Abstract
Hemophagocytic lymphohistiocytosis (HLH) is characterized by fever, splenomegaly, jaundice, and pathologic findings of hemophagocytosis in bone marrow or other tissues such as the lymph nodes and liver. Pleocytosis, or the presence of elevated protein levels in cerebrospinal fluid, could be helpful in diagnosing HLH. However, the pathologic diagnosis of the brain is not included in the diagnostic criteria for this condition. In the present report, we describe the case of a patient diagnosed with HLH, in whom the brain pathology, but not the bone marrow pathology, showed hemophagocytosis. As the diagnosis of HLH is difficult in many cases, a high level of suspicion is required. Moreover, the pathologic diagnosis of organs other than the bone marrow, liver, and lymph nodes may be a useful alternative.
Keywords: Hemophagocytic lymphohistiocytosis, Central nervous system, Brain
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a systemic disease characterized by hemophagocytic syndrome accompanied by fever, hepatosplenomegaly, jaundice, cytopenia, low fibrinogen level, high ferritin level, and hypertriglyceridemia. HLH comprises two different conditions that may be indistinguishable. Familial HLH is a group caused by the mutation of the gene encoding perforin (PRF1)1) or UNC13D gene2), STX11 gene3), or others. Secondary HLH is a group that develops as a result of strong immunological activation after either infection, malignancy, or rheumatoid disorders.
According to the HLH-2004 diagnostic guideline, diagnosis of HLH is done by clinical findings and laboratory results. Such diagnosis is supported by hemophagocytosis in the bone marrow or other tissues such as spleen, liver, or lymph node4). However, in atypical cases that do not fulfill the diagnostic criteria of HLH, histiocytes or macrophages found in the cerebrospinal fluid (CSF) can be helpful in the diagnosis. This report describes a rare HLH case who presented with prolonged fever and seizures, whose brain pathology but not bone marrow pathology showed hemophagocytosis. The institutional review board of Seoul National University Hospital approved this study (E-1307-039-501).
Case report
A 5-year-old boy visited Seoul National University Children's Hospital (SNUCH) in August 2010 with fever for six weeks and intermittent seizure for five weeks. The patient had visited another hospital five weeks ago with fever and generalized-tonic-clonic seizures. On initial CSF examination, no specific abnormalities were found: four white blood cell (WBC)/mm3, protein 15 mg/dL, glucose 65 mg/dL. T2 weighted brain magnetic resonance imaging (MRI) showed high signal intensity at the right internal capsule area (Fig. 1A). Initially he was treated with antiviral agents and antiepileptic drugs (AEDs) under the assumption of encephalitis. Neutropenia developed one week after admission, and left cervical lymph node enlargement was found three weeks after admission. Four weeks after the admission, abdominal distension developed and hepatosplenomegaly was found.
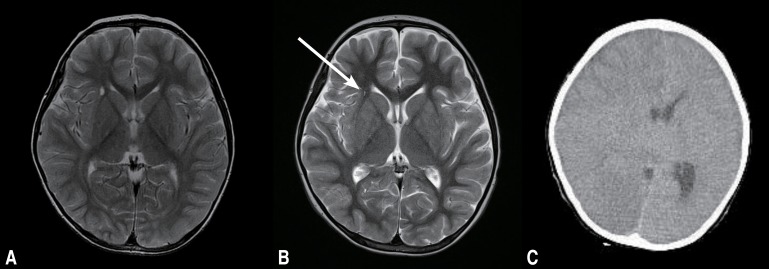
Fig. 1. Brain magnetic resonance imaging findings. (A) The initial brain magnetic resonance image (transverse T2-weighted image) shows focal high signal intensity at the anterior limb of the right internal capsule, but no abnormal focal lesion in the brain parenchyma. (B) Brain magnetic resonance image (transverse T2-weighted image) obtained after one month of disease progression. The area of the high signal intensity lesionlesion (arrow) had decreased, but it was still present. (C) Brain computed tomography performed at the 2nd hospital day showed severe bilateral cerebral edema with consequent transtentorial herniation and basal cistern obliteration. Linear high density along the falx and a low attenuation lesion in the right frontal lobe were also observed. Emergent craniotomy and lesionectomy were performed for control of increased intracranial pressure.
