Abstract
Reduction of long-term mortality risk, an important clinical outcome for people in alcohol dependence treatment, can rarely be established in randomized controlled trials (RCTs). We calculated the reduction in all-cause mortality risk using data from short-term (6 and 12 months) double-blind RCTs comparing as-needed nalmefene treatment to placebo, and mortality risks from meta-analyses on all-cause-mortality risk by reduction of drinking in people with alcohol dependence. A reduction in drinking in the RCTs was defined by shifts in drinking risk levels established by the European Medicines Agency. Results showed that the reduction of drinking in the nalmefene group was associated with a reduction in mortality risk by 8% (95% CI: 2%, 13%) when compared to the placebo group. Sensitivity analyses confirmed a significant effect. Thus comparing the difference between nalmefene and placebo in reduction in drinking levels with results on all-cause mortality risk from meta-analyses indicated a clinically relevant reduction in mortality risk. Given the high mortality risk of people with alcohol dependence, abstinence or a reduction in drinking have been shown to reduce mortality risk and should be considered treatment goals.
Keywords: Alcohol dependence, alcohol treatment, clinical relevance, mortality, meta-analysis, nalmefene, placebo
Introduction
One of the key criteria in the process of approving a new drug is clinical relevance of the results. Randomized controlled trials (RCTs) are usually limited to several months in length, and sample sizes have been calculated based on surrogate endpoints like drinking behavior, such as abstinence periods or reduction of drinking (European Medicines Agency, 2010; Food and Drug Administration, 2015). Clinical benefits or clinical relevance in terms of reduction of morbidity or mortality have to be established indirectly in most cases as the underlying trials are time-limited (e.g. efficacy trials and safety trials up to 12–15 months; see European Medicines Agency, 2010; Food and Drug Administration, 2015). For abstinence, it has been demonstrated that stable abstinence over such time predicts long term abstinence (Dawson et al., 2007; Weisner et al., 2003) and various clinical relevant endpoints such as functionality, co-morbidity, or mortality (Kaskutas et al., 2014; Roerecke et al., 2013).
For reduction of drinking, which can be used for evaluating efficacy of treatment with respect to clinical relevance, it seems harder to find a standard. The treatment goal of reduction in drinking is less well established and informed by a lower level of evidence and consensus. For the US, the Food and Drug Administration has proposed the standard of no heavy drinking days (Delucchi and Weisner, 2010; Food and Drug Administration, 2015; Sanchez-Craig et al., 1995). In Europe, the EMA (European Medicines Agency, 2010) has created categories of drinking levels associated with different risks for relevant clinical outcomes, mainly based on chronic disease and injury (World Health Organization, 2000). A recent publication applied principles outlined in the EMA guideline for evaluation of clinical relevance of reduction of alcohol consumption in clinical trials to biomarkers and quality of life measures (Aubin et al., 2015). Of special importance seems mortality as the most severe endpoint associated with alcohol use disorders in general and alcohol dependence in particular (Harris and Barraclough, 1998; Roerecke and Rehm, 2013, 2014). The work of Laramée et al. can be considered as examples (François et al., 2014; Laramée et al., 2014) to demonstrate how the differences found in clinical trials (Van den Brink et al., 2013, 2014) would lead to clinically relevant outcomes, if they persisted, or if the effects were found in larger samples (see also Barbosa et al., 2010 for a modeling of different outcomes, such as quality-adjusted life years and costs).
All the studies cited above share one assumption: that the risk curves for various disease or cause of death categories (for an overview, please see Rehm et al., 2010; Shield et al., 2013) can actually be applied to changes in consumption by individuals over time. Thus, if 100 g pure alcohol/day is associated with a certain risk for liver disease incidence or liver disease mortality, and 30 g with a lower risk based on epidemiological studies (Rehm et al., 2010), then it is assumed that if an individual switches from 100 g to 30 g pure alcohol per day, this individual would reduce his or her risk accordingly. While this assumption is plausible, there are not enough studies to show that real reductions by individuals were associated with risk reductions. However, there are enough studies with all-cause mortality. Meta-analyses of these studies have shown that a reduction in drinking (odds ratio [OR] = 0.41, 95% CI: 0.34, 0.50), regardless of achieving actual abstinence, was associated with a reduction in average mortality risk after treatment for alcohol dependence, although abstinence showed the strongest association with reduced mortality (Roerecke et al., 2013). We used an indirect approach to estimate the most important indicator for clinical relevance (namely mortality risk) for nalmefene treatment in comparison to a placebo. We are assuming that the reduced drinking levels in the RCTs is maintained on average 3.5 years, which is the average assessment of drinking levels after baseline in the meta-analyses by Roerecke et al. (2013). Because both reduced drinking and abstinence have been associated with a reduced mortality risk in comparison to continued heavy drinking, two scenarios (Scenario I and II) were used to estimate mortality risk.
