Abstract
We report a chimeric monoclonal antibody (mAb) directed to a neo-epitope that is exposed in the IgG lower hinge following proteolytic cleavage. The mAb, designated 2095–2, displays specificity for IdeS-generated F(ab’)2 fragments, but not for full-length IgG or for closely-related F(ab’)2 fragments generated with other proteases. A critical component of the specificity is provided by the C-terminal amino acid of the epitope corresponding to gly-236 in the IgG1 (also IgG4) hinge. By its ability to bind to IdeS-cleaved anti-CD20 mAb, mAb 2095–2 fully restored antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) against WIL2-S cells to the otherwise inactive anti-CD20 IgG1 F(ab’)2 fragment. Similarly, 2095–2 reinstated ADCC against MDA-MB-231 cells to an anti-CD142 IgG1 F(ab’)2 fragment. mAb 2095–2 was also capable of eliciting both CDC and ADCC to IgG4 F(ab’)2 fragments, an IgG subclass that has weaker ADCC and CDC when intact relative to intact IgG1. The in vitro cell-based efficacy of 2095–2 was extended to the in vivo setting using platelets as a cell clearance surrogate. In a canine model, the co-administration of 2095–2 together with IdeS-generated, platelet-targeting anti-CD41/61 F(ab’)2 fragment not only restored platelet clearance, but did so at a rate and extent of clearance that exceeded that of intact anti-CD41/61 IgG at comparable concentrations. To further explore this unexpected amplification effect, we conducted a rat study in which 2095–2 was administered at a series of doses in combination with a fixed dose of anti-CD41/61 F(ab’)2 fragments. Again, the combination, at ratios as low as 1:10 (w/w) 2095–2 to F(ab’)2, proved more effective than the anti-CD41/61 IgG1 alone. These findings suggest a novel mechanism for enhancing antibody-mediated cell-killing effector functions with potential applications in pathologic settings such as tumors and acute infections where protease activity is abundant.
Keywords: IgG fragments, antibody-dependent cell-mediated cytotoxicity, chimeric antibody, complement-dependent cytotoxicity, hinge region
Abbreviations
- ADCC
antibody-dependent cell-mediated cytotoxicity
- CDC
complement-dependent cytotoxicity
- FACS
fluorescence-activated cell sorter
- GluV8
glutamyl endopeptidase V8
- IdeS
Immunoglobulin G-degrading enzyme of Streptococcus pyogenes
- mAb
monoclonal antibody
- MMP
matrix metalloproteinase
- PBMC
peripheral blood mononuclear cell
Introduction
Proteolytically cleaved IgGs present cryptic epitopes for autoimmune recognition.1 Accordingly, a majority of healthy individuals possess autoantibodies that bind to specific sites of cleavage in the IgG hinge region.2 This class of autoantibody targets the C-terminal ends of Fab and F(ab′)2 fragments, as well as certain intermediates, that derive from human and bacterial enzymes.2 Proteases associated with cancer, inflammation, and infectious diseases are represented in the group.3,4 These autoantibodies also bind to single-chain peptide analogs of the IgG1 hinge possessing defined C-termini that correspond to sites generated in IgG by protease action.2,5 Comparable autoimmune profiles were not detected against the hinge region of intact IgG2 or to cleavage site analog peptides with the opposing free N-termini.6
The hinge of IgG is the flexible domain that connects the two antigen binding Fab components to the Fc domain. The Fc provides structure to recruit and bridge immune cells and complement to achieve the eradication of pathologic cells.7,8 As expected, the effector functions of IgGs are largely negated if the Fc region is fully removed by proteolytic action –a circumstance requiring the scission of both hinge heavy chains.2 A surprising finding was that a comparable loss-of-function ensued from a single proteolytic scission in only one of the hinge chains.9,10 In some circumstances, it appeared that a single proteolytic cleavage of IgG may be the predominant product when cleavage occurs on cell surfaces.9 In an extension of these findings, it was shown that a single proteolytic cleavage of trastuzumab, a clinically-indicated monoclonal antibody (mAb) therapeutic for cancer, resulted in reduced immune effector function and in vivo efficacy.10
A function for serum autoantibodies that target sites of proteolytic cleavage in IgG was suggested by the demonstration that their binding to cleaved IgGs could restore in vitro cell killing activity to inactive mAb fragments.2 A related in vivo example of the phenomenon was provided by a primate model in which circulating platelet numbers decreased when a platelet-directed monoclonal F(ab′)2 fragment encountered high titers of autoantibodies directed against the lower hinge pepsin cleavage site.11 The anti-hinge autoantibodies in both of the above cases were polyclonal, serum-derived immunoglobulins. It has been proposed that anti-hinge autoantibodies may thereby provide host immunity with a defense pathway to combat the local inactivation of IgGs in the proteolytic environments that can surround pathogenic cells.1,3 Human anti-hinge (HAH) autoantibodies have also been detected in patients with chronic inflammatory disorders such as rheumatoid arthritis and inflammatory bowel disease,12 where patients also have elevated levels of proteases capable of cleaving IgGs (e.g., MMP-3, human neutrophil elastase).13 In the case of rheumatoid arthritis, it has been suggested that rather than aiding host immune responses against invasive cells, HAH can instead augment the pathology associated with cleaved self-reactive antibodies.14 These recent observations add to previous literature on the presence of human anti-hinge antibodies in pathologies as diverse as cold agglutination,15 HIV,16 systemic lupus erythematous,17 and cancer,18 as well as suggestions for immunoregulatory functions.19 Nevertheless, the functional roles of serum anti-hinge antibodies remain obscure, due, in part, to their polydispersity and polyclonal nature.1
The preceding evidence for a connection between the proteolytic cleavage of IgGs and anti-hinge autoantibodies led us to investigate the potential utility of a monoclonal anti-hinge antibody. For the present study a mAb, designated 2095–2 in its chimerized version, was generated against the site at which the bacterial IdeS protease (S. pyogenes) specifically cleaves the human IgG1 hinge between gly-236 and gly-237.20,21 mAb 2095-2 possessed no demonstrable binding activity against intact IgG1. This mAb was characterized with regard to its ability to restore function to IdeS-cleaved IgGs in a panel of in vitro cell-killing assays. For in vivo purposes, a platelet clearance model was configured in both canines and rats to establish the extent and timing of functional restoration to an IdeS-cleaved fragment of an anti-platelet mAb that targets CD41/61. These models provided evidence for subtle dose response inter-relationships between cleaved IgG and 2095–2. By comparison to the intact anti-platelet mAb administered alone, the addition of the anti-hinge mAb to the inactive fragment revealed instances of unexpected functional amplification for the combination approach.