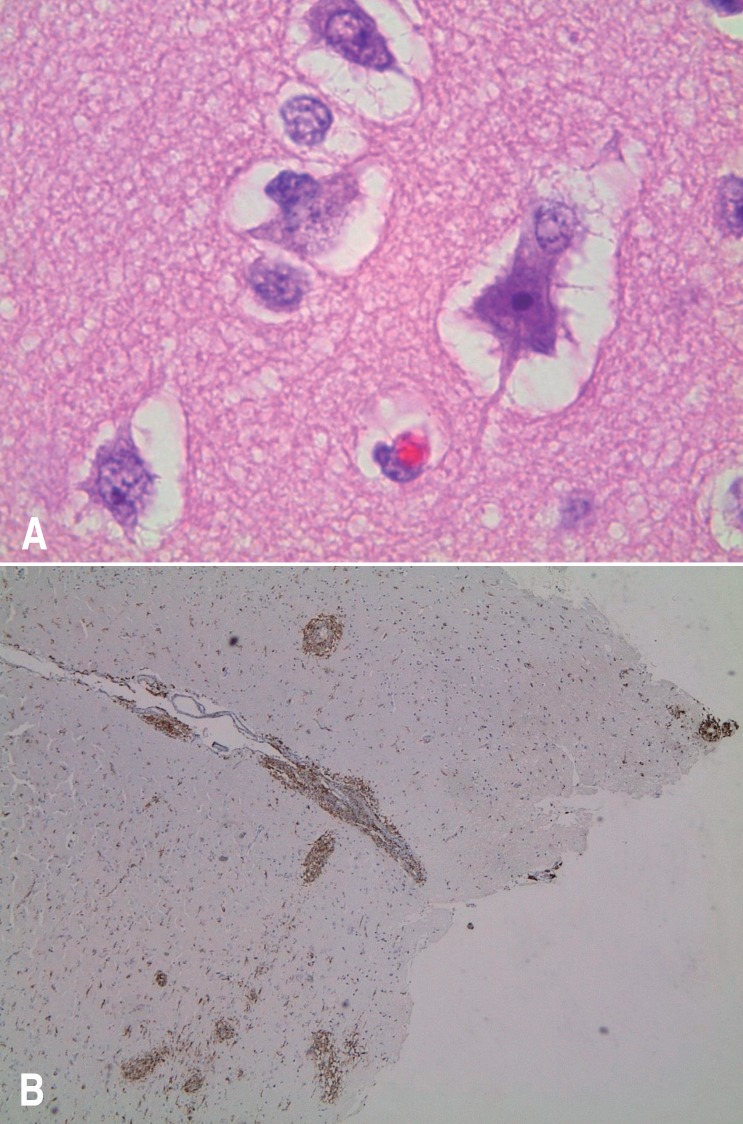
The patient was transferred to SNUCH with seizures and fever persisting for one month. Laboratory findings showed anemia and thrombocytopenia with increased reticulocyte count, elevated aspartate aminotransferase, alanine aminotransferase, and C-reactive protein. Ferritin level was 15,671 ng/mL, fibrinogen level was 139 mg/dL, and triglycerides level was 285 mg/dL. Peripheral blood cell morphology showed left-shifted neutrophils, atypical lymphocytes, toxic neutrophils, and vacuolated neutrophils. Epstein-Barr virus (EBV) IgG antibody was positive, while IgM antibody was negative. His follow-up brain MRI done on the day of admission showed decreased size of focal lesion with high signal intensity at the anterior limb of right internal capsule (Fig. 1B). By the second hospital day, he showed altered mentality with anisocoric pupil and sluggish light reflexes. Brain computed tomography showed severe bilateral cerebral edema and consequent transtentorial herniation (Fig. 1C). To control intracranial pressure, emergent craniotomy with decompressive right temporal lobectomy was performed. Under the suspicion of HLH, bone marrow examination was done, which showed hypercellular marrow with increased number of megakaryocytes. However, no evidence of HLH was found. EBV polymerase chain reaction was found to be negative. On brain pathology, there was perivascular and leptomeningeal lymphohistiocytic infiltration with hemophagocytosis and some red neurons with parenchymal hemorrhage. CD68 immunohistochemistry revealed perivascular and leptomeningeal histiocytic infiltration, which were consistent with HLH (Fig. 2). There was no mutation detected on the gene analysis of PRF1 and UNC13D. On the basis of clinical features, the patient was diagnosed with HLH. Hemophagocytosis found in the brain biopsy specimen supported such diagnosis. The etiology is not confirmed but secondary HLH might be more possible given his age and clinical course.
Fig. 2. Pathological findings. (A) A hemophagocyte observed in the brain specimen (temporal lobe) (H&E, ×1,000). (B) CD68 immunohistochemistry indicated perivascular and leptomeningeal histiocytic infiltration (×40).
