
| Lower Northeast | 2 | 2 | 3 | 13 | 665 | 982 | 97 | 1,764 | ||||||
| Mid-Atlantic | 2 | 3 | 5 | 2 | 14 | 7 | 6 | 42 | 45 | 24 | 1,034 | 3,928 | 556 | 5,668 |
| Southeast | 4 | 5 | 8 | 46 | 78 | 11 | 45 | 99 | 224 | 1,985 | 2,180 | 414 | 5,099 | |
| Upper Midwest | 1 | 1 | 3 | 3 | 5 | 14 | 5 | 30 | 81 | 63 | 4 | 210 | ||
| South-Central | 29 | 23 | 11 | 74 | 132 | 124 | 36 | 112 | 114 | 2,803 | 1,185 | 8 | 4,651 | |
| Midwest | 3 | 3 | 10 | 10 | 1 | 2 | 8 | 192 | 296 | 121 | 646 | |||
| Central-Mountain | 1 | 1 | 1 | 1 | 4 | |||||||||
| Pacific-Southwest | 26 | 26 | ||||||||||||
| Total | 2 | 40 | 36 | 22 | 150 | 234 | 151 | 137 | 269 | 415 | 6,813 | 8,650 | 1,202 | 18,121 |
Based on 26 records of ticks removed from military personnel at McClellan Airforce Base in Sacramento between 1995 and 1998.
Based on the collection of six adult ticks from an unknown host in the city of Hamilton in 1937.
Based on the collection of six larval ticks from an unknown host in Concord County in 1978.
Based on the collection of 35 nymphal and 7 larval ticks from an unknown host in McCook County in 1997.
The names of states in which the species is currently established, and the associated decade in which establishment was first documented, are in bold.
Based on our classification criteria, A. americanum is established in 653 counties distributed across 32 states and the District of Columbia. These counties are concentrated primarily in the Southeast and South-Central regions (which account for 38 and 31%, respectively, of the counties where A. americanum is classified as established) in a band stretching southwest from southern Iowa into Texas and southeast from southern Illinois into Florida (Fig. 1). The remaining counties where A. americanum is classified as established are distributed largely across states in the Midwest region (15% of counties) and along a narrow coastal margin up the eastern seaboard from South Carolina into southern and central New York (12% of counties where A. americanum is classified as established are split between the Lower Northeast and Mid-Atlantic regions).
Fig. 1.
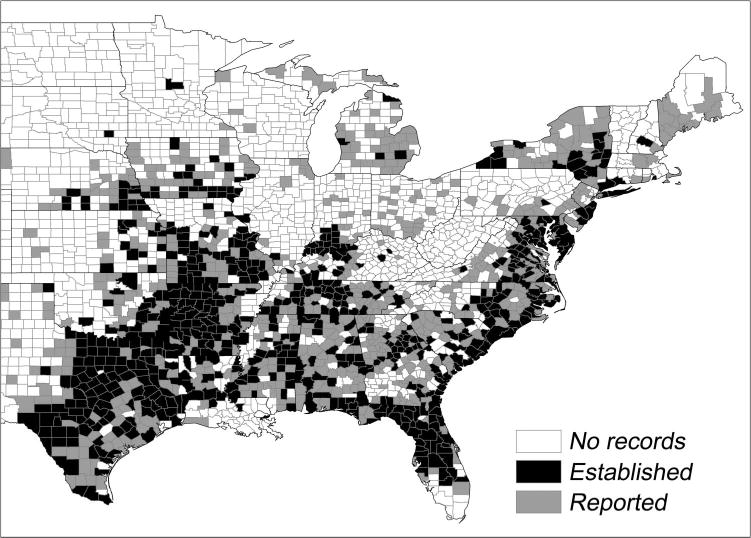
Spatial distribution of counties in which A. americanum is known to be established or reported, cumulative from the 1890s to present time.
In addition to the counties where A. americanum is classified as established, the species is considered reported in 647 counties distributed across 36 states. Of these states, six contain counties with the reported classification status but lack counties where A. americanum is classified as established: Colorado, Maine, Massachusetts, Ohio, Wisconsin, and Wyoming. Across all 18,121 records (county–collection year combinations) in our database, 31% were made in counties in the Mid-Atlantic region, 28% in counties in the Southeast region, 26% in counties in the South-Central region, and 10% in counties in the Lower Northeast region. None of the remaining five regions (Central-Mountain, Midwest, Pacific-Southwest, Upper Mid-west, and Upper Northeast) was associated with >4% of records.
