Abstract
Light signals regulate seedling morphological changes during de-etiolation through the coordinated actions of multiple light-sensing pathways. Previously we have shown that red-light-induced hypocotyl growth inhibition can be reversed by addition of dim blue light through the action of phototropin 1 (phot1). Here we further examine the fluence-rate relationships of this blue light effect in short-term (hours) and long-term (days) hypocotyl growth assays. The red stem-growth inhibition and blue promotion is a low-fluence rate response, and blue light delays or attenuates both the red light and far-red light responses. These de-etiolation responses include blue light reversal of red or far-red induced apical hook opening. This response also requires phot1. Cryptochromes (cry1 and cry2) are activated by higher blue light fluence-rates and override phot1's influence on hypocotyl growth promotion. Exogenous application of auxin transport inhibitor naphthylphthalamic acid abolished the blue light stem growth promotion in both hypocotyl growth and hook opening. Results from the genetic tests of this blue light effect in auxin transporter mutants, as well as phytochrome kinase substrate mutants indicated that aux1 may play a role in blue light reversal of red light response. Together, the phot1-mediated adjustment of phytochrome-regulated photomorphogenic events is most robust in dim blue light conditions and is likely modulated by auxin transport through its transporters.
Keywords: cryptochrome, photomorphogenesis, phytochrome, phototropin, red light
Introduction
In the developing seedling the transition from a dark growth program to a light growth program includes a well described set of physiological changes. The process of photomorphogenesis is typified by hypocotyl/stem growth inhibition, apical hook opening, chloroplast development, cotyledon expansion and other responses that are finely tuned to match the ambient light environment. Light quality, quantity, direction, and duration are all the key parameters in guiding this transition. Blue (B), red (R), and far-red (FR) light induce the most conspicuous changes. These alterations begin with photons activating specific photoreceptors, the cryptochromes (crys) and phototropins (phots) sensing B and UV-A light, and the phytochromes (phys), responsive to R and FR light. The different light signals initiate specific transduction events that work independently, cooperatively, or even counteractively to modulate photomorphogenic events.1-3
There is substantial evidence of genetic, physical and biochemical interaction between photosensors during developmental transitions. The phys are required for cry-mediated stem inhibition induced by B light.1,4,5 Similarly, B light-induced phototropic responses likely require coordinated actions of several photoreceptors.3,6 The degree of phot-mediated phototropic bending at least partially depends on the phy-antagonizing effect on hypocotyl agravitropism.7-9 Additionally, crys also work together with phots to modulate phototropism according to the fluence rate of B light.6 On the other hand, R or FR light-induced hypocotyl growth inhibition is most likely an event solely mediated by phys.2,10 Addition of high fluence rate B to R or FR light background generally enhances this inhibition through crys. These are examples where light inputs are complementary, additive or synergistic, otherwise known as “coaction.”
While B light responses typically advance photomorphogenesis, in rare cases it has been shown that phototropin directs processes that retard development. When dim B light (0.1 μmol/m2s) is delivered simultaneously with R light (<70 μmol/m2s), the R light-induced hypocotyl inhibition reverses and the stem growth rate is enhanced.11,12 Genetic tests indicate that this effect is mediated by phot1 and requires the presence of NPH3.12 These findings present special cases in plant development where coaction between multiple photosensory systems guides seedling acclimation to the light environment.
This study examines the phot1-mediated B light reversal of phytochrome responses in greater detail. Fluence-rate / response and time course experiments detail the interactions between phototropin 1 and other sensory systems with respect to hypocotyl growth rates and hook opening. Pharmacological and genetic tests show that the B light response is likely mediated by redundant transporters. These tests provide additional information about interaction between low-fluence-rate B light and opposition of R-light-mediated events during early photomorphogenic development.
Results
Dim B light opposes red-induced hypocotyl growth inhibition and apical hook opening
A previous report showed that dim B light could attenuate R or FR light induced hypocotyl growth rate inhibition when applied simultaneously.12 The present study expands these findings to further characterize B and R/FR light interaction during photomorphogenic development. Apical hook opening and chlorophyll biosynthesis are 2 events associated with R light induced de-etiolation. Figure 1A shows that addition of dim B light to the background of R light can eliminate hypocotyl growth inhibition. The simultaneous change in hook opening was also affected. Seedlings treated with R light for 180 min possessed an average hook angle of 67.6 (±9.5) degrees. However, when a dim B light (0.1 μmol/m2s) was co-irradiated with R light, the hook angle was only 28.1 (±6.3) degrees after 180 min (Fig. 1B). Eventually, the apical hook fully opened with no visible differences in both experimental light after 24 h (data not shown). The same effect on the apical hook opening was also observed when B light was delivered with FR light (Fig. 1C). No differences in chlorophyll accumulation kinetics were observed (Fig. S1).
Figure 1.