Results
An anti-hinge mAb directed against the IdeS cleavage site displays specificity for IdeS-generated F(ab’)2 fragments, but not for full-length IgG or F(ab’)2 fragments generated with MMP-3 or GluV8
We had previously shown that HAH autoantibodies can be detected in the serum of normal humans.2,5 These polyclonal HAH autoantibodies displayed specificity for cleaved IgGs, but were not cross-reactive with the intact IgG counterpart.2 We had further demonstrated that HAH autoantibodies could restore antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) function to cell-bound F(ab’)2 fragments.2 To generate a monoclonal anti-hinge antibody, we immunized rabbits with a peptide analog of the human IgG1 hinge region with a C-terminal amino acid corresponding to the IdeS cleavage site (i.e., G236 in Kabat numbering aligned to the EU sequence22). As shown in Figure 1A, the rabbit anti-hinge mAb 91–2 detected an IdeS-generated F(ab’)2 fragment, but not the intact IgG1 counterpart or MMP-3 or GluV8-generated F(ab’)2 fragments. To demonstrate that mAb 91–2 was capable of restoring immune effector function to IdeS cleaved F(ab’)2 fragments, we performed an anti-hinge rescue CDC assay using anti-CD20 rituximab against WIL2-S target cells. As shown in Figure 1B, the rabbit anti-hinge mAb restored CDC function to an IdeS-generated F(ab’)2 fragment of rituximab. The rabbit mAb 91–2 was then engineered to have a human IgG1 constant region, and this mAb was designated 2095–2. As shown in Figure 1C, 2095–2 demonstrated specificity for an IdeS-generated F(ab’)2 fragment, but not for intact IgG1 or F(ab’)2 fragments generated with MMP-3 or GluV8. These results indicate that we have generated a variable region specific for the IdeS cleavage site in human IgG1, and that chimerization does not affect the binding specificity.
Figure 1.
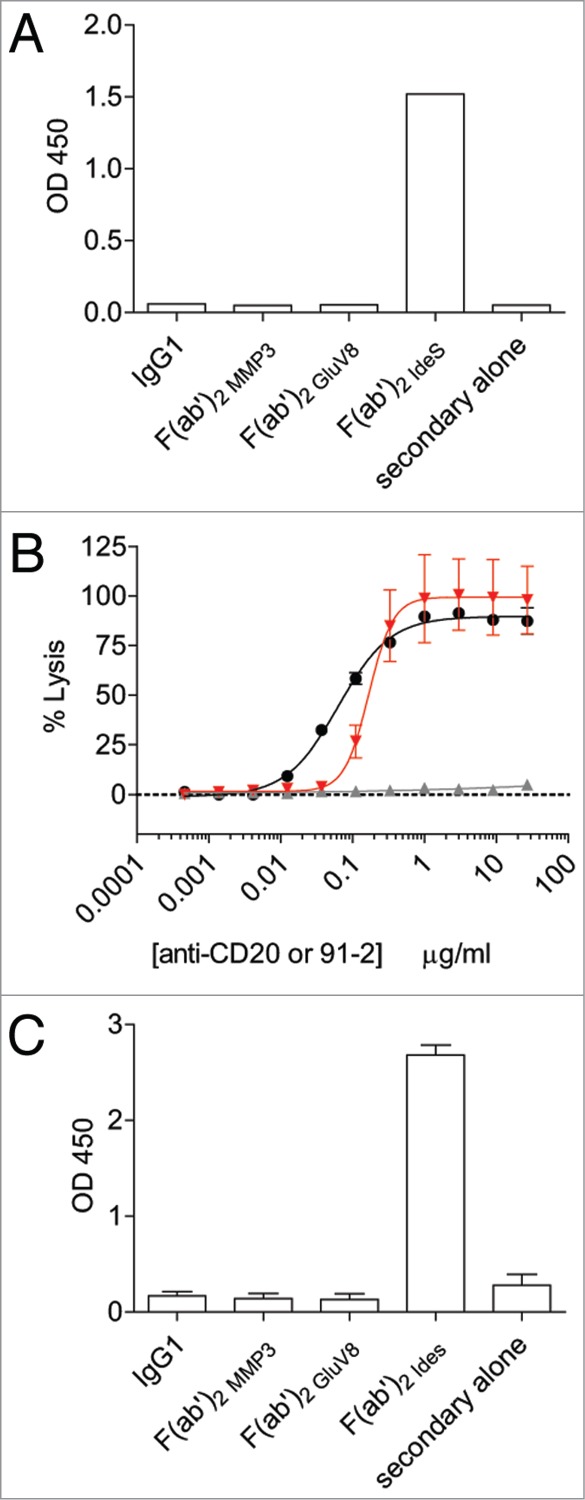
Generation of a monoclonal antibody specific for IdeS cleavage site in the lower hinge of human IgG1. (A) The fully rabbit anti-hinge mAb 91–2 bound to IdeS-generated F(ab’)2 fragments, but not to intact IgG1 and F(ab’)2 fragments generated with MMP-3 or GluV8 in a plate-based ELISA assay. Data are representative of two independent experiments (duplicate measurements per experiment). (B) CDC activity was measured using rituximab IgG1 (black circles), rituximab IgG1 F(ab’)2 IdeS (solid gray up triangles), and a fixed concentration of rituximab IgG1 F(ab’)2 IdeS (1 μg/ml) in the presence of serially-diluted 91–2 (solid red down triangles). Data are representative of two independent experiments (triplicate measurements per experiment). (C) The rabbit/human chimeric anti-hinge mAb 2095–2 displayed the same specificity as the fully rabbit mAb 91–2. Data are representative of two independent experiments (duplicate measurements per experiment).
2095–2 restores ADCC and CDC only to F(ab’)2 fragments generated with IdeS