Among the four data sources used to create our database, records obtained from the Web of Science search of the published literature (13% of all records) collectively had broader and more evenly distributed spatiotemporal coverage compared with those obtained from the NVSL database (33%), the USNTC database (3%), or the VectorMap database (51%). For example, although the total number of states represented by records from each data source was fairly similar, the majority of records from the USNTC, NVSL, and VectorMap databases were clustered within a relatively small number of states: 71% of the records in the USNTC database were associated with six of the database’s 32 states (Arkansas, Florida, Georgia, Mississippi, Oklahoma, and Texas), 69% of NVSL records were associated with 2 of 21 states (Florida and Texas), and 74% of VectorMap records were associated with 3 of 31 states (Maryland, New Jersey, and Virginia). In contrast, none of the 31 states represented in the Web of Science-derived database was associated with >9% of records in that database. Literature-derived records were associated with 87 (76%) of the 115 years represented in the full database compared with 79 (69%), 21 (18%), and 15 (13%) years for the USNTC, NVSL, and VectorMap records, respectively. Because of the relatively more even spatial distribution and broader temporal coverage of literature-derived records, and because these records are most readily accessible to the public, we used records obtained through the Web of Science search to describe spatiotemporal patterns and tick collection methodologies for the data presented in the following sections.
Spatiotemporal Patterns
After all Web of Science-derived classifications for A. americanum at the county and decade level had been made cumulative, there were 3,893 total decadal records for established or reported status. These were summed within regions and decades to examine spatiotemporal trends by individual decade (Supp Fig. 1A [online only]) and cumulatively over time (Supp Fig. 1B [online only]). Between the 1890s and 2000s, 35 and 30% of the records were associated with states in the Southeast and South-Central regions, respectively. The Mid-Atlantic and Midwest regions each accounted for roughly 10%, and none of the three remaining regions (Lower Northeast, Upper Midwest, and Upper Northeast) accounted for 8%. The number of records per decade was low (<22 per region) and fairly consistent among all seven regions from the 1890s until the 1930s, when the number of records in the South-Central and Southeastern regions began to increase (Supp Fig. 1A [online only] and Fig. 2). Beginning in the 1940s, the number of records per decade in the Southeastern region grew exponentially to reach 315 in the 2000s, the highest number of records in that decade across all regions by nearly double (Supp Fig. 1A [online only] and Fig. 2). In the South-Central region, the number of records per decade increased almost fivefold between the 1930s and 1950, but thereafter the decadal increases were slower (by an average factor of 1.6) to peak at 173 records in the 2000s. With the exception of gradual but limited increases in the Mid-Atlantic region, the number of records per decade remained low in other regions between the 1950s and the 1980s, whereas the number of records per decade in the Lower Northeast, Midwest, and Upper Midwest regions increased rapidly in the 1990s and 2000s.
The number of publications reporting collection of A. americanum was at least three in each decade between the 1890s and 1930s, but then rose to ≈20 per decade in the 1940s, 1950s, and 1960s (Fig. 3). Beginning in the 1970s, the number increased gradually by a factor of roughly 1.6 (average of 23 publications) per decade through the 2000s. Relative to a general publication trend during this period in the field of entomology, characterized by searching the Web of Science by decade using the subject word “entomology,” the number of A. americanum-related publications over time deviated from expectation only during the 1940s and 1950s, when there were more publications on this tick species than expected. The number of counties per decade in which A. americanum collections occurred remained low (<30) between the 1890s and 1940s. It spiked to 135 in the 1950s and then fluctuated between 65 and 154 until the 1990s, when it tripled to ≈300. The 2000s saw a further increase of 33% to 399 counties. By comparison, the number of collection records per decade (some publications include multiple collection records) was higher in every decade following the 1940s and increased at a greater and more consistent rate beginning in the 1970s, ending at nearly 750 in the 2000s.
Fig. 3.