Methods and materials
In this report we used participants with a high or very high drinking risk level (DRL, as defined by WHO; European Medicines Agency, 2010) at both the screening visit (covering drinking in the prior 4 weeks) and the randomization visit, which corresponds to the population indicated for the use of nalmefene (resulting in n = 641 from 6-months RCTs and n = 183 from the 1-year RCT with at least one valid post-randomization assessment of drinking). In addition to abstinence, the following DRLs were used for patient assessment throughout the RCTs: 1–20 g pure alcohol per day, >20–40 g, >40–60 g, >60 g (women); 1–40 g, >40–60 g, >60–100 g, >100 g (men) for low, medium, high, and very high risk, respectively (from European Medicines Agency, 2010). Abstinence or low DRL were defined as a reduced drinking at the end of each trial. Missing values were handled using the last observation carried forward for the main analyses (Scenarios I and II). In sensitivity analyses, a mixed model repeated measures (MMRM) was used. Data from the RCTs are displayed in Tables 1 and 2.
Table 1.
Drinking risk level at baseline and month 6 from two 6-month double-blind randomized controlled trials.
| Time point |
Women (n = 217) |
Men (n = 424) |
||
|---|---|---|---|---|
| Drinking risk level | Placebo | Nalmefene | Placebo | Nalmefene |
| Baseline | ||||
| High | 25 | 29 | 109 | 91 |
| Very high | 86 | 77 | 102 | 122 |
| Month 6 | ||||
| Abstinence or low | 30 | 33 | 71 | 96 |
| Medium or above | 81 | 73 | 140 | 117 |
Note: Drinking risk level (DRL, low, medium, high, very high) as defined in the Methods section. Missing values were handled using the last observation carried forward.
Table 2.
Drinking risk level at baseline and month 13 from the 1-year double-blind randomized controlled trials.
| Time point |
Women (n = 42) |
Men (n = 141) |
||
|---|---|---|---|---|
| Drinking level | Placebo | Nalmefene | Placebo | Nalmefene |
| Baseline | ||||
| High | 4 | 10 | 19 | 63 |
| Very high | 6 | 22 | 13 | 46 |
| Month 13a | ||||
| Abstinence or low | 3 | 19 | 13 | 63 |
| Medium or above | 7 | 13 | 19 | 46 |
13 months of 28 days.
Note: Drinking risk level (DRL, low, medium, high, very high) as defined in the Methods section. Missing values were handled using the last observation carried forward.
Data sources
We used data from two sources for this analysis.
The first source was data from two double-blind RCTs (NCT00811720; NCT00812461) investigating drinking levels up to 6 months (Gual et al., 2013; Mann et al., 2013; Van den Brink et al., 2013) and one double-blind RCT (NCT00811941) investigating drinking levels up to 1 year (Van den Brink et al., 2014) comparing nalmefene versus placebo (both in combination with psychosocial support). All RCTs received ethics approval and all patients gave informed written consent.
Briefly, the two 6-months RCTs were conducted in Germany, Finland, Sweden, and Austria (Mann et al., 2013), and in Belgium, Czech Republic, France, Italy, Poland, Portugal, and Spain (Gual et al., 2013) from December 2008 to July 2010. The 1-year RCT was conducted between March 2009 and September 2010 in the Czech Republic, Estonia, Hungary, Latvia, Lithuania, Poland, Russia, Slovakia, Ukraine, and the UK (Van den Brink et al., 2014). Main eligibility criteria were (Gual et al., 2013; Mann et al., 2013; Van den Brink et al., 2014): ⩾18 years of age, primary diagnosis of alcohol dependence (American Psychiatric Association, 2000) assessed by the Mini-International Neuropsychiatric Interview (Lecrubier et al., 1997), and blood alcohol level <0.02% at screening.