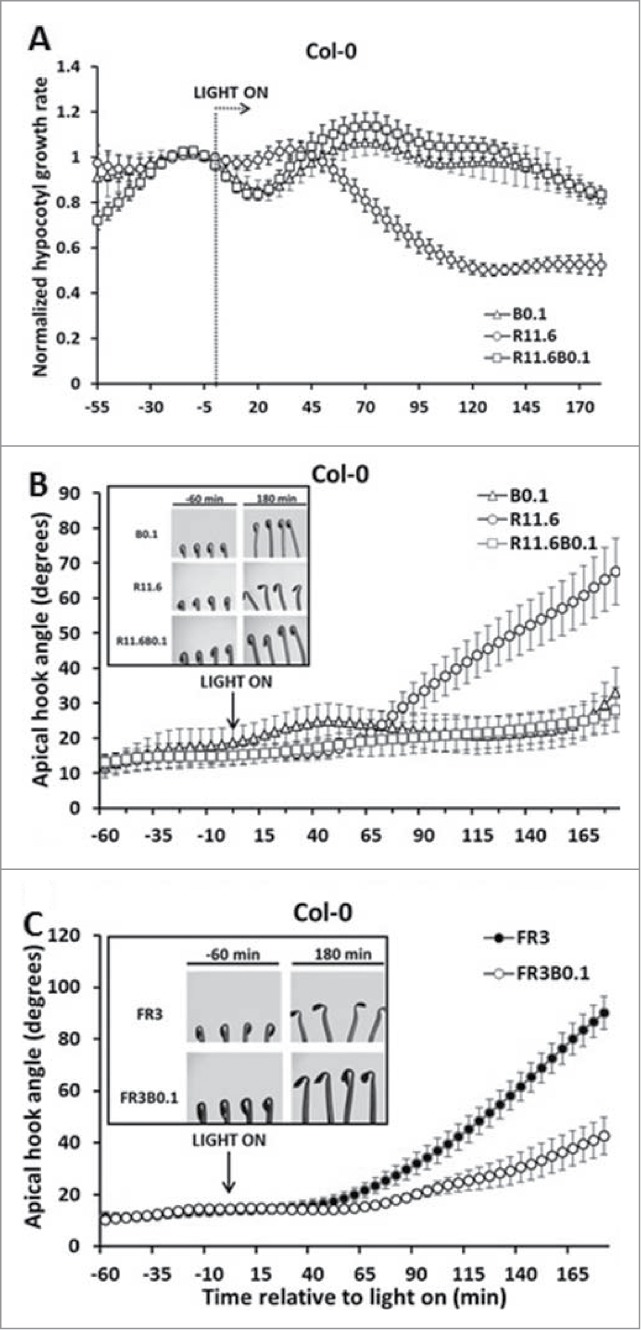
Dim B light attenuates R light-induced hypocotyl inhibition and hook opening. R11.6 = R light, 11.6 μmol/m2s, B0.1 = B light, 0.1 μmol/m2s. Error bars represent SEM (8≤n≤12). (A) B light attenuates R light-induced hypocotyl growth inhition; (B) B light attenuates R light-induced apical hook opening. (C) B light attenuates FR light-induced apical hook opening.
Blue light fluence rate/response in the modulation of red de-etiolation responses
The results from Figure 1 show that a R light response may be negated by a dim B light pulse. To characterize the fluence-rate /response relationship of this phenomenon, the R light grown seedlings were treated with co-illumination of B light at different fluence rates. The results in Figure 2 show that a fluence rate of 1.5 μmol/m2s B light applied in conjunction with the R treatment was sufficient to partially reverse the R-light-mediated growth inhibition (Fig. 2A). Increasing B light fluence rate to 10 μmol/m2s enhanced R-light-induced stem inhibition. Fluence rate-response experiments on hook opening show that increasing B light fluence rate to 1.5 μmol/m2s also delayed the hook opening relative to R light alone. However, the higher fluence rate of B light (≥10 μmol/m2s) accelerated the R light-induced hook opening process (Fig. 2B).
Figure 2.
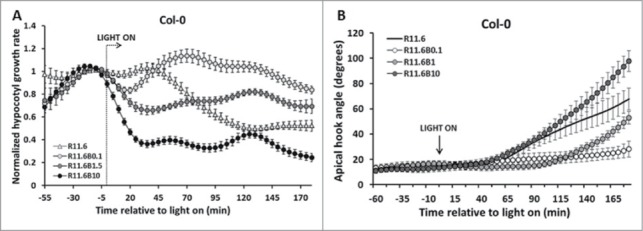
B light fluence rate response in R light-induced hypocotyl inhibition and hook opening. (A) Increasing B light fluence rate reduced the amplitude of B-negation of R light stem response in the short-term assay; (B) R light stimulates apical hook opening (dark circles), addition of dim B light delays R induced hook opening (open circles). Increasing B light fluence rate to 1 μmol/m2s still attenuates R light-induced hook opening (light gray circles), but 10 μmol/m2s B light enhanced R light-induced hook opening process (dark gray circles).
The roles of crys and phot1 in the B-light-mediated adjustment of hook opening response
The phot1 receptor has been shown to mediate the dim B reversal of R/FR light-induced hypocotyl inhibition. The R-light-induced hook opening kinetics were also examined in the phot1 mutant background. The results in Figure 3A and B showed that slight delays in the onset of hook opening, and slower opening in FR, were found between R/FR light and R/FR light plus dim B light treated mutant seedlings. The cry receptors are responsible for higher-fluence rate seedling responses to B light. Tests in cry1 and cry2 mutants show that R-light-induced hook opening by addition of high B (≥10 μmol/m2s) is largely impaired in mutant seedlings (Fig. 3C).