We next wanted to assess the specificity of 2095–2 in the context of cell-surface bound F(ab’)2 fragments generated with IdeS, GluV8, or MMP-3. Figure 2A demonstrates that F(ab’)2 fragments of rituximab generated with the 3 aforementioned proteases all bound to the WIL2-S target cells at nearly identical levels. However, when 2095–2 was applied to the WIL2-S cells opsonized with the 3 different F(ab’)2 fragments, 2095–2 was only detected on cells opsonized with IdeS-generated F(ab’)2 fragments (Fig. 2B). Accordingly, a fixed concentration of 3 μg/ml of 2095–2 was only capable of restoring ADCC function to IdeS-generated F(ab’)2 fragments and not to MMP-3 or GluV8-generated F(ab’)2 fragments (Fig. 2C). A CDC assay was next employed using WIL-2 cells as target cells and intact anti-CD20 IgG1 and F(ab’)2 fragments generated with IdeS, GluV8, or MMP-3. As was shown with ADCC, a fixed concentration of 5 μg/ml of 2095–2 only restored CDC function to IdeS-generated F(ab’)2 fragments, whereas no restoration of function was seen with 2095–2 co-incubated with GluV8 or MMP-3 F(ab’)2 fragments (Fig. 2D). These results demonstrate that, in the context of F(ab’)2 fragments bound to the surface of a cell, 2095–2 only displayed binding specificity and restoration of function activity to IgG1 cleaved with IdeS.
Figure 2.
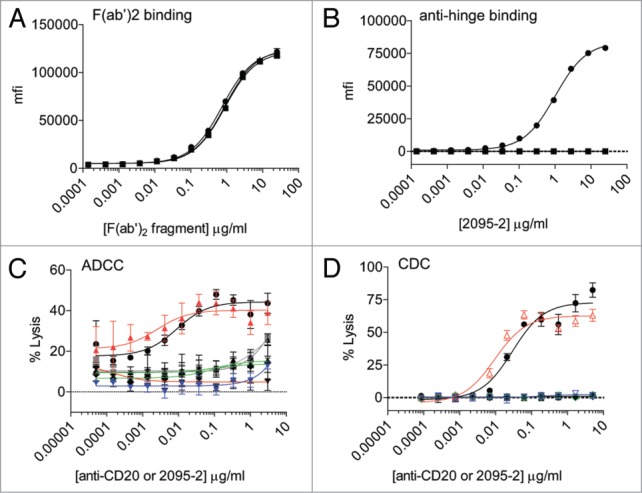
2095–2 specificity on cell-bound F(ab’)2 fragments and ADCC/CDC restoration of function. (A) F(ab’)2 fragments of rituximab generated with IdeS (solid black circles), GluV8 (solid black up triangles), and MMP-3 (solid black squares) displayed comparable binding to WIL2-S cells. (B) The anti-hinge mAb 2095–2 bound to cells opsonized with IdeS-generated F(ab’)2 fragments (solid black circles), but not to GluV8-generated F(ab’)2 fragments (solid black up triangles) or MMP-3-generated (solid black squares). Data from (A) and (B) are representative of two independent experiments. (C) The anti-hinge mAb 2095–2 was capable of restoring ADCC function to IdeS-generated F(ab’)2 fragments (open up red triangles), but not to GluV8-generated F(ab’)2 fragments (open green diamonds) or MMP-3-generated F(ab’)2 fragments (open down blue triangles). Addition of 2095–2 alone (solid gray squares) or F(ab’)2 IdeS fragments (solid black up triangles), F(ab’)2 GluV8 fragments (solid black diamonds), or F(ab’)2 MMP-3 fragments (solid black down triangles) alone did not result in appreciable ADCC activity. Data are representative of 2 independent experiments (duplicate measurements per experiment). (D) The anti-hinge mAb 2095–2 was capable of restoring CDC function to anti-CD20 IdeS-generated F(ab’)2 fragments (open up red triangles), but not to GluV8-generated F(ab’)2 fragments (open green diamonds) or MMP-3-generated F(ab’)2 fragments (open down blue triangles). Addition of 2095–2 alone (solid gray squares) or F(ab’)2 IdeS fragments (solid black up triangles), F(ab’)2 GluV8 fragments (solid black diamonds), or F(ab’)2 MMP-3 fragments (solid black down triangles) alone did not result in appreciable CDC activity. Data are representative of 2 independent experiments (triplicate measurements per experiment).
