Figure 1.
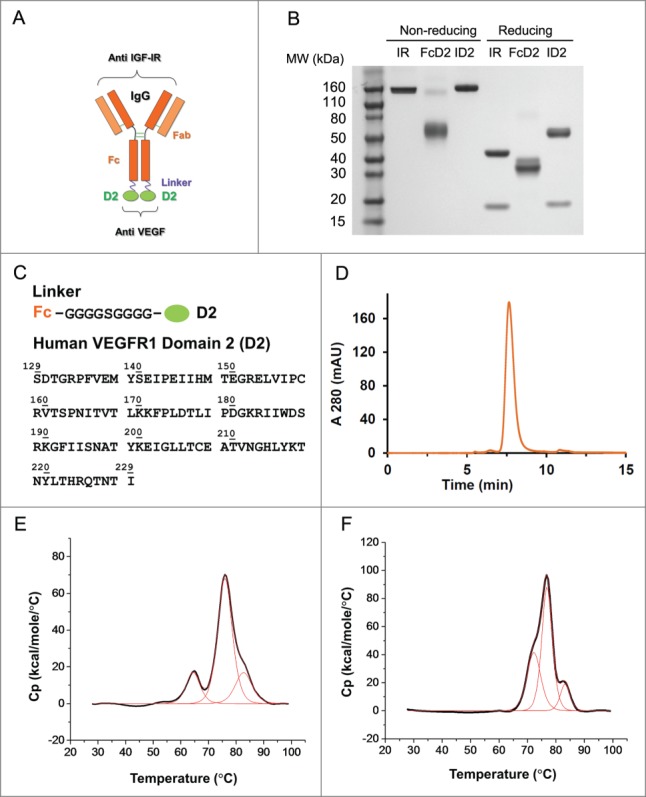
Design and molecular structure of bi-functional antibody receptor domain fusion molecule with VEGF capture (bi-AbCap). (A) Cartoon illustration of the designed bi-AbCap fusion molecule. Orange: Anti IGF-IR IgG backbone, IR mAb; Purple: polypeptide linker; Green: extracellular domain 2 of human VEGFR1 (D2). An FcD2 control molecule was made containing the regions of the hinge, Fc, linker and D2. (B) SDS-PAGE of IR mAb, FcD2 and bi-AbCap ID2. (C) Top: linker sequence: G4SG4; Bottom: the D2 domain used for fusion encompassing amino acids 129–229 of hVEGFR1. (D) ID2 elutes as a mono-disperse peak by size exclusion chromatograpy (SEC). (E) ID2 displays 3 melting temperatures (Tm) when the thermal stability of ID2 measured by differential scanning calorimetry (DSC). Tm1 = 64.4 ± 0.0°C, Tm2 = 76.0 ± 0.0°C, Tm3 = 82.7 ± 0.1°C. (F) IR mAb displays 3 Tms by DSC: Tm1 = 72.2 ± 0.1°C, Tm2 = 76.8 ± 0.0°C, Tm3 = 83.2 ± 0.1°C.