Figure 3.
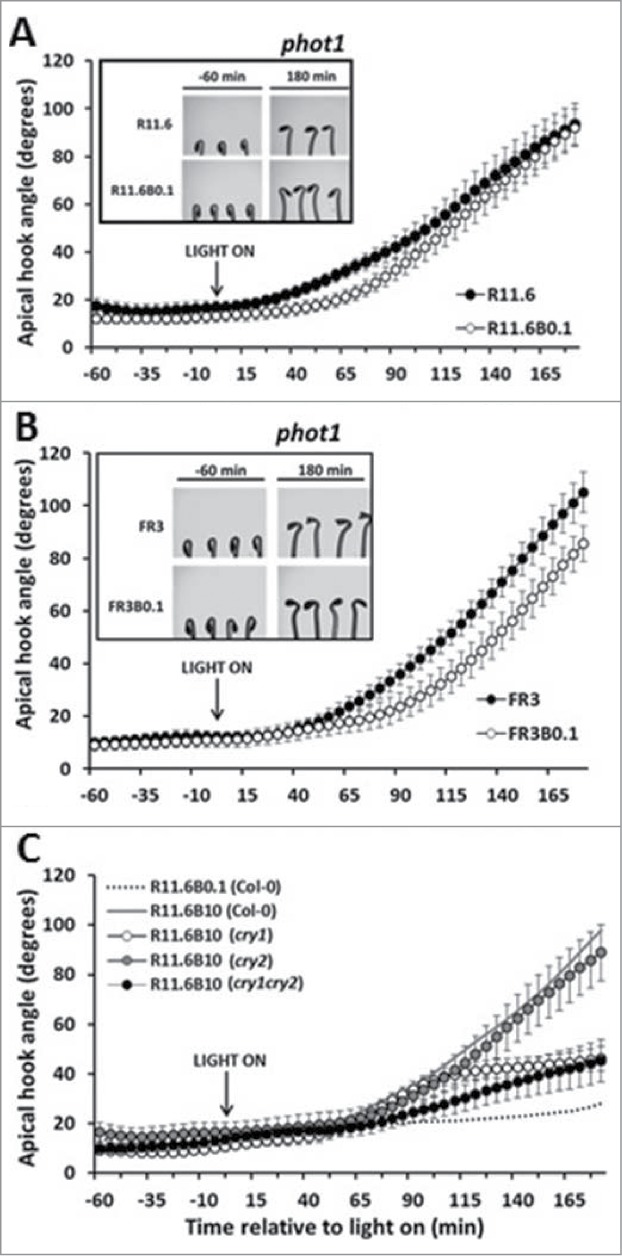
Roles of phot1 and cry in the blue attenuation of red induced hook opening. The dim B light attenuation of R light (A) or FR light (B)-induced hook opening response was absent in phot1 mutant background. Inset: R/FR or R/FR plus B light treated seedlings at the beginning (−60 min relative to light on) and the end (180 min) of one representative short-term experiment; (C) The enhanced R light-induced hook opening by higher fluence rate B light was normal in cry2 (gray circles) mutant, but was partially impaired in cry1 (open circles) and cry1cry2 (dark circles) mutants.
B reversal of R light-induced responses is blocked by NPA
The fact that very low fluence-rate B light can negate or delay R-light-induced stem growth inhibition and apical hook opening lead us to speculate that auxin transport might play a role in this response. To test this hypothesis, we included the polar auxin transport inhibitor N-1-Naphthylphthalamic Acid (NPA) into the media. The same light treatments were applied to seedlings grown on media containing different concentrations of NPA. The results in Figure S2A showed that at an NPA concentration of 0.5 μM, the absolute hypocotyl growth rate kinetics were similar between NPA treated and untreated seedlings. However, NPA B light negation of the R light response was reduced at this NPA concentration (Fig. S2A). When the NPA concentration was increased to 1 μM, the B light effect was completely absent, and the R-induced hypocotyl growth inhibition was slightly enhanced (Fig. 4A). NPA delayed and slowed hook opening kinetics in response to R light at 2 different concentrations (1 and 5 μM) in our short-term assays (Fig. S2B and Fig. 4B). No effect of dim B light on the R light response was observed. Taken together, these results indicate that polar auxin transport may play a role in B-light attenuation of R light-mediated de-etiolation events.
Figure 4.
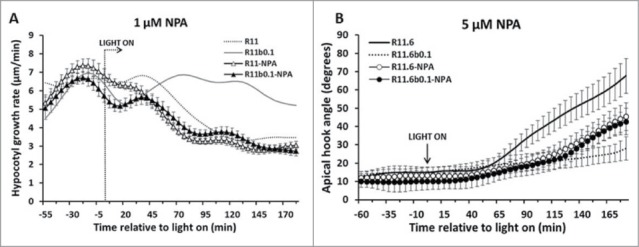
B light attenuation of R-mediated hypocotyl and hook responses require normal auxin transportation. (A) The B light negation of R-light-induced hypocotyl growth inhibition is fully suppressed by 1 μM NPA; (B) The R-light-induced hook opening process was abolished when treated with 5 μM NPA. In panel (A), open triangles represent the absolute hypocotyl growth rate under R light (11.6 μmol/m2s) conditions; dark triangles represent the hypocotyl growth rate under R light plus dim B light (0.1μmol/m2s) conditions. In panel (B), open circles represent the hook opening kinetics under R light conditions; dark circles represent the hook opening kinetics under R light plus dim B light conditions. Wild-type Col-0 in response to R light (dotted line) and R light plus dim B light (gray line) without NPA treatment were included for comparison in all panels.