2095–2 is capable of restoring ADCC and CDC functions to IgG4 F(ab’)2 fragments generated with IdeS
PBMC-based ADCC assays in the 2–4 h range are driven by FcγRIIIa expressed on NK cells,23,24 and among the IgG1, IgG2, and IgG4 subclasses, IgG1 demonstrates the highest affinity for FcγRIIIa.25 Since IdeS is capable of cleaving all of the human IgG subclasses within the lower hinge region,21 we sought to determine if 2095–2 could restore function to F(ab’)2 fragments from each subclass. ADCC assays were set-up using MDA-MB-231 target cells with anti-CD142 mAbs on the human IgG1, IgG2, and IgG4 subclasses. The IgG1 anti-CD142 mAb elicited ADCC, whereas the IgG2 and IgG4 subclasses had limited to undetectable ADCC activity (Fig. 3A). Addition of the 2095–2 mAb alone did not result in detectable ADCC activity. To determine if 2095–2 could rescue ADCC activity against different IgG subclasses, MDA-MB-231 cells were opsonized with IgG1, IgG2, and IgG4 IdeS-generated F(ab’)2 fragments in the presence of a fixed concentration of 3 μg/ml of 2095–2. As shown in Figure 3B, 2095–2 was capable of restoring anti-CD142 ADCC activity to an IgG1 F(ab’)2 fragment, but not to an IgG2 F(ab’)2 fragment. In contrast, 2095–2 was capable of restoring ADCC to the IgG4 F(ab’)2 fragment. A similar CDC assay was set-up where WIL2-S cells were opsonized with either IgG1, IgG2, or IgG4 subclass anti-CD20 mAbs and corresponding IdeS-generated F(ab’)2 fragments. Consistent with the ADCC results, a fixed concentration of 5 μg/ml 2095–2 was capable of restoring CDC activity to both F(ab’)2 fragment IgG1 and IgG4 anti-CD20 mAbs, but not to an IgG2 F(ab’)2 fragment (Fig. 3D). These results demonstrate that anti-hinge antibodies have the ability to restore ADCC and CDC functions to IgG4 IdeS-generated F(ab’)2 fragments where the intact parental IgG4 mAb had limited to undetectable activity.
Figure 3.
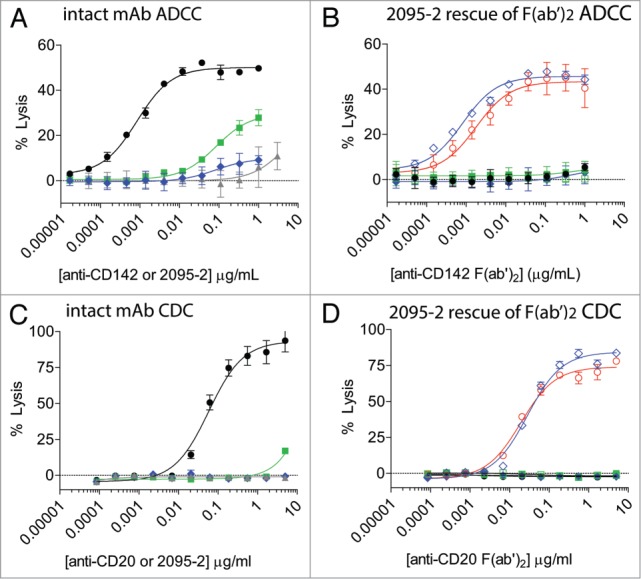
2095–2 restores ADCC and CDC function to both IdeS-generated IgG1 F(ab’)2 fragments and IgG4 F(ab’)2 fragments, but not to IgG2 F(ab’)2 fragments. (A) The intact anti-CD142 IgG1 mAb elicited ADCC against MDA-MB-231 cells (solid black circles), whereas anti-CD142 IgG2 (solid green squares), anti-CD142 IgG4 (solid blue diamonds), and 2095–2 alone (solid gray up diamonds) showed minimal ADCC activity. (B) Anti-CD142 IdeS-generated F(ab’)2 fragments of IgG1 (solid black circles), IgG2 (solid green squares), and IgG4 (solid blue diamonds) did not elicit detectable ADCC. A fixed concentration of 3 μg/ml of 2095–2 restored ADCC to anti-CD142 IdeS-generated F(ab’)2 fragments of IgG1 (open red circles) and IgG4 (open blue diamonds). Data in (A) and (B) are representative of three independent experiments (duplicate measurements per experiment). (C) The intact anti-CD20 IgG1 mAb elicited CDC against WIL2-S cells (solid black circles), whereas anti-CD20 IgG2 (solid green squares), anti-CD20 IgG4 (solid blue diamonds), and 2095–2 alone (solid gray up diamonds) displayed minimal CDC activity. (D) Anti-CD20 IdeS-generated F(ab’)2 fragments of IgG1 (solid black circles), IgG2 (solid green squares), and IgG4 (solid blue diamonds) did not elicit detectable CDC. A fixed concentration of 5 μg/ml of 2095–2 restored CDC to anti-CD20 IdeS-generated F(ab’)2 fragments of IgG1 (open red circles) and IgG4 (open blue diamonds). Data in (C) and (D) are representative of two independent experiments (triplicate measurements per experiment).