Under the diagnosis of HLH, the patient was treated with chemotherapy of HLH-2004 regimen4) suggested by the Histiocyte Society which is based on etoposide, dexamethasone, cyclosporine A, and intrathecal chemotherapy with methotrexate and prednisolone in selected patients. In addition, CSF analysis and intrathecal chemotherapy was done at two days after the initiation of the HLH-2004 regimen due to brain involvement of HLH. CSF findings showed one WBC/mm3, and the protein level of CSF was 126 mg/dL. Intrathecal methotrexate and hydrocortisone were administered four times in total. In the last follow-up CSF analysis (done after the 4th intrathecal chemotherapy), CSF protein level was decreased to 93 mg/dL and WBC level was 2/mm3. After eight weeks of initial therapy, his mentality improved but left side hemiplegia persisted with motor power grade III. Seizures were controlled with AEDs during the chemotherapy.
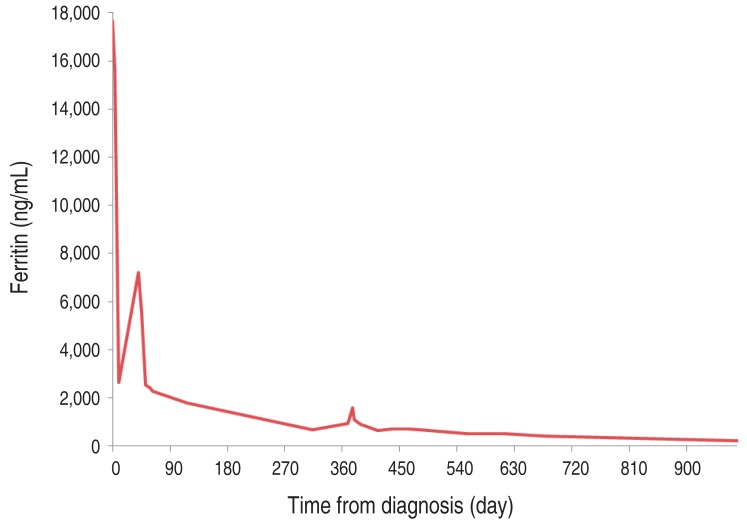
There was no evidence of HLH reactivation for over 28 months after completion of chemotherapy, and his ferritin improved significantly (Fig. 3). Now he is being followed up with AEDs for seizure controls and his left hemiplegia is much improved.
Fig. 3. Trends in the ferritin level.
Discussion
Until now, there was one report about neuropathologic findings of central nervous system (CNS)-involved HLH patients confirmed with autopsy specimen5). In addition, there was a report of HLH which involved only CNS with pathological confirmation, without other systemic symptoms6). Most recently, there was a report of two CNS-involved HLH cases in Korea7). In current case, HLH was diagnosed on the basis of clinical features and lab findings. The unique finding of this study was that bone marrow pathology did not show the features of HLH, but the pathology of brain showed hemophagocytosis, supporting the diagnosis. The patient's initial ferritin level was 15,671 ng/mL, in accordance with the result of a recent report which showed ferritin levels above 10,000 ng/mL are specific and sensitive for HLH8). Elevated protein level of CSF also supported the diagnosis of HLH9).
On the basis of the present report, we suggest that the diagnostic criteria of HLH-2004 may need some updating. In some cases, the diagnostic criteria cannot be fulfilled even if HLH is strongly suspected clinically. A recent study showed that hemophagocytosis of bone marrow specimen has 83% sensitivity and 60% specificity only10), and another report showed HLH diagnosed after repetitive bone marrow biopsy11). Furthermore, some laboratory exams (e.g., soluble CD25 and NK-cell activity) are not available in many hospitals, making the diagnosis more difficult. In these circumstances, if hemophagocytosis is confirmed at organs other than bone marrow, liver and lymph node, it can be helpful in the diagnosis of HLH. HLH is known to be a fatal disease that has a high mortality rate if the diagnosis is delayed. Prompt recognition and early treatment are significant for HLH patient recovery. Therefore, the confirmation of hemophagocytosis in other organs may be helpful in the diagnosis of HLH when other clinical findings support.
CNS involvement is common in HLH, and the manifestation is highly variable: seizure, hemiplegia or tetraplegia, ataxia, cranial nerve palsy, signs of increased intracranial pressure, and coma. Thus, when a patient shows unexplained neurologic manifestations, HLH should also be considered in the differential diagnosis. However, reports showed that some HLH patients do not have neurological symptoms or signs but do have neuroradiological abnormal findings. These findings imply that neuroradiological abnormality may precede apparent clinical neurological manifestations12). HLH with CNS involvement has been reported in up to 50% of patients, and they have worse outcome with a poorer 5-year survival rate13). Therefore, evaluation for CNS should be done when HLH is diagnosed. CSF analysis is included in the HLH-2004 guideline, and the brain MRI is also recommended when reactivation or new onset of HLH. In addition, the present authors suggest that the brain MRI should be considered at the initial diagnosis.