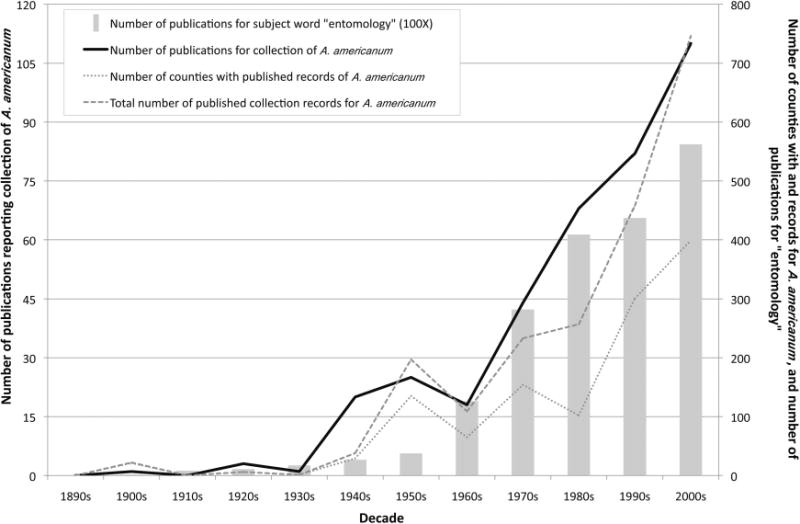
Number of publications and unique county records of collection for A. americanum plotted against decade of publication (based exclusively on records from the published literature). The number of publications (×100) for the subject word “entomology,” determined using a Web of Science literature search, is plotted as a reference trend.
Tick Collection Methodologies Used
The published literature yielded 2,388 database records that could be used to evaluate trends in tick collection methodology. Of these, 1,015 (43%) used flagging or dragging to collect ticks; 708 (30%) involved the collection of ticks from animals (not including deer); 421 and 419 (≈18% each) involved the collection of ticks from deer or humans, respectively; 405 (17%) used CO2 trapping to collect ticks; 61 (3%) were associated with ticks submitted to reference centers; and 351 (15%) failed to report a collection method (note that percentages do not sum to 100, as many studies used multiple methods).
Discussion
We present a compilation of data on field collection records for A. americanum in the continental United States. The resulting map outputs (Fig. 1 and Supp Fig. 1 [online only]) display the spatial distribution of counties in which A. americanum is known to be established or reported, by individual decade and cumulative by decade, from the 1890s to present time. Because these maps present distribution patterns for A. americanum at the county scale, they represent a marked improvement over previous maps of the generalized (smoothed) distribution of the species across the continental United States (http://www.cdc.gov/ ticks/geographic_distribution.html#lone-star, Childs and Paddock 2003, Yabsley 2010). Moreover, as we used the Dennis et al. (1998) scheme for classifying a tick species as established versus reported in a given county, the presented spatial distribution for counties with A. americanum can be compared with that presented for I. scapularis by Dennis et al. (1998). Our map based on cumulative data to present time (Fig. 1) reveals clusters of counties in which A. americanum is classified as established in numerous states in the South-Central and Southeastern regions. Such clusters also occur along the eastern seaboard as far north as New York State. Counties in which A. americanum is classified as reported occur as far north as Michigan’s Upper Peninsula, inland New York State, and Maine and west into north Texas, Nebraska, and Kansas (Fig. 1; Anderson and Magnarelli 1980, Means and White 1997, Keirans and Lacombe 1998, Walker et al. 1998, Ijdo et al. 2000, Mixson et al. 2006). The general impression is of a species with a distributional center in the southern and southeastern parts of the eastern United States but that also occurs further north, especially along the Atlantic Coast and into the Midwest.
The data and maps presented herein have several important limitations that must be considered when attempting to draw conclusions from them. First, they represent compilations of collection records based on convenience sampling rather than systematic surveys for A. americanum. In the absence of systematic sampling efforts, distribution maps for a given species may reflect the intensity of collection efforts rather than the true distribution of the species itself. For example, the map in Fig. 1 displays several clusters of counties in the south without records of A. americanum within what otherwise appears to be and are probably areas of continuous distribution. Conversely, extensive field surveys and compilations of collection records at the state level (e.g., in Nebraska and Iowa, Bartholomew et al. 1995, Lingren et al. 2005, Cortinas and Spomer 2013) result in portions of these states appearing as distinct geographic foci for A. americanum. We consider it likely that, had similar efforts been undertaken in neighboring states to the south or east, Nebraska and Iowa would have appeared less like geographic outliers and more like the northern edge of a continuous distribution. Similarly, while our map and that of Dennis et al. (1998) for I. scapularis shows a paucity of collection records for ticks in an area extending from eastern Virginia and western North Carolina north through West Virginia and into Pennsylvania, our results do not provide insight into whether the absence of A. americanum in these areas is owing to unfavorable environmental conditions or simply a lack of sampling. Related to this and similar to the approach of Dennis et al. (1998), we purposely did not seek information on counties that were sampled but failed to yield A. americanum because of the difficulty in evaluating the sensitivity of associated collection efforts.