2095–2 restores and amplifies cell-clearing function to a cleaved anti-platelet mAb
Antibody-mediated clearance of platelets has proven to be a useful model to assess the in vivo biological activity of different IgG subclasses,26 as well as Fc-engineered variants.27 For the purposes of this study, a model of platelet clearance in canines was devised using the chimeric anti-CD41/CD61 mAb, c7E3.28 A pilot phase was conducted to establish the dose requirements and the timing of platelet clearance with c7E3 IgG (data not shown). The results indicated that 0.01 mg/kg dose was ineffective in canines at 2 and 24 h and was indistinguishable from the saline group. A 0.05 mg/kg dose had little effect by 2 h, but at 24 h had resulted in > 90% platelet clearance. The 0.2 mg/kg dose of c7E3 IgG resulted in profound platelet clearance at 2 h that was then maintained at 24 h. These results pointed to a dose of 0.05 mg/kg for the c7E3 IdeS-generated F(ab’)2 fragment to compare with intact IgG and for subsequent concentration-effect combinations with the 2095–2 mAb.
The results obtained with c7E3 IgG and the F(ab’)2 fragment are presented in Figure 4. Intact c7E3 IgG administered at 0.05 mg/kg induced a decline in platelet number (∼70% clearance) with a nadir apparent at 24–48 h. Recovery of platelet numbers began at ∼72 h and slowly increased through the 96-h determination. An additional blood sample was incorporated into the study at 7 d (168 h) to allow a longer-term assessment of platelet recovery and showed that full recovery was achieved (in all applicable treatment groups). In contrast, the platelet numbers in the c7E3 F(ab’)2 group were not different from those in the saline control group. Likewise the cleavage site-specific mAb, 2095–2, when infused alone, had no measurable impact on circulating platelets. However, in the group that received c7E3 F(ab’)2 (0.05 mg/kg) plus mAb 2095–2 (0.5 mg/kg), a rapid decrease of platelet counts occurred such that at 2 h the effect was nearly maximal. This was a markedly faster clearance than observed with intact c7E3 IgG (0.05 mg/kg). In addition, the extent of platelet decline was greater in the c7E3 F(ab’)2 / 2095–2 combination group (2 and 24 h) compared with the intact c7E3 IgG group (24 h). These results demonstrated that 2095–2 not only remediated in vivo cell-clearance to an otherwise-ineffective anti-platelet F(ab’)2 fragment, but provided an apparent amplification of the IgG clearance mechanism.
Figure 4.
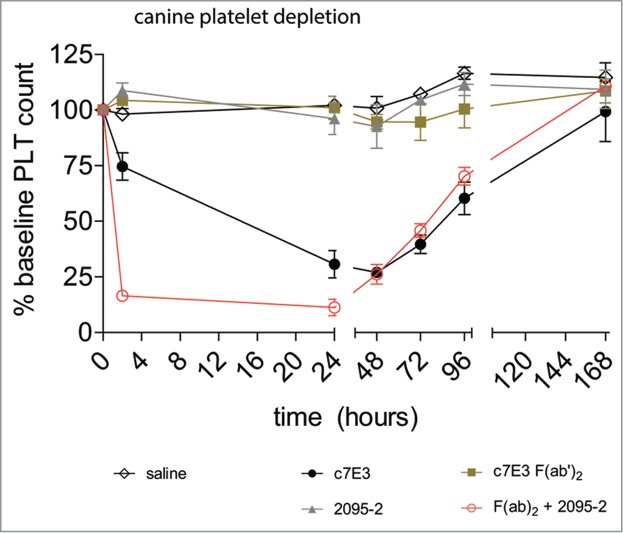
2095–2 restores in vivo platelet depletion to IdeS-generated F(ab’)2 fragments of c7E3 in canines. Treatment of canines with saline (open black diamonds), 0.05 mg/kg of 2095–2 alone (gray up triangles), and 0.05 mg/kg of IdeS-generated F(ab’)2 fragments of c7E3 alone (solid brown squares) did not result in platelet depletion, whereas treatment with 0.05 mg/kg of intact c7E3 (solid black circles) resulted in platelet depletion. The combined treatment of 0.05 mg/kg of c7E3 F(ab’)2 IdeS and 0.5 mg/kg of 2095–2 (open red circles) resulted in rapid platelet clearance. Data are representative of the average +/− SEM of three animals per group.