Genetic tests of B light mechanism
The directional flow of phytohormone auxin from cell to cell is mediated by specific auxin transporters. These transporters facilitate auxin molecules in (influx carriers) and out (efflux carriers) of a cell depending on external and internal signals. Some of these transporters, such as PINs, ABC transporters, and AUX/LAX have been indicated to modulate plant tropism response.14-19 Thus, we postulated that one or several of these transporters might mediate the B light negation of the R light response. To test this hypothesis, we measured stem growth and hook opening kinetics in several PIN transporter mutants (pin1, pin3, pin4, and pin7), one ABC transporter mutant (abcb19/pgp19), and one auxin influx transporter mutant (aux1). These mutants were chosen because of their cognate gene expression patterns in the hook/hypocotyl area.19-21 Meanwhile the auxin response factor mutant arf7/nph4 was also included for the test based on its role in phototropism response.22,23
Generally, the transporter mutants maintained a normal or near normal B light negation effect to R light. Specifically, pin1, pin3 and pin7 mutant seedlings responded to R light or the addition of dim B light like wild-type seedlings (Fig. S3A, B, and C). The pin4 mutant seedlings showed a reduction in the amplitude of the B light negation of R light-induced hypocotyl inhibition (Fig. 5A). The abcb19 mutant seedlings were tested at a higher R light fluence rate (50 μmol/m2s) in order to obtain robust stem inhibition. While the kinetics of hypocotyl growth inhibition were affected in abcb19 mutants, a B light effect was still observed (Fig. S3D). The aux1 mutant seedlings showed an enhanced R-light-induced hypocotyl inhibition response, but the B-light-negation of R-light-induced hypocotyl response is still observed (Fig. 5C). The auxin response factor mutant arf7/nph4 showed a greatly reduced R light hypocotyl inhibition response (also at 50 μmol/m2s). However, the addition of dim B light did partially negate the limited R light hypocotyl inhibition observed in nph4 (Fig. 5E).
Figure 5.
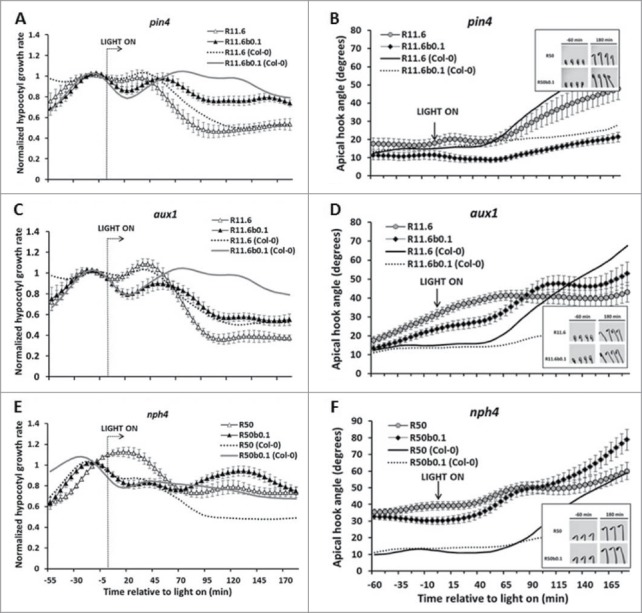
Genetic tests of the dim B light reversal of R light response in auxin transport and response mutants. The dim B light negation of R light-induced hypocotyl growth inhibition and hook opening responses were tested in pin4 (A and B), aux1 (C and D), and arf7/nph4 (E and F). Wild-type Col-0 in response to R light (dotted line) and R light plus dim B light (gray line) were included for comparison.
When apical hook opening was assessed, the pin4 mutant showed slightly delayed opening kinetics compared with wild-type plants in response to R light, and B light appears to reverse this trend (Fig. 5B). The arf7/nph4 mutants consistently presented a slightly open initial apical hook angle in darkness compared to wild-type seedlings (Fig. 5F). Interestingly, none of aux1, and arf7/nph4 mutants showed the B light attenuation of R light induced hook opening response (Fig. 5B, D, and E). These findings seem to be consistent with the results from NPA treatment tests, indicating that normal auxin transport or reponse is important for the development of the B negation of R responses.
Genetic tests of pks mutants
PKS protein family has been reported to serve as the signaling component in both phy signaling pathway and phototropism. It was therefore of interest to test the role of individual PKS proteins, if any, in the phot1-mediated B light negation response to R light. In order to obtain a robust R-light stem inhibition response, a higher R-light fluence rate (50 μmol/m2s) was used. All the pks mutants (pks1, pks2, pks4) showed approximately wild-type hypocotyl growth rate kinetics under higher R conditions, and all responded normally to the B light treatment (Fig. S4).