Additional limitations of our data include the fact that the records in our database represent a mixture of sampling efforts focusing primarily on A. americanum and others where this species was collected as a byproduct of attempts to collect other ticks species such as I. scapularis or D. variabilis. The latter case introduces an element of uncertainty with regards to the sensitivity of the sampling to detect A. americanum, given variation in phenology and microhabitat preferences among tick species. The collection methods represented in our database, including sampling of host-seeking ticks as well as removal of ticks from humans, domestic animals, and wildlife, vary in their sensitivity to collect ticks, and the predominant collection methodology undoubtedly has varied over time as well as between geographic areas and tick collectors. Finally, A. americanum can locally reach tremendous densities of host-seeking specimens (Hair and Howell 1970, Goddard and Varela-Stokes 2009), and our established versus reported classification scheme fails to capture the variability in tick abundance among the counties where it is considered established.
The aforementioned limitations and associated knowledge gaps underscore the need for future work on A. americanum, similar to that undertaken for I. scapularis, especially involving systematic field sampling to assess tick density across its geographic range and modeling of the predicted density at a fine (sub-county) scale (Diuk-Wasser et al. 2006, 2010). A fundamental contribution of our mapping effort is the identification of specific areas where surveys need to be undertaken to determine whether A. americanum is truly absent or if the perceived absence is simply owing to a lack of sampling or failure of collectors to submit specimens for identification or report collections. Specific geographic areas of interest in-clude—1) counties without records of A. americanum within areas that otherwise appear to be associated with continuous distributions (e.g., Mississippi, Alabama, and Georgia), 2) areas that represent the northern margin of the perceived distribution of A. americanum in the Northeast (e.g., Massachusetts, New Hampshire, and Vermont) and Upper Midwest (e.g., Wisconsin and Minnesota), 3) areas directly to the west of the distributional belt along the Mid-Atlantic coast (e.g., western areas of Pennsylvania and Virginia, West Virginia), and 4) areas that appear to represent the species’ western and northwestern range limits (e.g., South Dakota, Nebraska, and Kansas). Maps could subsequently be further enhanced through the addition of data on spatial variation in the abundance and density of A. americanum across its range.
The series of decadal and cumulative maps from the 1890s to present time for counties in which A. americanum is classified as established or reported (Supp Fig. 1 [online only]) shows an intriguing spatiotemporal pattern but must be interpreted with knowledge of general trends for collection of host-seeking ticks. For example, sharp increases in collection records for A. americanum can be expected to have occurred not only following the recognition in the 1990s of the important role of this tick species as a vector of E. chaffeensis (Yabsley 2010), but also following the recognition of vector status for notable pathogens of other tick species that co-occur with A. americanum. Examples of events that spurred substantial field efforts aiming to collect other tick species (host-seeking and attached to hosts) but also resulted in the collection of co-occurring A. americanum include the recognitions in the 1930s that D. variabilis is a vector of the causative agent of Rocky Mountain spotted fever (Parker et al. 1933) and in the 1980s that I. scapularis is a vector of the causative agent of Lyme disease in North America (Burgdorfer 1984, Piesman and Sinsky 1988). Thus, the notable increases in A. americanum records in the South-Central and Southeastern regions during the 1940s, 1950s, and 1960s (Supp Fig. 1A [on-line only]) probably reflect an increase in tick collection efforts focusing on D. variabilis, whereas the increases in A. americanum records in the Upper Mid-west and Lower Northeast during the 1990s (Supp Fig. 1A [online only]) likely were driven in large part by work that focused on I. scapularis. The factors driving the selection of tick survey locations during particular time periods should be kept in mind when considering the environmental and climatic determinants of A. americanum distribution and abundance.
Although our decadal maps are suggestive of a northward shift in the distribution of A. americanum in recent decades, more definitive evidence is needed to confirm such a shift. Such evidence could be pursued by repeating field sampling from previous decades in the same localities and with the same collection methods. In this respect, Ginsberg et al. (1991, 2002) noted that A. americanum comprised a greater proportion of total ticks flagged on Long Island and Fire Island, NY, in 1986, 1990, and 1994–2000 than in samples reported from previous studies in the 1940s (when A. americanum was not collected) and the 1970s when it occurred but was relatively less abundant than other species (Anastos 1947; Collins et al. 1949a,b; Good 1972, 1973).