In conclusion, this paper reports a case of successfully treated HLH, which presented with sustained high fever and seizure. The patient's brain pathology showed hemophagocytosis, although bone marrow did not represent the hallmark of HLH. From the experience of the present case, it is recommended that the diagnosis of HLH can be assisted with pathologic confirmation of tissues other than bone marrow, liver, and lymph node if the patient's clinical findings support HLH. Additionally, neuroradiologic study and CSF analysis should be considered at initial diagnosis of HLH because it can reveal the HLH involvement of brain even in the cases without neurological symptom.
Acknowledgments
This study was supported by grant no 04-2012-0580 from SNUH Research Fund.
Footnotes
Conflicts of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Stepp SE, Dufourcq-Lagelouse R, Le Deist F, Bhawan S, Certain S, Mathew PA, et al. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science. 1999;286:1957–1959. doi: 10.1126/science.286.5446.1957. [DOI] [PubMed] [Google Scholar]
- 2.Feldmann J, Callebaut I, Raposo G, Certain S, Bacq D, Dumont C, et al. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3) Cell. 2003;115:461–473. doi: 10.1016/s0092-8674(03)00855-9. [DOI] [PubMed] [Google Scholar]
- 3.zur Stadt U, Schmidt S, Kasper B, Beutel K, Diler AS, Henter JI, et al. Linkage of familial hemophagocytic lymphohistiocytosis (FHL) type-4 to chromosome 6q24 and identification of mutations in syntaxin 11. Hum Mol Genet. 2005;14:827–834. doi: 10.1093/hmg/ddi076. [DOI] [PubMed] [Google Scholar]
- 4.Henter JI, Horne A, Arico M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124–131. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 5.Henter JI, Nennesmo I. Neuropathologic findings and neurologic symptoms in twenty-three children with hemophagocytic lymphohistiocytosis. J Pediatr. 1997;130:358–365. doi: 10.1016/s0022-3476(97)70196-3. [DOI] [PubMed] [Google Scholar]
- 6.Shinoda J, Murase S, Takenaka K, Sakai N. Isolated central nervous system hemophagocytic lymphohistiocytosis: case report. Neurosurgery. 2005;56:187. [PubMed] [Google Scholar]
- 7.Baek HJ, Kook H, Han DK, Lee MC, Jeong TW, Hwang TJ. Unrelated stem cell transplantation after reduced-intensity conditioning plus rituximab for Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis with CNS involvement. Korean J Pediatr. 2009;52:725–729. [Google Scholar]
- 8.Allen CE, Yu X, Kozinetz CA, McClain KL. Highly elevated ferritin levels and the diagnosis of hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2008;50:1227–1235. doi: 10.1002/pbc.21423. [DOI] [PubMed] [Google Scholar]
- 9.Deiva K, Mahlaoui N, Beaudonnet F, de Saint Basile G, Caridade G, Moshous D, et al. CNS involvement at the onset of primary hemophagocytic lymphohistiocytosis. Neurology. 2012;78:1150–1156. doi: 10.1212/WNL.0b013e31824f800a. [DOI] [PubMed] [Google Scholar]
- 10.Goel S, Polski JM, Imran H. Sensitivity and specificity of bone marrow hemophagocytosis in hemophagocytic lymphohistiocytosis. Ann Clin Lab Sci. 2012;42:21–25. [PubMed] [Google Scholar]
- 11.Chiapparini L, Uziel G, Vallinoto C, Bruzzone MG, Rovelli A, Tricomi G, et al. Hemophagocytic lymphohistiocytosis with neurological presentation: MRI findings and a nearly miss diagnosis. Neurol Sci. 2011;32:473–477. doi: 10.1007/s10072-010-0467-2. [DOI] [PubMed] [Google Scholar]
- 12.Horne A, Trottestam H, Arico M, Egeler RM, Filipovich AH, Gadner H, et al. Frequency and spectrum of central nervous system involvement in 193 children with haemophagocytic lymphohistiocytosis. Br J Haematol. 2008;140:327–335. doi: 10.1111/j.1365-2141.2007.06922.x. [DOI] [PubMed] [Google Scholar]
- 13.Kim MM, Yum MS, Choi HW, Ko TS, Im HJ, Seo JJ, et al. Central nervous system (CNS) involvement is a critical prognostic factor for hemophagocytic lymphohistiocytosis. Korean J Hematol. 2012;47:273–280. doi: 10.5045/kjh.2012.47.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]