Main exclusion criteria were (Gual et al., 2013; Mann et al., 2013; Van den Brink et al., 2014): <6 heavy drinking days (⩾60 g/day for men and ⩾40 g/day for women; European Medicines Agency, 2010) in the 4 weeks before screening, average alcohol consumption below medium risk levels (for the two 6-month trials, the 12-month one including low risk levels), >14 consecutive abstinent days in the 4 weeks before screening, a score ⩾10 (indicating the need for medication-supported detoxification) on the revised version of the Clinical Institute Withdrawal Assessment for Alcohol (CIWA-Ar; Sullivan et al., 1989), aspartate aminotransferase or alanine aminotransferase values >3 times of upper normal limit, current DSM-IV Axis 1 disorder other than alcohol dependence, DSM-IV Axis II antisocial personality disorder, or recent (within 1 week prior to the screening) treatment with opioid agonists or partial agonists. In the 1-year RCT, patients with stable comorbid psychiatric disorders were eligible (Van den Brink et al., 2014). For a more detailed description of selection criteria, please see supplementary appendices in Mann et al. (2013), Van den Brink et al. (2014), and Gual et al. (2013).
One nalmefene tablet (18 mg) was to be taken on each day when risk of drinking was perceived, preferably 1–2 hours before the anticipated time of drinking. Drinking (daily number of standard drinks) and medication intake throughout the trials were recorded with Timeline Follow-back (Sobell and Sobell, 1992). Country-specific conversion factors were used to transform drinking into g/day, and patients were provided with a conversion card. In addition to nalmefene or placebo, all participants participated in a motivational and adherence-enhancing intervention (BRENDA, (Starosta et al., 2006; Volpicelli et al., 2001)) starting at randomization and at each subsequent site visit. No treatment goal was specified, i.e. abstinence and a reduction in drinking were accepted. Pre-defined primary outcome measures were change from baseline in total alcohol consumption and number of heavy drinking days. A total of 1711 patients were screened, and 1322 patients were randomized to as-needed nalmefene or placebo in the two 6-month RCTs, and 841 patients screened and 675 patients randomized in the 1-year RCT. The majority of these patients had not undergone previous treatment for alcohol dependence or withdrawal symptoms (Van den Brink et al., 2013, 2014).
Secondly, we used data from comprehensive published meta-analyses (Roerecke et al., 2013) as an indicator for mortality risk, and applied the pooled all-cause mortality risk reductions from these meta-analyses to the reductions in alcohol consumption observed in the above mentioned RCTs. In total, data from 16 primary studies were included in these meta-analyses, contributing to 755 observed deaths, with 4951 people at risk (Roerecke et al., 2013). The time from baseline to follow-up of drinking status (abstinence, reduced drinking, or relapse/continued heavy drinking) ranged from 1 to 15 years with a weighted mean of 3.5 years, and the time from baseline to mortality or end of study ranged from 3 to 16 years with a weighted mean of 8.8 years.
In Scenario I, we used pooled mortality risks based on reduced drinking including abstinence versus continued heavy drinking, and in Scenario II we used pooled mortality risks based on reduced drinking excluding abstinence versus continued heavy drinking. In other words: Scenario I used an OR of 0.41 (95% CI: 0.34, 0.50), based on reduced drinking including abstinence versus continued heavy drinking (Roerecke et al., 2013), and Scenario II, a more conservative analysis (considering that some patients became abstinent with nalmefene treatment during RCTs), used an OR of 0.61 (95% CI: 0.39, 0.94), based on reduced drinking excluding abstinence versus continued heavy drinking (Roerecke et al., 2013).
