Abstract
Reactive oxygen species (ROS) accumulate at the tip of growing pollen tubes. In Arabidopsis, NADPH oxidases RbohH and RbohJ are localized at the plasma membrane of pollen tube tip and produce ROS in a Ca2+-dependent manner. The ROS produced by Rbohs and Ca2+ presumably play a critical role in the positive feedback regulation that maintains the tip growth. Ultrastructural cytochemical analysis revealed ROS accumulation in the apoplast/cell wall of the pollen grains on the stigmatic papillae in the wild type, but not in the rbohH rbohJ double mutant, suggesting that apoplastic ROS derived from RbohH and RbohJ are involved in pollen tube elongation into the stigmatic papillae by affecting the cell wall metabolism.
Keywords: apoplast, arabidopsis thaliana, pollen tubes, respiratory burst oxidase homolog, reactive oxygen species
ROS Accumulate in the Apoplast of Pollen Tube Tip Upon Pollination
Reproduction is a central process for organisms to transfer the genetic information to the next generation. In higher plants, pollen grains set on the stigmatic papillae and germinate into the pistil, transport the male nuclei to ovules. Pollen tubes grow by tip growth, where the growth site is restricted to the tip, forming the long tubular structure.1 Reactive oxygen species (ROS) accumulate at the tip of growing pollen tubes and 2 NADPH oxidases, respiratory burst oxidase homolog H (RbohH) and RbohJ, specifically localized in the plasma membrane of the growing tip2 are involved in this accumulation.2-4 The ROS are suggested to stimulate the plasma membrane Ca2+-permeable channel(s) and induce Ca2+ influx into the cytoplasm.5 The cytosolic Ca2+ rise in turn activates the ROS-producing activity of Rboh proteins by direct binding to the EF-hand motifs and by phosphorylation through the Ca2+-dependent protein kinases, generating a positive feedback regulation that maintains the growth site to the tip during elongation (Fig. 1).2,6-8 However, the subcellular sites of ROS accumulation upon pollination remain unknown.
Figure 1.
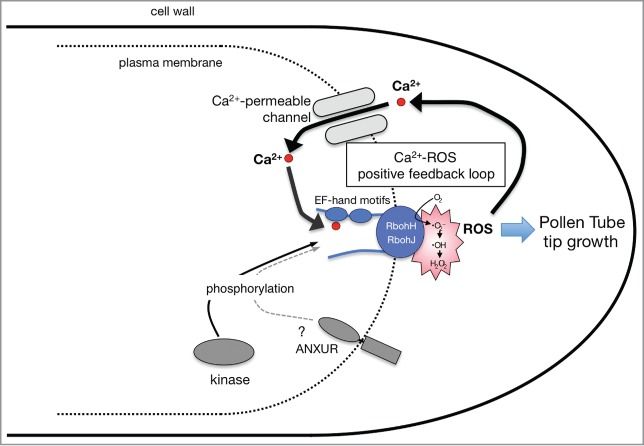
A schematic model of a positive feedback regulation at the tip of growing pollen tubes. Two NADPH oxidases, RbohH and RbohJ, located at the plasma membrane of the tip,2 producing ROS in the cell wall. Plasma membrane Ca2+-permeable channel(s) may be activated by ROS to induce Ca2+ influx into the cytosol as shown in root hairs.5 In turn, the ROS-producing activities of Rbohs are synergistically activated by direct binding of Ca2+ to their cytosolic EF-hand motifs and their phosphorylation by Ca2+-dependent protein kinases2 as also shown for other Rboh proteins.6-8 Phosphorylation of RbohH and/or RbohJ may also be mediated by ANXUR receptor-like kinases.3
To examine the subcellular sites of ROS accumulation and action upon pollination, we performed ultrastructural cytochemical analysis to detect ROS under a transmission electron microscopy. To visualize the ROS accumulation, Arabidopsis pollinated pistils were incubated with CeCl3 that reacts with ROS to produce electron-dense insoluble precipitate of cerium perhydroxides, Ce[OH]2OOH and Ce[OH]3OOH suitable for ultrastructural cytochemistry.9-11 Cerium deposition (CD) was detected in the apoplast/cell wall of the wild-type around the pollen tube contacting with the stigmatic papilla cell (Fig. 2A). Deposition of Ce was confirmed by the energy-dispersive X-ray spectroscopy (EDX) analysis (emission at 4.84 keV in Fig. 2B). In the rbohH-3 atrbohJ-2 double mutant, however, CD was impaired (Fig. 2C and D). These results suggest that ROS produced by RbohH and RbohJ accumulate in the apoplast/cell wall of the tip of pollen tubes.