Critical factors influencing the potential for A. americanum to establish and proliferate have been reviewed previously (Hair and Howell 1970, Childs and Paddock 2003, Paddock and Yabsley 2007) and include climate conditions, the presence of suitable habitat for tick development and host-seeking, and abundant populations of key vertebrate hosts. Second growth woodland with dense underbrush—which not only offer favorable temperature and humidity conditions for lone star ticks but are also preferred habitat of their most important host, the white-tailed deer (Clymer et al. 1970; Hoch et al. 1971; Semtner et al. 1971a,b; Smith 1977; Bloemer et al. 1988; Goddard and McHugh 1990)—is the habitat in which A. americanum tends to be most abundant (Hair and Howell 1970, Patrick and Hair 1978, Hair and Bowman 1986). This habitat occurs commonly throughout the forested portions of the eastern United States today. Indeed, extensive deforestation and hunting of deer during the 18th and 19th centuries in the eastern United States, followed by reforestation and subsequent increases in the abundance of white-tailed deer, may be the most important factors to explain the spatiotemporal patterns of A. americanum abundance over the last centuries (reviewed by Childs and Paddock 2003, Paddock and Yabsley 2007).
Reports from the mid-18th century of A. americanum occurring in abundance as far north as New York State (Fitch 1870) suggest that the species may now be in the process of reclaiming its historical range in the Upper Midwest and Northeast in the wake of reforestation and increasing deer populations. Future work to clarify the interplay of habitat characteristics, changes in land use patterns, deer abundance, and climate in determining the local abundance of A. americanum would be helpful in improving both targeted surveillance of this tick species and associated disease risk. Transect surveys extending from locations defined herein as representing the core current distributional area of the lone star tick into its northern range limits in the Midwest, Upper Midwest, and northeastern United States would be especially valuable.
Supplementary Material
Acknowledgments
We thank D. Gudex-Cross for help with location verification of geocoded collection records, R. Cortinas for discussions about the distribution of A. americanum in Nebraska, and staff of the NVSL Pathobiology laboratory for tick identification records included in CEAH’s tick geodatabase. L. Beati was supported by National Science Foundation (NSF) grant 1026146.
References Cited
- Anastos G. Hosts of certain New York ticks. Psyche. 1947;54:178–180. [Google Scholar]
- Anderson JF, Magnarelli LA. Vertebrate host relationships and distribution of ixodid ticks (Acari: Ixo-didae) in Connecticut, USA. J Med Entomol. 1980;17:314–323. doi: 10.1093/jmedent/17.4.314. [DOI] [PubMed] [Google Scholar]
- Barbour AG, Maupin GO, Teltow GJ, Carter CJ, Piesman J. Identification of an uncultivable Borrelia species in the hard tick Amblyomma americanum: possible agent of a Lyme disease-like illness. J Infect Dis. 1996;173:403–409. doi: 10.1093/infdis/173.2.403. [DOI] [PubMed] [Google Scholar]
- Bartholomew DM, Rowley WA, Novak MG, Platt KB. Ixodes scapularis and other ticks (Acari: Ixodidae) associated with Lyme disease in Iowa. J Vector Ecol. 1995;20:1–6. [Google Scholar]
- Bishopp FC, Trembley HL. Distribution and hosts of certain North American ticks. J Parasitol. 1945;31:1–54. [Google Scholar]
- Bloemer SR, Zimmerman RH, Fairbanks K. Abundance, attachment sites, and density estimators of lone star ticks (Acari: Ixodidae) infesting white-tailed deer. J Med Entomol. 1988;25:295–300. doi: 10.1093/jmedent/25.4.295. [DOI] [PubMed] [Google Scholar]