Derivation of mortality risk estimates
The data from the RCTs presented in Table 1 and 2 were combined with reported mortality risk estimates by drinking level after alcohol treatment from the meta-analyses of Roerecke et al. (2013) using the following formula for relative risk (RR) of mortality of nalmefene versus placebo:
where pna,i is the prevalence of DRLi in the nalmefene group, ppl,i is the prevalence of DRLi in the placebo group, and ORi is the OR for DRLi, which was the same for the nalmefene and placebo groups. With respect to confidence intervals, for each analysis, 100,000 Monte Carlo simulations were computed based on the following approach:
Two sources of uncertainty were entered: the prevalence of the respective category, and the OR,
The uncertainty of prevalence was derived from the SE as
The uncertainty of the OR was based on the meta-analyses from Roerecke et al. (2013).
As customary in analyses of OR, it was assumed that the distribution of the logarithmic risk was normally distributed (Fleiss et al., 2003; Rothman et al., 2008). The relative risks of mortality, nalmefene versus placebo, were pooled across trials using inverse-variance weighted DerSimonian–Laird random-effect models to allow for potential between-study heterogeneity (DerSimonian and Laird, 1986). Sensitivity analyses were performed varying the prevalence estimates from the RCTs with missing values imputed using individual patient-predicted values of total alcohol consumption (g pure alcohol/day) derived from a MMRM used in the primary analysis of total alcohol consumption in the nalmefene RCTs (Gual et al., 2013; Mann et al., 2013; Van den Brink et al., 2014). Between-study heterogeneity was quantified using the I2 statistic (Higgins and Thompson, 2002). All meta-analytical analyses were performed on the natural log scale in Stata statistical software, version 12 (Stata Corp, College Station, Texas).
Results
Scenario I
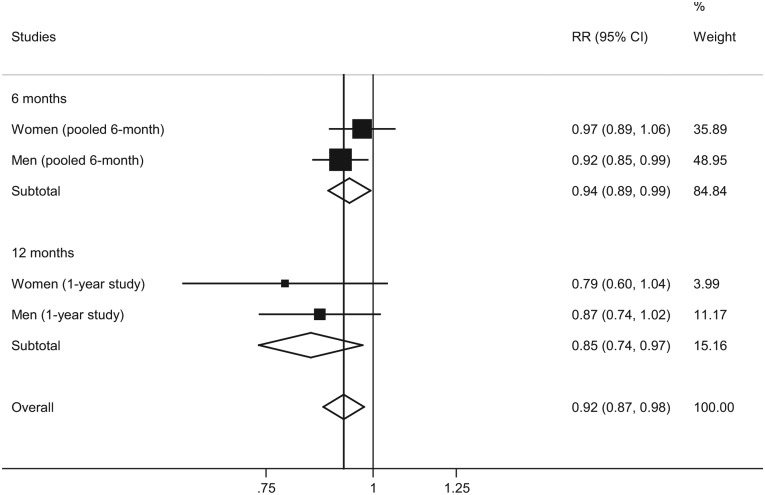
Assuming an OR of 0.41 (95% CI: 0.34, 0.50) for reduced drinking including abstinence, the 9-year estimated mortality risks in the nalmefene group versus the placebo group after combining the data from the nalmefene RCTs with the data from the meta-analysis are presented in Figure 1. The overall treatment effect of nalmefene versus placebo was predicted to reduce the estimated 9-year mortality risk on average by 8% (95% CI: 2%, 13%).
Figure 1.
Mortality risks (nalmefene versus placebo) assuming an OR = 0.41 for reduced drinking (including abstinence).
Note: I2 = 6%, p = 0.37 for overall analysis, random-effects model.