The suggestion of a novel amplification phenomenon led us to conduct a more detailed dose-response investigation for mAb 2095–2 combination therapy. For this purpose, we adopted a rat model of platelet clearance. The c7E3 F(ab’)2 fragment had been previously shown to bind to rat platelets, although with lower affinity compared with canine and primate platelets.29 In a similar approach to the above canine study, we first examined a range of doses of c7E3 IgG for its effects on rat platelet clearance and established that a dose of 3 mg/kg resulted in detectable platelet clearance in rats, whereas platelet depletion was minimal at a 1 mg/kg dose (data not shown). Based on those results, we chose a dose of 1 mg/kg for c7E3 F(ab’)2 with the goal of providing a maximum range for any potential restorative effects of mAb 2095–2. As shown in Figure 5A, intact c7E3 dosed at 3 mg/kg resulted in an ∼70% decline at 24 h whereas a 1 mg/kg dose was essentially ineffective. Figure 5B depicts the results of the combination experiments. As expected, c7E3 F(ab’)2 alone at 1 mg/kg did not induce a progressive decline of platelet numbers, but the same F(ab’)2 dose combined with 0.1 mg/kg 2095–2 decreased platelet numbers (1:10 (w/w) ratio or ∼1:7 molar ratio of 2095–2 to F(ab’)2). The kinetic profile obtained with the combination was not appreciably different from that observed with intact c7E3 IgG at 3 mg/kg (Fig. 5A). Further, increasing doses of 2095–2 (0.33, 1.0 and 3.0 mg/kg) greatly accelerated the rate of platelet clearance by c7E3 F(ab’)2, and all three combination regimens effectively reduced platelet numbers to near the limit of detection through 24 h. The results of the dose-ranging study in rats corroborated the earlier findings in canines and further pointed to an unexpected combinatorial mechanism of cell (platelet) clearance using an anti-hinge mAb approach.
Figure 5.
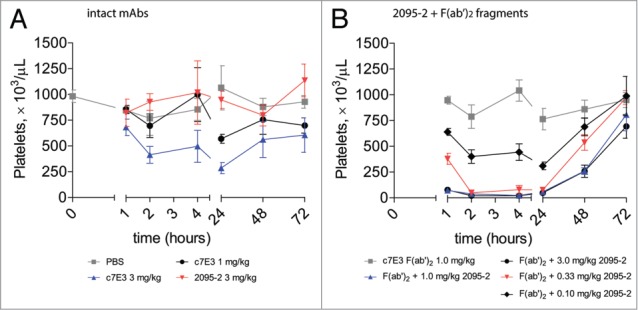
2095–2 amplifies in vivo platelet depletion to IdeS-generated F(ab’)2 fragments of c7E3 in rats compared with intact c7E3. (A) Treatment of rats with PBS (solid gray squares), 1 mg/kg of c7E3 (solid black circles), or 3 mg/kg of 2095–2 (solid red down triangles) did not result in platelet depletion, whereas treatment with 3 mg/kg of c7E3 (solid blue up triangles) displayed detectable platelet depletion. (B) Treatment of rats with 1 mg/kg of c7E3 F(ab’)2 fragment did not result in platelet depletion (solid gray squares), whereas the combination of 1 mg/kg of c7E3 F(ab’)2 fragment and 0.1 mg/kg of 2095–2 (solid black diamonds), 0.33 mg/kg of 2095–2 (solid red down triangles), 1 mg/kg of 2095–2 (solid blue up triangles), or 3 mg/kg of 2095–2 (solid black circles) resulted in a dose-dependent increase in platelet depletion. Data are representative of the average +/− SEM of three animals per group.
Discussion
The motivation for developing a mAb directed to cleavage site neo-epitopes in the IgG1 hinge stemmed from the many observations that human anti-hinge antibodies occur widely in the normal population.1,30 The roles of such polyclonal antibodies in health and disease remain obscure and have led to numerous correlations and hypotheses.1
We chose rabbits for anti-hinge mAb development when it was noted that strong polyclonal responses to the peptide antigens of interest were consistently obtained,31 and similar responses were not gained with immunizations in mice. Indeed, others have indicated that mice rarely generate anti-hinge autoantibodies.32 The rabbit immunogen was a cysteine-coupled KLH conjugate of the peptide CTAPPAPAPELLG with a free C-terminal glycine corresponding to the point of IdeS-mediated scission between gly-236 and gly-237 in the human lower hinge.21 The sequence corresponding to the core hinge of IgG1, CPPC, was changed to APPA in order to prevent inter and intra disulfide bonds within the peptide preparation. An obvious feature of this immunogen is that its C-terminal exposure of gly-236 corresponds with the end of IgG1 that remains bound to cellular targets after cleavage. In fact, we had found no similar serum reactivity to the opposing side of the cleavage site containing the free N-terminus (gly-237).6 The initial rabbit IgG (91–2) was engineered as a chimeric rabbit/human mAb (2095–2) with human constant domains and rabbit antigen-binding domains. The 2095–2 mAb displayed selective binding to antigens displaying the gly-236 terminus and negligible reactivity for extended sequences or to IgG hinge analogs ending at C-terminal sites exposed by unrelated proteases as was demonstrated in a recent publication where the structure of the 2095–2 Fab was detailed.33 Biotinylated-peptide lengths between 8 and 12 amino acids were variously and successfully employed in ELISA; length appeared to be relatively less important than the C-terminal residue. As previously noted, the use of peptide analogs facilitated an investigation of other sites of C-terminal immune recognition that could point to other potential proteolytic cleavage sites. The gly-236 terminus was identified as among the most antigenic sites in the hinge and is the site of IdeS cleavage of human IgG1 and IgG4.2
The use of a single-chain peptide as the immunogen for mAb 2095–2 disregarded the fact that the IgG hinge is composed of two parallel chains with disulfide bridges. This approach was chosen despite earlier suggestions regarding the presence of conformation-based epitopes for serum anti-hinge antibodies.34 The X-ray crystal structure of mAb 2095–2 in complex with the cleaved hinge peptide analog demonstrated why disulfide bonds or higher order structures did not come into play. The specificity of 2095–2 was shown to result from discreet interactions with the terminal four amino acids of the single-chain sequence; key importance given to the C-terminal glycine.33 The mAb 2095–2 was also shown only to bind to IgG F(ab’)2 fragments possessing the same carboxyl terminus. We further propose that single-chain immunogens can be considered more reflective of the IgG single-cleaved hinge in which a single C-terminus is exposed. As previously noted, single cleaved IgGs may be the predominant products of protease action on cell-bound antibodies.9 Nevertheless, the subject of single-cleaved IgG as the target for mAb 2095–2 binding in vitro and in vivo and the resulting functional restoration will be the subject of a subsequent communication.