- Breitschwerdt EB, Hegarty BC, Maggi RG, Lantos PM, Aslett DM, Bradley JM. Rickettsia rickettsii transmission by a lone star tick, North Carolina. Emerg Infect Dis. 2011;17:873–875. doi: 10.3201/eid1705.101530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W. The New Zealand white rabbit; an experimental host for infecting ticks with Lyme disease spirochetes. Yale J Biol Med. 1984;57:609–612. [PMC free article] [PubMed] [Google Scholar]
- Childs JE, Paddock CD. The ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Annu Rev Entomol. 2003;48:307–337. doi: 10.1146/annurev.ento.48.091801.112728. [DOI] [PubMed] [Google Scholar]
- Clymer BC, Howell DE, Hair JA. Animal hosts of economically important ticks (Acarina) in east-central Oklahoma. Ann Entomol Soc Am. 1970;63:612–613. [Google Scholar]
- Collins DL, Nardy RV, Glasgow RD. Some host relationships of Long Island ticks. J Econ Entomol. 1949a;42:110–112. doi: 10.1093/jee/42.1.110. [DOI] [PubMed] [Google Scholar]
- Collins DL, Nardy RV, Glasgow RD. Further notes on the host relationships of ticks on Long Island. J Econ Entomol. 1949b;42:159–160. doi: 10.1093/jee/42.1.159. [DOI] [PubMed] [Google Scholar]
- Cooley RA, Kohls GM. The genus Amblyomma in the United States. J Parasitol. 1944;30:77–111. [Google Scholar]
- Cortinas R, Spomer S. Lone star tick (Acari: Ixodidae) occurrence in Nebraska: historical and current perspectives. J Med Entomol. 2013;50:243–251. doi: 10.1603/me12207. [DOI] [PubMed] [Google Scholar]
- Dennis DT, Nekomoto TS, Victor JC, Paul WS, Piesman J. Reported distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the United States. J Med Entomol. 1998;35:629–638. doi: 10.1093/jmedent/35.5.629. [DOI] [PubMed] [Google Scholar]
- Diuk-Wasser MA, Gatewood AG, Cortinas MR, Yaremych-Hamer S, Tsao J, Kitron U, Hickling G, Brownstein JS, Walker E, Piesman J, et al. Spatio-temporal patterns of host-seeking Ixodes scapularis nymphs (Acari: Ixodidae) in the United States. J Med Entomol. 2006;43:166–176. doi: 10.1603/0022-2585(2006)043[0166:spohis]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Diuk-Wasser MA, Vourc’h G, Cislo P, Hoen AG, Melton F, Hamer SA, Rowland M, Cortinas R, Hickling GJ, Tsao JI, et al. Field and climate-based model for predicting the density of host-seeking nymphal Ixodes scapularis, an important vector of tick-borne disease agents in the eastern United States. Glob Ecol Biogeogr. 2010;19:504–514. [Google Scholar]
- Felz MW, Durden LA, Oliver JH., Jr Ticks parasitizing humans in Georgia and South Carolina. J Parasitol. 1996;82:50–508. [PubMed] [Google Scholar]
- Fitch A. Fourteenth report on the noxious, beneficial and other insects of the state of New York. Trans N Y State Agric Soc. 1870;30:355–381. [Google Scholar]
- Fritzen CM, Huang JJ, Westby K, Freye JD, Dunlap B, Yabsley MJ, Schardein M, Dunn JR, Jones TF, Moncayo AC. Infection prevalences of common tick-borne pathogens in adult lone star ticks (Amblyomma americanum) and American dog ticks (Dermacentor variabilis) in Kentucky. Am J Trop Med Hyg. 2011;85:718–723. doi: 10.4269/ajtmh.2011.10-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg HS, Ewing CP, Oconnell AF, Bosler EM, Daley JG, Sayre MW. Increased population densities of Amblyomma americanum (Acari, Ixodidae) on Long Island, New York. J Parasitol. 1991;77:493–495. [PubMed] [Google Scholar]
- Ginsberg HS, Butler M, Zhioua E. Effect of deer exclusion by fencing on abundance of Amblyomma americanum (Acari: Ixodidae) on Fire Island, New York, USA. J Vector Ecol. 2002;27:215–221. [PubMed] [Google Scholar]
- Goddard J. Experimental infection of lone star ticks, Amblyomma americanum (L.), with Rickettsia parkeri and exposure of guinea pigs to the agent. J Med Entomol. 2003;40:686–689. doi: 10.1603/0022-2585-40.5.686. [DOI] [PubMed] [Google Scholar]