Scenario II
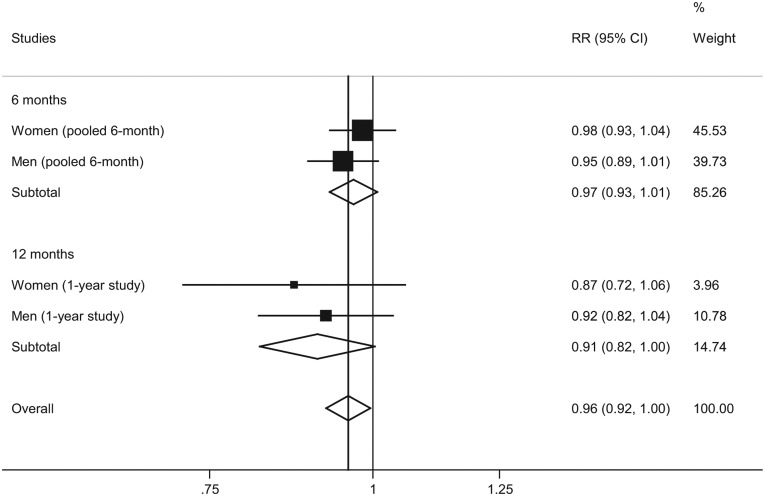
Assuming an OR of 0.61 (95% CI: 0.39, 0.94) for reduced drinking (based on studies with data on reduced drinking, excluding studies with only abstinence data; see Roerecke et al., 2013), the mortality risks in the nalmefene group versus the placebo group after combining the data from the nalmefene RCTs with the data from the meta-analysis are presented in Figure 2. The overall treatment effect of nalmefene versus placebo was predicted to reduce the estimated 9-year mortality risk on average by 4% (95% CI: 0%, 8%).
Figure 2.
Mortality risks (nalmefene versus placebo) assuming an OR = 0.61 for reduced drinking (excluding abstinence).
Note: I2 = 0%, p = 0.54 for overall analysis, random-effects model.
Sensitivity analyses
Sensitivity analyses were performed using the DRLs with missing values imputed using individual patient-predicted values of total alcohol consumption as described above. For Scenario I, the overall treatment effect of nalmefene versus placebo was predicted to reduce the estimated 9-year mortality risk on average by 9% (RR: 0.91; 95% CI: 0.86, 0.96); and for the Scenario II, the overall treatment effect was 5% (RR: 0.95; 95% CI: 0.91, 0.99). Thus, the predicted mortality reductions were slightly higher when using MMRM imputation for missing values than using a last observation carried forward imputation for handling missing values in the nalmefene RCTs.
Discussion
Before discussing the results and implications of the study, we would like to point out potential limitations.
Limitations
Data included in the original meta-analysis on reduced drinking were derived from 16 published studies with various definitions of reduced drinking, including abstinence. The low DRL can be seen as a conservative interpretation of reduced drinking; many of the studies included in the original meta-analyses by Roerecke et al. (2013) had more lenient definitions and higher thresholds as reduced drinking after alcohol treatment. Thus, it is justified to use low DRL to define reduced drinking. However, while the definition of reduced drinking is conservative with medium DRL considered as no improvement in heavy drinking, the proportion of abstainers in the studies included in the meta-analyses of Roerecke et al. (2013) was higher than that in the nalmefene RCTs. Therefore, Scenario II was performed using the OR for reduced drinking excluding abstinence (based on studies with data on reduced drinking, excluding studies with only abstinence data; see Roerecke et al., 2013). This estimate is conservative, as there were abstinence outcomes observed in the nalmefene RCTs. Furthermore, we are assuming that any reduction in drinking during the RCTs is maintained after the conclusion of the trials. The mean assessment of drinking status after treatment was 3.5 years in the meta-analyses used to estimate mortality risks associated with a reduction in drinking levels, and mortality was ascertained after a mean of 8.8 years after baseline. The second assumption is that the RCTs and studies included in the meta-analyses have comparable populations. While all participants in both RCTs and the meta-analyses received treatment for alcohol use disorders, many of the studies in the meta-analyses were from an in-patient treatment setting and thus may comprise of more severe cases of alcohol use disorders. However, no other systematic examinations have been published and thus we used the best data available for our study.