Discussion
Phytochrome is a classic example of how two wavelengths of light can work in opposition to tailor physiological resposnes to the ambient environment. Such push-pull interactions allow for tailored responses, especially at critical developmental transitions, such as the switch from etiolated to autotrophic growth. Here we expand on previous findings by Wang et al.12 that show that B light can either enhance or negate a R light response, depending on fluence rate. Dim B light signals eliminate the R light response through phot1 action, while higher fluence rates override this inhibition through the cryptochromes.
Early light-induced morphological and physiological changes enable a young seedling to prepare for autotrophic growth. This transition is mediated by multiple photo-sensing systems that couple the light environment to changes in gene expression and physiological response. The phy receptor family (phyA-E) mainly responds to R and FR wavebands. In the initial stage of R light-induced hypocotyl growth inhibition, phyA mediates the fast growth inhibition response which happens within 1-3 hrs after R light onset.10,12 The phyB receptor contributes after hours of illumination for a persistent effect. Addition of B light to R light background activates 2 sets of B light photosenory systems, the crys and the phots.
The cry-mediated B light response generally accelerates/enhances the process of R-light-induced early photomorphogenic events. Such cry effects are most robust under high (>10 μmol m−2 s−1) fluence rate of B light and similar results are seen in this work (Fig. 2A and 3C). Tests of cry mutants for the hook opening response suggest that cry1 plays the major role in stimulating hook opening process. This observation is inconsistent with an earlier report that indicates cry1 does not affect hook opening kinetics under high B light irradiation,13 likely because the present work was performed with simultaneous R and B light treatment.1,4,5
In contrast, in the R light environment the phot1 receptor mediates two opposing responses based on B light fluence rate. In dim B light alone, phot1 mediates rapid growth inhibition within minutes of treatment.24 However, the same treatment in the presence of R light opposes stem growth inhibition.12 The addition of dim B light slows R-light mediated hook opening. One possible explanation is that dim B light provided from above acts as a phototropic guide, causing the plant to extend toward the light without guidance from a side-oriented signal. At higher fluence rates the inhibitory effects of blue light overcome the upward phototropic movement.
Auxin regulates tropic response. Disruption of auxin transport by the auxin transport inhibitor NPA suppresses both phototropic and gravitropic responses.14,25,26 The maintenance of apical hook requires the presence of auxin gradient at the concave side of the hook, and application of NPA eliminates this auxin gradient and stimulates hook opening of dark grown seedlings.21 Additionally, the hypocotyl elongation of light-grown Arabidopsis seedlings also requires auxin transport.25 The fact that NPA can suppress both B-light-mediated attenuation of R-light-induced hypocotyl inhibition and hook opening in our experiments indicates a possible involvement of auxin transport in such responses.
R light not only regulates auxin biosynthesis, but also affects its transport, content, and distribution.27 The phot1-mediated negation of R-light-induced hypocotyl and hook responses is not likely mediated by phot1 antagonizing phy-regulated gene expression, but instead, might be a result of phot1 modulation of auxin transporter localization and activity within a cell.12 In the latter scenario, phot1 and its signal transducer NPH3 may modify the cellular distribution of PIN3 and PIN2 in hypocotyl and root, respectively.15,28 Mutation of PIN3 gene reduces the hypocotyl curvature toward continuous unilateral white light illumination.14,15 However, a recent study suggests that PIN3 and PIN7 mediate the pulse-induced first positive phototropic response, but not the continuous-light-induced second positive phototropic response when the seedlings were placed vertically on the media.18 Similarly, mutations in ABCB19 lead to the abnormal localization of PIN1 transporter, which ultimately enhances hypocotyl phototropism.29 It has been shown that phot1 inhibits ABCB19 activity by direct phosphorylation of this protein in a HeLa cell system.17 Reduction of ABCB19 transporter activity suppresses basipetal auxin transport, resulting in more auxin channeled to lateral directions through PIN3 depending on light directions.17
It was of interest to determine if any of these transporters affected the B light hypocotyl and hook responses in the R environment. The pin1, pin3, pin7, and abcb19 showed normal B-light-negation of the R light hypocotyl inhibition response. Only pin4 showed slight reductions in the amplitude of the response. This result agrees with previous findings,18 and indicates likely redundant roles in the response. The aux1 was originally discovered as root agravitropic mutant, and its role in unilateral BL-induced hypocotyl phototropism is subtle when ARF7/NPH4 is functional.19,30,31 Meanwhile, AUX1 has also been suggested to facilitate auxin transport from the source (leaf) to the sink (root) in Arabidopsis seedlings.32 The enhanced R light-induced hypocotyl inhibition in aux1 mutant indicates AUX1 may antagonize R light hypocotyl response by maintaining a proper auxin concentration in hypocotyl cells. B light might not be able to fully attenuate the enhanced R light inhibition to the wild-type level under such scenario.