- Goddard J, McHugh CP. Impact of a severe tick infestation at Little Rock AFB, Arkansas on Volant Scorpion military training. Mil Med. 1990;155:277–280. [PubMed] [Google Scholar]
- Goddard J, Varela-Stokes AS. Role of the lone star tick, Amblyomma americanum (L.), in human and animal diseases. Vet Parasitol. 2009;160:1–12. doi: 10.1016/j.vetpar.2008.10.089. [DOI] [PubMed] [Google Scholar]
- Good NE. Tick locality and host records from Long Island and southeastern New York State. Entomol News. 1972;83:165–168. [PubMed] [Google Scholar]
- Good NE. Ticks of eastern Long Island: notes on host relations and seasonal distribution. Ann Entomol Soc Am. 1973;66:240–243. [Google Scholar]
- Hair JA, Bowman JL. Behavioral ecology of Amblyomma americanum (L) In: Hair JA, Sauer JR, editors. Morphology, physiology, and behavioral biology of ticks. Ellis Horwood Limited; Chichester: 1986. pp. 406–427. [Google Scholar]
- Hair JA, Howell DE. Lone star ticks: their biology and control in Ozark recreation areas. Okla State Univ Agric Exp Stat Bull. 1970;B-679:1–47. [Google Scholar]
- Hoch AL, Hair JA, Barker RW. Measurement of physical parameters to determine suitability of modified woodlots as lone star tick habitat. J Med Entomol. 1971;8:725–730. doi: 10.1093/jmedent/8.6.725. [DOI] [PubMed] [Google Scholar]
- Hopla CE. Experimental studies on tick transmission of tularemia organisms. Am J Hyg. 1953;58:101–118. doi: 10.1093/oxfordjournals.aje.a119585. [DOI] [PubMed] [Google Scholar]
- Ijdo JW, Wu CY, Magnarelli LA, Stafford KC, Anderson JF, Fikrig E. Detection of Ehrlichia chaffeensis DNA in Amblyomma americanum ticks in Connecticut and Rhode Island. J Clin Microbiol. 2000;38:4655–4656. doi: 10.1128/jcm.38.12.4655-4656.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirans JE, Lacombe EH. First records of Amblyomma americanum, Ixodes (Ixodes) dentatus, and Ixodes (Ceratixodes) uriae (Acari: Ixodidae) from Maine. J Parasitol. 1998;84:629–631. [PubMed] [Google Scholar]
- Lingren A, Rowley WA, Thompson C, Gilchrist M. Geographic distribution of ticks (Acari: Ixodidae) in Iowa with emphasis on Ixodes scapularis and their infection with Borrelia burgdorferi. Vector-borne Zoonotic Dis. 2005;5:219–226. doi: 10.1089/vbz.2005.5.219. [DOI] [PubMed] [Google Scholar]
- Loftis AD, Reeves WK, Spurlock JP, Mahan SM, Troughton DR, Dasch GA, Levin ML. Infection of a goat with a tick-transmitted Ehrlichia from Georgia, USA, that is closely related to Ehrlichia ruminantium. J Vector Ecol. 2006;31:213–223. doi: 10.3376/1081-1710(2006)31[213:ioagwa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Masters EJ, Grigery CN, Masters RW. STARI, or Masters disease: lone star tick-vectored Lyme-like illness. Infect Dis Clin N Am. 2008;22:361–376. doi: 10.1016/j.idc.2007.12.010. [DOI] [PubMed] [Google Scholar]
- McMullan LK, Folk SM, Kelly AJ, MacNeil A, Goldsmith CS, Metcalfe MG, Batten BC, Albarino CG, Zaki SR, Rollin PE, et al. A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med. 2012;367:834–841. doi: 10.1056/NEJMoa1203378. [DOI] [PubMed] [Google Scholar]
- Means RG, White DJ. New distribution records of Amblyomma americanum (L.) (Acari: Ixodidae) in New York State. J Vector Ecol. 1997;22:133–145. [PubMed] [Google Scholar]
- Merten HA, Durden LA. A state-by-state survey of ticks recorded from humans in the United States. J Vector Ecol. 2000;25:102–113. [PubMed] [Google Scholar]