Implications
While clinical studies with usual 6 months duration cannot show clinical relevance on major outcomes such as mortality, combining trial data on reduction in drinking levels and mortality risks associated with such reductions allowed the estimation of clinical relevance. In short, we could show that the reduction of drinking following treatment with nalmefene versus placebo had clinical relevance with respect to mortality, when combined with results from meta-analyses of all relevant clinical studies. In other words: the relative drinking level reductions observed in patients with a high or very high DRL at screening and randomization between compared arms from the nalmefene RCTs (Gual et al., 2013; Mann et al., 2013; Van den Brink et al., 2013, 2014) are large enough to expect reduced mortality in the future. The question is, how the effect size of the present study compares to other effect sizes. First, the effect sizes of pharmacological treatment for alcohol use disorders in general compare favorable to the effects of other treatments such as psychotherapy (Miller et al., 2003; Rehm et al., 2013). The only intervention with higher mortality gains would be brief interventions in certain hospital settings, i.e. for people with high risk of mortality (McQueen et al., 2011; Rehm and Roerecke, 2013). Second, the absolute gain for society would be huge in regions like Europe, where more than 3% of the adult population fulfill the criteria of alcohol dependence (Rehm et al., 2015a), and most of them would fulfill the criteria of high or very high DRL (Rehm et al., 2015b). Finally, the effect size against placebo of the underlying studies was similar to effect sizes for other treatments, e.g. for depression treatment (effect size: numbers needed to treat; Arroll et al., 2009).
While the expected reductions of mortality are relatively small, any statistically significant reduction of mortality is important, especially given the high mortality risk associated with patients in alcohol treatment (Roerecke and Rehm, 2013). The results also indicate that reduction of drinking can be clinically relevant on the long term, even if drinking levels are not reduced to abstinence. This result is important, since many people with alcohol use disorders either are unable or do not want to choose abstinence as a treatment goal and some of them will not attend treatment for that reason (e.g., Heather et al., 2010; Hodgins et al., 1997). On the other hand, treatment goal and results may change during treatment or post-treatment, and reduction of drinking was not necessarily associated with less long-term success (see also Ambrogne, 2002; Sanchez-Craig and Lei, 1986; Sanchez-Craig et al., 1984). The proportion of people with alcohol use disorders receiving treatment overall is low (Alonso et al., 2004, Manthey et al., in press; Rehm et al., 2012), and important public health improvements could be made, if more people sought treatment (Rehm et al., 2013). The presented results clearly support this line of reasoning (see also Nutt and Rehm, 2014), and it is hoped that the introduction of pharmacological agents such as nalmefene will lead to higher treatment rates and better survival of people with alcohol dependence.
Footnotes
Declaration of Conflicting Interests: MR declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. JR disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: grants, personal fees, and other (membership nalmefene board at the time when the manuscript was conceptualized) from Lundbeck, outside the submitted work. PS, PL and NR are Lundbeck employees.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: MR was a post-doctoral fellow at the Centre for Addiction and Mental Health (CAMH). JR received salary from the CAMH, the University of Toronto, and the Technische Universität Dresden, plus research funding from EU, Lundbeck, NIH, and WHO during the time of writing the manuscript, none specifically for the work undertaken on the manuscript. PS, PL and NR are employees of Lundbeck, which sponsored this study.
References
- Alonso J, Angermeyer MC, Bernert S, et al. (2004) Use of mental health services in Europe: results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatr Scand 109: 47–54. [DOI] [PubMed] [Google Scholar]
- Ambrogne JA. (2002) Reduced-risk drinking as a treatment goal: what clinicians need to know. J Subst Abuse Treat 22: 45–53. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2000) Diagnostic and Statistical Manual of Mental Disorders, 4th ed., text revision. Washington, DC: American Psychiatric Association. [Google Scholar]