The ability of the 2095–2 mAb to restore in vitro complement function and antibody-dependent cell-killing to F(ab’)2 fragments was predicted based on a similar restoration of cell-killing by human serum anti-hinge polyclonal antibody preparations.2,5 Cell-bound antibodies with a proteolytic nick in the hinge likely present the cleavage site epitope in an advantageous orientation for recognition by anti-hinge antibodies. For the in vivo testing of this concept, we adopted platelet clearance as a convenient and quantifiable model system. An intact anti-CD41/61 IgG induced time-dependent reductions in circulating platelet numbers in both canines and rats whereas equivalent doses of an IdeS-generated F(ab’)2 fragment were ineffective. The results obtained with the combinations of mAb 2095–2 and the F(ab’)2 fragment, however, could not have been anticipated with regard to the rapidity and extent of platelet clearance. In both the canine and rat models, there was a strong suggestion of an amplification effect such that the F(ab’)2 fragment + 2095–2 combination was more effective than the intact anti-CD41/61 mAb by itself. In the rat dose-response experiment, lower doses of 2095–2 compared with the F(ab’)2 fragment were sufficient to achieve amplification of platelet clearance. Thus, the platelet model provided evidence for a surprising level of cell clearance in vivo that surpassed the activity of related cell-killing combinations in vitro. The results point to a novel treatment paradigm for the reduction or eradication of pathologic cell types and studies are underway to explore this potential in models of cancer and infectious diseases.
The detailed mechanism for the restoration of function by mAb 2095–2 remains to be fully understood. However, one likely contribution for the anti-hinge mAb is a restitution of target cell connectivity to immune cells or complement through the intact Fc domain of 2095–2. If true, such a mechanism implies that, even in its cleaved state, the cell-bound antibody can mediate the eradication of the target cell – in this case via the anti-hinge intermediary mAb. This conjecture finds support in the observation that mAb 2095–2 introduces cell-killing capacity to an IgG4 F(ab’)2 – a subclass with weak NK cell-mediated ADCC and CDC activity as the intact IgG4 relative to intact IgG1. Furthermore, in the solved crystal structure of the Fab of 2095–2 in complex with a peptide analog of the IgG1 hinge, the core recognition motif was determined as a 4 amino acid sequence corresponding to the lower hinge region of IgG1, ELLG.33 The corresponding sequence in IgG4 contains phe-234 in contrast to the IgG1 leu-234. This discrepancy in sequence did not affect the ability of 2095–2 to restore function to an IgG4 F(ab’)2 fragment.
The unexpected finding of in vivo amplification for mAb 2095–2 + F(ab’)2 prompted attempts to understand this phenomenon in mechanistic terms. Among these, it might be conjectured that the complex of the anti-hinge mAb with the cell-bound F(ab’)2 fragment could position a “surrogate” Fc domain at an extended distance from the cell surface to, in some way, facilitate recruitment of immune cells or complement. Alternatively, an enhanced flexibility of the 2-antibody “sandwich” might assist those same immune surveillance and recruitment systems. Or, the bivalent anti-hinge mAb could be envisioned as a bridge between two clipped cell-bound antibodies and thereby reorient the cell surface targets or take advantage of avidity binding not available to primary antibodies. Other explanations can likely be put forth. Nevertheless, we suggest that the novel findings from this study point to a means to translate a well-documented human serum anti-IgG hinge immune paradigm into a therapeutic regimen for the eradication of pathological entities.
Materials and Methods
Reagents
WIL2-S cells and MDA-MB-231 cells were obtained from American Type Culture Collection. WIL2-S cells were cultured in RPMI 1640 (Life Technologies, cat.no. 61870) and 10% FBS (Life Technologies, cat.no. 10437), and MDA-MB-231 cells were cultured in DMEM containing glutamax (Life Technologies, cat.no. 10569), 1X nonessential amino acids (Life Technologies, cat.no.11140), and 10% FBS. The IgG1 anti-CD142 mAb (CDR grafted, humanized) contains the same V-region as the murine anti-human CD142 mAb TF8–5G9, which came from the Scripps Research Institute and has been previously described.35,36 The heavy and light chains of the anti-CD142 mAb were engineered onto the IgG2 and IgG4 subclasses by molecular cloning. The V-region DNA sequences for the anti-CD20 mAb were the same as those used in rituximab (VL GenBank accession number AR015962 and VH GenBank accession number AR000013). The anti-CD41/CD61 mAb (also known as GPIIb/IIIa and αIIbβ3), c7E3 was previously described.28 The heavy and light chains of the anti-CD20 mAb were engineered onto the IgG2 and IgG4 subclasses by molecular cloning.