The ARF7/NPH4 has been shown to have altered phototropic and gravitropic responses, and thus may directly function in hypocotyl differential growth by modulating auxin responsiveness.22,23,33 The reduced R light (50 μmol/m2s) hypocotyl inhibition in nph4 mutant indicates NPH4 may partly mediate the initial R light hypocotyl inhibition growth. Even under reduced hypocotyl inhibition conditions, B light can still oppose this response (Fig. 5E). The nph4 mutant showed larger initial apical hook angle compared with wild-type plant, indicating its important role in maintaining folded apical hook, which is consistent with previous report.33 It is likely that some of the auxin responsive genes that regulated by ARF7/NPH4 are mediating the B and R light hook opening response at the end point. On the other hand, the normal auxin transport may also be important for the B light negation of hook opening response. Results from pharmacological and genetic tests support such hypothesis.
Several studies have demonstrated that the PKS proteins serve as a molecular link between phy- and phot- meidated responses.34,35 Therefore, we tested the hypothesis that the B light-attenuation of R light hypocotyl response was mediated by one of the PKS proteins. Results reveal no significant differences between mutant and wild-type seedlings. Although these data do not support for the hypothesis, it does not exclude the possibility that multiple PKS proteins might redundantly participate in this response.
Materials and Methods
Plant materials
The Arabidopsis thaliana (hereafter Arabidopsis) photoreceptor mutants tested in this study are identical to those previously assessed 12: cry1-304, cry2-1, phot1-3 (nph1-3). The nph3-6 mutant seeds were obtained from Dr. Mannie Liscum. The pks1-1, pks2-1, and pks4-1 single mutant seeds were obtained from Dr. Christian Fankhauser. The pin3-5 (CS9364), pin4-3 (CS9368), pin7-2 (CS9366), and arf7/nph4 (CS24607) were ordered from the Arabidopsis Biological Resource Center (ABRC) at Ohio State University (Columbus, OH). The abcb19-3 mutant seeds were provided by Dr. Edgar Spalding. The pin1 (CS86744) and aux1-7 (CS3074) mutant seeds are gifts from Dr. Bala Rathinasabapathi. All genotypes tested in this study are in Columbia (Col-0) background, which was used as wild-type.
Light sources and treatments
Light treatments were generated using narrow-bandwidth LED arrays (Light Emitting Computers, Victoria, BC Canada). The peak wavelengths of B light, R light, and FR light are 470, 660, and 730 nm. Light fluence rates were measured using a LI-COR LI-250 photometer with a PAR sensor (LI-COR, Lincoln, NE).
Time course hypocotyl growth and hook opening kinetics
Arabidopsis seeds were surface-sterilized by covering them with 95% ethanol on blotting paper in a laminar flow hood. Upon drying, the seeds were distributed in Petri dishes on minimal media (1mM KCl and 1mM CaCl2) solidified with 1% Difco agar (Beckton, Dickinson and Co, Sparks, MD). The plates were covered in foil and then stratified for 48-72 h at 4°C. The seeds were then treated with 2 h of fluorescent white light (16 μmol/m2s) at 23°C to synchronize germination. Seedlings were grown in absolute darkness at 23°C for 40-48 h. Newly-emerged etiolated seedlings were used for imaging, and images were acquired in a 5-min-interval and analyzed according to the method described.13 For the auxin transport inhibition experiments, germinating seedlings grown on minimal media without NPA were transferred onto minimal media supplemented with NPA (Sigma-Aldrich, St. Louis, MO) at different concentrations. Seedlings were acclimated 40–60 min in darkness on NPA before light experiments.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Drs. Mannie Liscum, Christian Fankhauser, Edgar Spalding, and Bala Rathinasabapathi for supplying mutant seeds for this study.
Funding
This work was supported by the funding from National Science Foundation Grant # IOS-0746756 (KMF).
References
- 1. Casal JJ, Mazzella MA. Conditional synergism between cryptochrome 1 and phytochrome B is shown by the analysis of phyA, phyB, and hy4 simple, double, and triple mutants in Arabidopsis. Plant Physiology 1998; 118:19; PMID:9733522; http://dx.doi.org/ 10.1104/pp.118.1.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Neff MM, Chory J. Genetic interactions between phytochrome A, phytochrome B, and cryptochrome 1 during Arabidopsis development. Plant Physiology 1998; 118:27-35; PMID:9733523; http://dx.doi.org/ 10.1104/pp.118.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Casal JJ. Phytochromes, cryptochromes, phototropin: photoreceptor interactions in plants. Photochem Photobiol 2000; 71:1-11; PMID:10649883; http://dx.doi.org/ 10.1562/0031-8655(2000)071%3c0001:PCPPII%3e2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 4. Ahmad M, Cashmore AR. The blue-light receptor cryptochrome 1 shows functional dependence on phytochrome A or phytochrome B in Arabidopsis thaliana. Plant J 1997; 11:421-7; PMID:9107032; http://dx.doi.org/ 10.1046/j.1365-313X.1997.11030421.x. [DOI] [PubMed] [Google Scholar]