- Arroll B, Elley CR, Fishman T, et al. (2009) Antidepressants versus placebo for depression in primary care. Cochrane Database Syst Rev 8: CD007954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubin HJ, Reimer J, Nutt DJ, et al. (2015) Clinical relevance of as-needed treatment with nalmefene in alcohol-dependent patients. Eur Addict Res 21: 160–168. [DOI] [PubMed] [Google Scholar]
- Barbosa C, Taylor B, Godfrey C, et al. (2010) Modelling lifetime QALYs and health care costs from different drinking patterns over time: a Markov model. Int J Methods Psychiatr Res 19: 97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Grant BF. (2007) Rates and correlates of relapse among individuals in remission from DSM-IV alcohol dependence: a 3-year follow-up. Alcohol Clin Exp Res 31: 2036–2045. [DOI] [PubMed] [Google Scholar]
- Delucchi KL, Weisner C. (2010) Transitioning into and out of problem drinking across seven years. J Stud Alcohol Drugs 71: 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency (2010) Guideline on the Development of Medicinal Products for the Treatment of Alcohol Dependence. London: European Medicines Agency. [Google Scholar]
- Fleiss JL, Levin B, Cho Paik M. (2003) Statistical Methods for Rates and Proportions, 3rd ed. Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- Food and Drug Administration (2015) Alcoholism: Developing Drugs for Treatment.Guidance for Industry. Silver Spring, MD: Food and Drug Administration. [Google Scholar]
- François C, Laramée P, Rahhali N, et al. (2014) The clinical relevance of reducing alcohol consumption in alcohol dependence: a predictive microsimulation model. Eur Addict Res 20: 269–284. [DOI] [PubMed] [Google Scholar]
- Gual A, He Y, Torup L, et al. (2013) A randomised, double-blind, placebo-controlled, efficacy study of nalmefene, as-needed use, in patients with alcohol dependence. Eur Neuropsychopharmacol 23: 1432–1442. [DOI] [PubMed] [Google Scholar]
- Harris EC, Barraclough B. (1998) Excess mortality of mental disorder. Br J Psychiatry 173: 11–53. [DOI] [PubMed] [Google Scholar]
- Heather N, Adamson SJ, Raistrick D, et al. (2010) Initial preference for drinking goal in the treatment of alcohol problems. I. Baseline differences between abstinence and non-abstinence groups. Alcohol Alcohol 45: 128–135. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG. (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- Hodgins DC, Leigh G, Milne R, et al. (1997) Drinking goal selection in behavioral self-management treatment of chronic alcoholics. Addict Behav 22: 247–255. [DOI] [PubMed] [Google Scholar]
- Kaskutas LA, Borkman TJ, Laudet A, et al. (2014) Elements that define recovery: the experiential perspective. J Stud Alcohol Drugs 75: 999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laramée P, Brodtkorb TH, Rahhali N, et al. (2014) The cost-effectiveness and public health benefit of nalmefene added to psychosocial support for the reduction of alcohol consumption in alcohol dependent patients with high/very high drinking risk levels: a Markov model. BMJ Open 4: e005376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecrubier Y, Sheehan DV, Weiller E, et al. (1997) The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI. Eur Psychiatry 12: 224–231. [Google Scholar]
- Mann K, Bladström A, Torup L, et al. (2013) Extending the treatment options in alcohol dependence: a randomized controlled study of as-needed nalmefene. Biol Psychiatry 73: 706–713. [DOI] [PubMed] [Google Scholar]
- Manthey J, Gual A, Jakubczyk A, et al. (in press) Alcohol use disorders in Europe: a comparison of general population and primary health care prevalence rates. J Subst Use. [Google Scholar]
- McQueen J, Howe TE, Allan L, et al. (2011) Brief interventions for heavy alcohol users admitted to general hospital wards. Cochrane Database Syst Rev 8: CD005191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, Wilbourne PL, Hettema JE. (2003) What works? A summary of alcohol treatment outcome research. In: Hester RK, Miller WR. (eds) Handbook of Alcoholism Treatment Approaches, 3rd ed. Boston, MA: Allyn and Bacon. [Google Scholar]
- Nutt DJ, Rehm J. (2014) Doing it by numbers: a simple approach to reducing the harms of alcohol. J Psychopharmacol 28: 3–7. [DOI] [PubMed] [Google Scholar]
- Rehm J, Roerecke M. (2013) Reduction of drinking in problem drinkers and all-cause mortality. Alcohol Alcohol 48: 509–513. [DOI] [PubMed] [Google Scholar]