Figure 2.
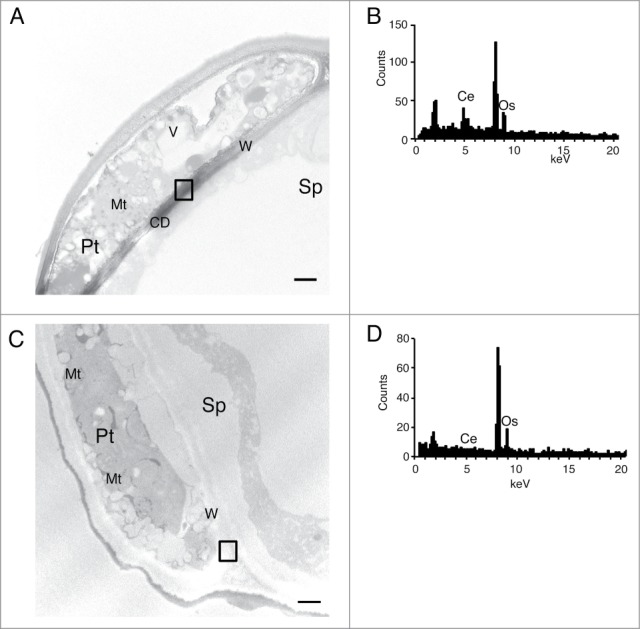
Localization of cerium deposition in the stigmas at 30 min after pollination. (A) A part of a cross section of the stigmatic papilla cell pollinated with wild-type pollen grain. The cerium deposition existed in the cell wall around a pollen tube contacting with stigmatic papilla. (B) The X-ray spectrum of the squares in (A). Ce emissions (4.84 keV) were detected in the high contrast areas. (C) A part of a cross section of the stigmatic papilla cell pollinated with the rbohH-3 atrbohJ-2 pollen grain. The cerium deposition was impaired in the cell wall around the pollen tube. (D) The X-ray spectrum of the squares in (C). Os emission (8.91 keV) was detected both in (C) and (D). CD: cerium deposition, W: cell wall, Mt: mitochondria, V: vacuole, Pt: pollen tube, Sp: stigmatic papilla cell, Ce: cerium, Os: osmium, Scale bar = 500 nm
Both pollen tube tip growth and fertility are severely impaired in the rbohH rbohJ double mutants, indicating that ROS at the apex of pollen tubes are involved in the regulation of pollen tube tip growth.2-4 Apoplastic ROS are suggested to play a role in cell wall loosening in maize coleoptiles, leaves, and roots.12-14 ROS-mediated oxidative scission of cell wall polysaccharides may be involved in the cell wall loosening.15 Therefore, the apoplastic ROS accumulation in the cell wall upon pollination may help pollen tubes germinating into the stigmatic papillae cells as well as pollen tube elongation. During the tip growth, localization of RbohH and RbohJ appear to be restricted to the tip of growing pollen tubes as shown for RbohC in the root hairs,8 and they may help construction of newly formed cell walls to be flexible at the tip. Alternatively, ROS may cross-link formation to reorganize the cell wall for reorganization. Physiological significance of the localized ROS production in the cell wall of the growing tip should be an important topic for future research.