- Mixson TR, Campbell SR, Gill JS, Ginsberg HS, Reichard MV, Schulze TL, Dasch GA. Prevalence of Ehrlichia, Borrelia, and Rickettsial agents in Amblyomma americanum (Acari: Ixodidae) collected from nine states. J Med Entomol. 2006;43:1261–1268. doi: 10.1603/0022-2585(2006)43[1261:poebar]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Paddock CD, Yabsley MJ. Wildlife and emerging zoonotic diseases: the biology, circumstances and consequences of cross-species transmission. Vol. 315. Springer Berlin; Berlin: 2007a. Ecological havoc, the rise of white-tailed deer, and the emergence of Amblyomma americanum – associated zoonoses in the United States; pp. 289–324. [DOI] [PubMed] [Google Scholar]
- Parker RR, Philip CB, Jellison WL. Potentialities of tick transmission in relation to geographical occurrence in the United States. Am J Trop Med Hyg. 1933;13:341–379. [Google Scholar]
- Patrick CD, Hair JA. White-tailed deer utilization of three different habitats and its influence on lone star tick populations. J Parasitol. 1978;64:1100–1106. [Google Scholar]
- Piesman J, Eisen L. Prevention of tick-borne diseases. Annu Rev Entomol. 2008;53:323–43. doi: 10.1146/annurev.ento.53.103106.093429. [DOI] [PubMed] [Google Scholar]
- Piesman J, Sinsky RJ. Ability of Ixodes scapularis, Dermacentor variabilis, and Amblyomma americanum (Acari: Ixodidae) to acquire, maintain, and transmit Lyme disease spirochetes (Borrelia burgdorferi) J Med Entomol. 1988;25:336–339. doi: 10.1093/jmedent/25.5.336. [DOI] [PubMed] [Google Scholar]
- Reichard MV, Edwards AC, Meinkoth JH, Snider TA, Meinkoth KR, Heinz RE, Little SE. Confirmation of Amblyomma americanum (Acari: Ixodidae) as a vector for Cytauxzoon felis (Piroplasmorida: Theile-riidae) to domestic cats. J Med Entomol. 2010;47:890–896. doi: 10.1603/me10013. [DOI] [PubMed] [Google Scholar]
- Semtner PJ, Barker RW, Hair JA. Ecology and behavior of lone star tick (Acarina: Ixodidae). 2. Activity and survival in different ecological habitats. J Med Entomol. 1971a;8:719–725. doi: 10.1093/jmedent/8.6.719. [DOI] [PubMed] [Google Scholar]
- Semtner PJ, Howell DE, Hair JA. Ecology and behavior of lone star tick (Acarina: Ixodidae. 1. Relationship between vegetative habitat type and tick abundance and distribution in Cherokee Co., Oklahoma. J Med Entomol. 1971b;8:329–335. doi: 10.1093/jmedent/8.3.329. [DOI] [PubMed] [Google Scholar]
- Smith JS. A survey of ticks infesting white-tailed deer in 12 southeastern states. M.S. thesis, University of Georgia, Athens, GA 1977 [Google Scholar]
- Stromdahl EY, Hickling GJ. Beyond Lyme: aetiology of tick-borne human diseases with emphasis on the south-eastern United States. Zoonoses Public Health. 2012;59:48–64. doi: 10.1111/j.1863-2378.2012.01475.x. [DOI] [PubMed] [Google Scholar]
- Stromdahl EY, Williamson PC, Kollars TM, Evans SR, Barry RK, Vince MA, Dobbs NA. Evidence of Borrelia lonestari DNA in Amblyomma americanum (Acari: Ixodidae) removed from humans. J Clin Microbiol. 2003;41:5557–5562. doi: 10.1128/JCM.41.12.5557-5562.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Office of Management and Budget. Circular A-105: Standard Federal Regions, Executive Office of the President, Office of Management and Budget. 1974:1–6. [Google Scholar]
- Varela-Stokes AS. Transmission of bacterial agents from lone star ticks to white-tailed deer. J Med Entomol. 2007;44:478–483. doi: 10.1603/0022-2585(2007)44[478:tobafl]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Walker ED, Stobierski MG, Poplar ML, Smith TW, Murphy AJ, Smith PC, Schmitt SM, Cooley TM, Kramer CM. Geographic distribution of ticks (Acari: Ixodidae) in Michigan, with emphasis on Ixodes scapularis and Borrelia burgdorferi. J Med Entomol. 1998;35:872–882. doi: 10.1093/jmedent/35.5.872. [DOI] [PubMed] [Google Scholar]
- Yabsley MJ. Natural history of Ehrlichia chaffeensis: vertebrate hosts and tick vectors from the United States and evidence for endemic transmission in other countries. Vet Parasitol. 2010;167:136–148. doi: 10.1016/j.vetpar.2009.09.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