- Rehm J, Allamani A, Elekes Z, et al. (2015a) General practitioners recognizing alcohol dependence: a large cross-sectional study in six European countries. Ann Fam Med 13: 28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Anderson P, Barry J, et al. (2015b) Prevalence of and potential influencing factors for alcohol dependence in Europe. Eur Addict Res 21: 6–18. [DOI] [PubMed] [Google Scholar]
- Rehm J, Baliunas D, Borges GL, et al. (2010) The relation between different dimensions of alcohol consumption and burden of disease: an overview. Addiction 105: 817–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Shield KD, Rehm MX, et al. (2012) Alcohol Consumption, Alcohol Dependence, and Attributable Burden of Disease in Europe: Potential Gains from Effective Interventions for Alcohol Dependence. Toronto, Canada: Centre for Addiction and Mental Health. [Google Scholar]
- Rehm J, Shield KD, Rehm MX, et al. (2013) Modelling the impact of alcohol dependence on mortality burden and the effect of available treatment interventions in the European Union. Eur Neuropsychopharmacol 23: 89–97. [DOI] [PubMed] [Google Scholar]
- Rehm J, Taylor B, Mohapatra S, et al. (2010) Alcohol as a risk factor for liver cirrhosis: a systematic review and meta-analysis. Drug Alcohol Rev 29: 437–445. [DOI] [PubMed] [Google Scholar]
- Roerecke M, Rehm J. (2013) Alcohol use disorders and mortality: a systematic review and meta-analysis. Addiction 108: 1562–1578. [DOI] [PubMed] [Google Scholar]
- Roerecke M, Rehm J. (2014) Cause-specific mortality risk in alcohol use disorder treatment patients: a systematic review and meta-analysis. Int J Epidemiol 43: 906–919. [DOI] [PubMed] [Google Scholar]
- Roerecke M, Gual A, Rehm J. (2013) Reduction of alcohol consumption and subsequent mortality in alcohol use disorders: systematic review and meta-analysis. J Clin Psychiatry 74: e1181–e1189. [DOI] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S, Lash TL. (2008) Modern Epidemiology, 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins. [Google Scholar]
- Sanchez-Craig M, Lei H. (1986) Disadvantages to imposing the goal of abstinence on problem drinkers: an empirical study. Br J Addict 81: 505–515. [DOI] [PubMed] [Google Scholar]
- Sanchez-Craig M, Annis HM, Bornet AR, et al. (1984) Random assignment to abstinence and controlled drinking: evaluation of a cognitive-behavioral program for problem drinkers. J Consult Clin Psychol 52: 390–403. [DOI] [PubMed] [Google Scholar]
- Sanchez-Craig M, Wilkinson DA, Davila R. (1995) Empirically based guidelines for moderate drinking: 1-year results from three studies with problem drinkers. Am J Public Health 85: 823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shield KD, Parry C, Rehm J. (2013) Chronic diseases and conditions related to alcohol use Alcohol Res 35: 155–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. (1992) Timeline follow-back: a technique for assessing self-reported ethanol consumption. In: Allen J, Litten RZ. (eds) Measuring Alcohol Consumption: Psychosocial and Biological Methods. Totowa, NJ: Humana Press. [Google Scholar]
- Starosta AN, Leeman RF, Volpicelli JR. (2006) The BRENDA model: integrating psychosocial treatment and pharmacotherapy for the treatment of alcohol use disorders. J Psychiatr Pract 12: 80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, et al. (1989) Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict 84: 1353–1357. [DOI] [PubMed] [Google Scholar]
- Van den Brink W, Aubin HJ, Bladström A, et al. (2013) Efficacy of as-needed nalmefene in alcohol-dependent patients with at least a high drinking risk level: results from a subgroup analysis of two randomized controlled 6-month studies. Alcohol Alcohol 48: 570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Brink W, Sørensen P, Torup L, et al. (2014) Long-term efficacy, tolerability and safety of nalmefene as-needed in patients with alcohol dependence: a 1-year, randomised controlled study. J Psychopharmacol 28: 733–744. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Pettinati HM, McLellan AT, et al. (2001) Combining Medication and Psychosocial Treatments for Addictions: the BRENDA Approach. New York: The Guilford Press. [Google Scholar]
- Weisner C, Ray GT, Mertens JR, et al. (2003) Short-term alcohol and drug treatment outcomes predict long-term outcome. Drug Alcohol Depend 71: 281–294. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2000) International Guide for Monitoring Alcohol Consumption and Related Harm. Geneva, Switzerland: World Health Organization. [Google Scholar]