Materials and Methods
The wild type Columbia (Col) and the T-DNA insertion mutants of rbohH-3 (SALK_136917) and rbohJ-2 (SAIL_31_D07) were used for this study. Detection of ROS accumulation sites was performed as previously described:9,10 Stigmas at 30 min after pollination with wild-type pollen grains or mutant pollen grains were washed twice in the 3-(N-morpholino) propanesulphonic acid (MOPS) buffer and incubated in freshly prepared 5 mm CeCl3 in the Mops buffer at pH 7.2 for 1 h. After brief washing in Mops, the stigmas were fixed for 1 h in 2.5% glutaraldehyde (V/V) in 50 mM sodium cacodylate (CAB) buffer, pH 7.2. After fixation, samples were washed in CAB and post-fixed for 1 h in 1% (v/v) osmium tetroxide in CAB. Samples were then washed with water, dehydrated through a graded ethanol series (50–100%) and embedded in Spurr's resin as previously described.16 Ultra-thin sections, cut with an ultramicrotome (Ultracut UTF, Leica, Germany), were observed with or without Pb staining by a transmission electron microscope (EDS2000, Hitachi, Tokyo, Japan). Cytochemical control specimens were processed in incubation medium without cerium and resulted in no similar deposition. Energy-dispersive X-ray spectroscopy (EDX) analysis was performed using NORAN EDX (Thermo Scientific, USA) fitted with EDS2000. The accelerating voltage was 200 kV and data collection was 30 live seconds. The electron micrographs shown are typical of the 50 micrographs obtained for each stigma.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed
Acknowledgments
We thank Prof. Dr. Seiji Takayama (Nara Institute of Science and Technology) and Prof. Dr. Tetsuya Higashiyama (Nagoya University) for comments and encouragement.
Funding
This work was supported in part by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan [Grants-in-Aid for Young Scientist (B) to H.K. (No. 21770054), for Scientific Research in Innovative Areas to H.K. (No. 21200068), and for Scientific Research on Priority Areas to K.K. (No. 21117516 and 23117718), and Nanotechnology Platform Program for the Research Center for Ultra-High Voltage Electron Microscopy (Nanotechnology Open Facilities), Osaka University].
Reference
- 1. Qin Y, Yang Z. Rapid tip growth: insights from pollen tubes. Semin Cell Dev Biol 2011; 22:816-24; PMID:21729760; http://dx.doi.org/ 10.1016/j.semcdb.2011.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kaya H, Nakajima R, Iwano M, Kanaoka MM, Kimura S, Takeda S, Kawarazaki T, Senzaki E, Hamamura Y, Higashiyama T, et al. Ca2+-activated reactive oxygen species production by arabidopsis RbohH and RbohJ is essential for proper pollen tube tip growth. Plant Cell 2014; 26:1069-80; PMID:24610725; http://dx.doi.org/ 10.1105/tpc.113.120642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boisson-Dernier A, Lituiev DS, Nestorova A, Franck CM, Thirugnanarajah S, Grossniklaus U. ANXUR receptor-like kinases coordinate cell wall integrity with growth at the pollen tube tip via NADPH oxidases. PLoS Biol 2013; 11:e1001719; PMID:24302886; http://dx.doi.org/ 10.1371/journal.pbio.1001719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lassig R, Gutermuth T, Bey TD, Konrad KR, Romeis T. Pollen tube NAD(P)H oxidases act as a speed control to dampen growth rate oscillations during polarized cell growth. Plant J 2014; 78:94-106; PMID:24506280; http://dx.doi.org/ 10.1111/tpj.12452 [DOI] [PubMed] [Google Scholar]