- 5. Mas P, Devlin PF, Panda S, Kay SA. Functional interaction of phytochrome B and cryptochrome 2. Nature 2000; 408:207-11; PMID:11089975; http://dx.doi.org/ 10.1038/35041583 [DOI] [PubMed] [Google Scholar]
- 6. Whippo CW, Hangarter RP. Second positive phototropism results from coordinated co-action of the phototropins and cryptochromes. Plant Physiol 2003; 132:1499-507; PMID:12857830; http://dx.doi.org/ 10.1104/pp.102.018481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lariguet P, Fankhauser C. Hypocotyl growth orientation in blue light is determined by phytochrome A inhibition of gravitropism and phototropin promotion of phototropism. Plant J 2004; 40:826-34; PMID:15546364; http://dx.doi.org/ 10.1111/j.1365-313X.2004.02256.x. [DOI] [PubMed] [Google Scholar]
- 8. Rösler J, Klein I, Zeidler M. Arabidopsis fhl/fhy1 double mutant reveals a distinct cytoplasmic action of phytochrome A. Proc Natl Acad Sci 2007; 104:10737-42; http://dx.doi.org/ 10.1073/pnas.0703855104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kami C, Hersch M, Trevisan M, Genoud T, Hiltbrunner A, Bergmann S, Fankhauser C. Nuclear phytochrome A signaling promotes phototropism in Arabidopsis. The Plant Cell Online 2012; 24:566-76; http://dx.doi.org/ 10.1105/tpc.111.095083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parks BM, Spalding EP. Sequential and coordinated action of phytochromes A and B during Arabidopsis stem growth revealed by kinetic analysis. P Natl Acad Sci USA 1999; 96:14142; http://dx.doi.org/ 10.1073/pnas.96.24.14142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Takemiya A, Inoue S-i, Doi M, Kinoshita T, Shimazaki K-i. Phototropins promote plant growth in response to blue light in low light environments. The Plant Cell Online 2005; 17:1120-7; http://dx.doi.org/ 10.1105/tpc.104.030049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Y, Maruhnich SA, Mageroy MH, Justice JR, Folta KM. Phototropin 1 and cryptochrome action in response to green light in combination with other wavelengths. Planta 2013; 237:225-37; PMID:23007554; http://dx.doi.org/ 10.1007/s00425-012-1767-y [DOI] [PubMed] [Google Scholar]
- 13. Wang L, Uilecan IV, Assadi AH, Kozmik CA, Spalding EP. HYPOTrace: Image analysis software for measuring hypocotyl growth and shape demonstrated on Arabidopsis seedlings undergoing photomorphogenesis. Plant Physiol 2009; 149:1632-7; PMID:19211697; http://dx.doi.org/ 10.1104/pp.108.134072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Friml J, Wiśniewska J, Benková E, Mendgen K, Palme K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 2002; 415:806; PMID:11845211; http://dx.doi.org/ 10.1038/415806a. [DOI] [PubMed] [Google Scholar]
- 15. Ding Z, Galvan-Ampudia CS, Demarsy E, Langowski L, Kleine-Vehn J, Fan Y, Morita MT, Tasaka M, Fankhauser C, Offringa R, et al. Light-mediated polarization of the PIN3 auxin transporter for the phototropic response in Arabidopsis. Nat Cell Biol 2011; 13:447-52; PMID:21394084; http://dx.doi.org/ 10.1038/ncb2208. [DOI] [PubMed] [Google Scholar]
- 16. Rakusová H, Gallego-Bartolomé J, Vanstraelen M, Robert HS, Alabadí D, Blázquez MA, Benková E, Friml J. Polarization of PIN3-dependent auxin transport for hypocotyl gravitropic response in Arabidopsis thaliana. The Plant Journal 2011:no-no. [DOI] [PubMed] [Google Scholar]
- 17. Christie JM, Yang H, Richter GL, Sullivan S, Thomson CE, Lin J, Titapiwatanakun B, Ennis M, Kaiserli E, Lee OR, Adamec J, et al. phot1 inhibition of ABCB19 primes lateral auxin fluxes in the shoot apex required for phototropism. PLoS biology 2011; 9:e1001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Haga K, Sakai T. PIN auxin efflux carriers are necessary for pulse-induced but not continuous light-induced phototropism in Arabidopsis. Plant Physiology 2012; 160:763-76; PMID:22843667; http://dx.doi.org/ 10.1104/pp.112.202432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stone BB, Stowe-Evans EL, Harper RM, Celaya RB, Ljung K, Sandberg G, Liscum E. Disruptions in AUX1-dependent auxin influx alter hypocotyl phototropism in Arabidopsis. Mol Plant 2008; 1:129-44; PMID:20031920; http://dx.doi.org/ 10.1093/mp/ssm013. [DOI] [PubMed] [Google Scholar]
- 20. Žádníková P, Petrášek J, Marhavý P, Raz V, Vandenbussche F, Ding Z, Schwarzerová K, Morita MT, Tasaka M, Hejátko J, et al. Role of PIN-mediated auxin efflux in apical hook development of Arabidopsis thaliana. Development 2010; 137:607-17; http://dx.doi.org/ 10.1242/dev.041277. [DOI] [PubMed] [Google Scholar]