- 5. Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JD, et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 2003; 422:442-6; PMID:12660786; http://dx.doi.org/ 10.1038/nature01485 [DOI] [PubMed] [Google Scholar]
- 6. Kimura S, Kaya H, Kawarazaki T, Hiraoka G, Senzaki E, Michikawa M, Kuchitsu K. Protein phosphorylation is a prerequisite for the Ca2+-dependent activation of arabidopsis NADPH oxidases and may function as a trigger for the positive feedback regulation of Ca2+ and reactive oxygen species. Biochim Biophys Acta 2012; 1823:398-405; PMID:22001402; http://dx.doi.org/ 10.1016/j.bbamcr.2011.09.011 [DOI] [PubMed] [Google Scholar]
- 7. Ogasawara Y, Kaya H, Hiraoka G, Yumoto F, Kimura S, Kadota Y, Hishinuma H, Senzaki E, Yamagoe S, Nagata K, et al. Synergistic activation of the arabidopsis NADPH oxidase atrbohD by Ca2+ and phosphorylation. J Biol Chem 2008; 283:8885-92; PMID:18218618; http://dx.doi.org/ 10.1074/jbc.M708106200 [DOI] [PubMed] [Google Scholar]
- 8. Takeda S, Gapper C, Kaya H, Bell E, Kuchitsu K, Dolan L. Local positive feedback regulation determines cell shape in root hair cells. Science 2008; 319:1241-4; PMID:18309082; http://dx.doi.org/ 10.1126/science.1152505 [DOI] [PubMed] [Google Scholar]
- 9. Bestwick CS, Brown IR, Bennett MH, Mansfield JW. Localization of hydrogen peroxide accumulation during the hypersensitive reaction of lettuce cells to Pseudomonas syringae pv phaseolicola. Plant Cell 1997; 9:209-21; PMID:9061952; http://dx.doi.org/ 10.1105/tpc.9.2.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iwano M, Che FS, Goto K, Tanaka N, Takayama S, Isogai A. Electron microscopic analysis of the H2O2 accumulation preceding hypersensitive cell death induced by an incompatible strain of pseudomonas avenae in cultured rice cells. Mol Plant Pathol 2002; 3:1-8; PMID:20569303; http://dx.doi.org/ 10.1046/j.1464-6722.2001.00087.x [DOI] [PubMed] [Google Scholar]
- 11. Briggs RT, Drath DB, Karnovsky ML, Karnovsky MJ. Localization of NADH oxidase on the surface of human polymorphonuclear leukocytes by a new cytochemical method. J Cell Biol 1975; 67:566-86; PMID:407; http://dx.doi.org/ 10.1083/jcb.67.3.566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liszkay A, van der Zalm E, Schopfer P. Production of reactive oxygen intermediates O2.-, H2O2, and.OH by maize roots and their role in wall loosening and elongation growth. Plant Physiol 2004; 136:3114-23; PMID:15466236; http://dx.doi.org/ 10.1104/pp.104.044784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rodriguez AA, Grunberg KA, Taleisnik EL. Reactive oxygen species in the elongation zone of maize leaves are necessary for leaf extension. Plant Physiol 2002; 129:1627-32; PMID:12177475; http://dx.doi.org/ 10.1104/pp.001222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schopfer P. Hydroxyl radical-induced cell-wall loosening in vitro and in vivo: implications for the control of elongation growth. Plant J 2001; 28:679-88; PMID:11851914; http://dx.doi.org/ 10.1046/j.1365-313x.2001.01187.x [DOI] [PubMed] [Google Scholar]
- 15. Fry SC. Oxidative scission of plant cell wall polysaccharides by ascorbate-induced hydroxyl radicals. Biochem J 1998; 332 (Pt 2):507-15; PMID:9601081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Che FS, Iwano M, Tanaka N, Takayama S, Minami E, Shibuya N, et al. Biochemical and morphological features of rice cell death induced by pseudomonas avenae. Plant Cell Physiol 1999; 40:1036-45; http://dx.doi.org/ 10.1093/oxfordjournals.pcp.a029485 [DOI] [Google Scholar]