- 21. Wu G, Cameron JN, Ljung K, Spalding EP. A role for ABCB19-mediated polar auxin transport in seedling photomorphogenesis mediated by cryptochrome 1 and phytochrome B. The Plant Journal 2010; 62:179-91; PMID:20088903; http://dx.doi.org/ 10.1111/j.1365-313X.2010.04137.x. [DOI] [PubMed] [Google Scholar]
- 22. Liscum E, Briggs WR. Mutations of Arabidopsis in potential transduction and response components of the phototropic signaling pathway. Plant Physiology 1996; 112:291-6; PMID:8819327; http://dx.doi.org/ 10.1104/pp.112.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harper RM, Stowe-Evans EL, Luesse DR, Muto H, Tatematsu K, Watahiki MK, Yamamoto K, Liscum E. The NPH4 locus encodes the auxin response factor ARF7, a conditional regulator of differential growth in aerial Arabidopsis tissue. The Plant Cell Online 2000; 12:757-70; http://dx.doi.org/ 10.1105/tpc.12.5.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Folta KM, Spalding EP. Unexpected roles for cryptochrome 2 and phototropin revealed by high-resolution analysis of blue light-mediated hypocotyl growth inhibition. The Plant Journal 2001; 26:471-8; PMID:11439133; http://dx.doi.org/ 10.1046/j.1365-313x.2001.01038.x. [DOI] [PubMed] [Google Scholar]
- 25. Jensen PJ, Hangarter RP, Estelle M. Auxin transportis required for hypocotyl elongation in light-grown but not dark-grown Arabidopsis. Plant Physiology 1998; 116:455-62; PMID:9489005; http://dx.doi.org/ 10.1104/pp.116.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nagashima A, Uehara Y, Sakai T. The ABC subfamily B auxin transporter AtABCB19 is involved in the inhibitory effects of N-1-Naphthyphthalamic Acid on the phototropic and gravitropic responses of Arabidopsis hypocotyls. Plant and Cell Physiology 2008; 49:1250-5; PMID:18556728; http://dx.doi.org/ 10.1093/pcp/pcn092. [DOI] [PubMed] [Google Scholar]
- 27. Nagashima A, Suzuki G, Uehara Y, Saji K, Furukawa T, Koshiba T, Sekimoto M, Fujioka S, Kuroha T, Kojima M, et al. Phytochromes and cryptochromes regulate the differential growth of Arabidopsis hypocotyls in both a PGP19-dependent and a PGP19-independent manner. The Plant Journal 2008; 53:516-29; PMID:18086281; http://dx.doi.org/ 10.1111/j.1365-313X.2007.03358.x. [DOI] [PubMed] [Google Scholar]
- 28. Wan Y, Jasik J, Wang L, Hao H, Volkmann D, Menzel D, Mancuso S, Baluška F, Lin J. The signal transducer NPH3 integrates the phototropin1 photosensor with PIN2-based polar auxin transport in Arabidopsis root phototropism. The Plant Cell Online 2012; 24:551-65; http://dx.doi.org/ 10.1105/tpc.111.094284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Noh B, Bandyopadhyay A, Peer WA, Spalding EP, Murphy AS. Enhanced gravi-and phototropism in plant mdr mutants mislocalizing the auxin efflux protein PIN1. Nature 2003; 423:999-1002; PMID:12827205; http://dx.doi.org/ 10.1038/nature01716. [DOI] [PubMed] [Google Scholar]
- 30. Bennett MJ, Marchant A, Green HG, May ST, Ward SP, Millner PA, Walker AR, Schulz B, Feldmann KA. Arabidopsis AUX1 gene: A permease-like regulator of root gravitropism. Science 1996; 273:948-50; PMID:8688077; http://dx.doi.org/ 10.1126/science.273.5277.948. [DOI] [PubMed] [Google Scholar]
- 31. Marchant A, Kargul J, May ST, Muller P, Delbarre A, Perrot-Rechenmann C, Bennett MJ. AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. The EMBO Journal 1999; 18:2066-73; PMID:10205161; http://dx.doi.org/ 10.1093/emboj/18.8.2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marchant A, Bhalerao R, Casimiro I, Eklöf J, Casero PJ, Bennett M, Sandberg G. AUX1 promotes lateral root formation by facilitating Indole-3-Acetic Acid distribution between sink and source tissues in the Arabidopsis seedling. The Plant Cell Online 2002; 14:589-97; http://dx.doi.org/ 10.1105/tpc.010354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stowe-Evans EL, Harper RM, Motchoulski AV, Liscum E. NPH4, a conditional modulator of auxin-dependent differential growth responses in Arabidopsis. Plant Physiology 1998; 118:1265-75; PMID:9847100; http://dx.doi.org/ 10.1104/pp.118.4.1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lariguet P, Schepens I, Hodgson D, Pedmale UV, Trevisan M, Kami C, de Carbonnel M, Alonso JM, Ecker JR, Liscum E, et al. PHYTOCHROME KINASE SUBSTRATE 1 is a phototropin 1 binding protein required for phototropism. Proc Nal Acad Sci 2006; 103:10134-9; http://dx.doi.org/ 10.1073/pnas.0603799103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Demarsy E, Schepens I, Okajima K, Hersch M, Bergmann S, Christie J, Shimazaki K, Tokutomi S, Fankhauser C. Phytochrome Kinase Substrate 4 is phosphorylated by the phototropin 1 photoreceptor. The EMBO Journal 2012; 31:3457-67; PMID:22781128; http://dx.doi.org/ 10.1038/emboj.2012.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