Generation of recombinant 2095–2
Rabbits were immunized with a peptide analog of the IdeS-cleaved human IgG1 hinge with a C-terminus residue corresponding to gly-236 (Kabat numbering system aligned to the human IgG1 EU sequence22). Rabbit hybridomas were generated,37 and the antibody heavy chain (HC) and light chain cell (LC) genes were molecularly cloned from a culture of a hybridoma cell line secreting a rabbit mAb (91–2) that bound to IdeS-cleaved human IgG1 F(ab’)2 fragments. Following sequence analysis, the single HC and two LC variable region gene fragments were cloned into mammalian expression plasmids containing human IgG1 or human kappa constant region coding sequences, respectively, to generate chimerized rabbit/human full antibody protein. The HC plasmids were transfected into HEK-293 cells in independent wells with each of the LC expression plasmids. After 7 d, the conditioned media containing the secreted antibody was assayed by an anti-Fc (Jackson ImmunoResearch, cat.no. 109–035–098) ELISA to measure recombinant antibody expression levels. The protein from the C2095LC2/HC co-transfection demonstrated robust binding to IdeS-generated F(ab’)2 fragments, whereas the protein generated containing the alternative LC (2095–1) did not demonstrate specific binding. Based on this data, recombinant 2095–2 is defined by variable regions C2095-HC and C2095-LC2.
ELISA
Duplicate 96-well plates were coated with 2 μg/ml of anti-CD20 (described above) intact IgG1, IdeS-generated F(ab’)2 fragments, GluV8-generated F(ab’)2 fragments, or MMP-3-generated F(ab’)2 fragments overnight at 4°C in PBS. Plates were washed 3 times with 150 mM NaCl + 0.02% Tween 20 (Sigma, cat.no. P7949). Plates were blocked in PBS + 3% bovine serum albumin for 1 h at room temperate. The rabbit anti-hinge mAb, 91–2, was added at 1 μg/ml for 1 h at room temperature, plates were washed 3 times, followed by addition of a donkey anti-rabbit horse radish peroxidase (HRP) secondary antibody (Pierce, cat.no. 31458) diluted 1:10,000 for 1 h at room temperature. Plates were washed 3 times, and HRP was detected with by the addition of 50 μl of TMB (Fitzgerald, cat.no. 31458). The reaction was stopped by the addition of 50 μl of 0.5 M HCl. Plates were read at 450 nm on a SpectraMax plus 384 plate-reader (Molecular Devices). A biotinylated version of the rabbit/human chimeric mAb, 2095–2, was added to separate plates at 1 μg/ml for 1 h at room temperature. Plates were washed 3 times and 2 μg/ml streptavidin-HRP (Jackson ImmunoResearch, cat.no. 016–030–084) for 1 h at room temperate. Plates were washed 3 times, and HRP was detected with by the addition of 50 μl of TMB (Fitzgerald, cat.no. 31458). The reaction was stopped by the addition of 50 μl of 0.5 M HCl. Plates were read at 450 nm on a SpectraMax plus 384 plate-reader (Molecular Devices). The charts were generated using GraphPad Prism v5.
Cell binding assays
IdeS (Genovis custom order), MMP-3 (Janssen R&D, LLC) or GluV8 (Biocentrum, cat.no. E-006) were used to cleave rituximab, and F(ab’)2 fragments were obtained after purification with protein A. The purity of the F(ab’)2 fragments was confirmed using capillary electrophoresis. For cell-binding experiments, 2×105 WIL2-S cells were stained with increasing concentrations of F(ab’)2 fragments in FACS buffer (BD Biosciences, cat.no. 554657) for 30 min at 4°C, washed twice with PBS, and then detected with either 10 μg/ml A647-conjugated 2095–2 or 10 μg/ml A647 goat anti-human Heavy + Light F(ab’)2 (Jackson ImmunoResearch, cat.no. 109–606–003). After 30 min at 4°C, cells were washed twice with PBS and then acquired on a flow cytometer (Becton Dickinson LSRFortessa). Data were analyzed using Flowjo (Treestar) and graphed using GraphPad Prism v5. Data were log-transformed and fitted to a sigmoidal dose-response curve.
ADCC assays
For the WIL2-S ADCC assays, 0.5×106 human PBMCs were used as effector cells and 1 ×104 BATDA-labeled (PerkinElmer, cat.no. C136–100) WILS2-S cells were used as target cells in a 50:1 ratio. Cells were incubated with increasing concentrations of anti-CD20 IgG1 or anti-CD20 F(ab’)2 fragments generated with IdeS, MMP-3 or GluV8 in the presence or absence of a fixed concentration of 3 μg/ml anti-hinge mAb 2095–2, a concentration intended to approximate the estimated amount of circulating HAH in normal human serum.2 Human PBMCs, labeled WIL-2S cells and the indicated antibody concentrations were combined in 200 μl total 10% FCS in RPMI 1640 supplemented with Glutamax (Life Technologies, cat.no. 35050), 10% heat-inactivated FBS, 0.1 mM nonessential amino acids (Life Technologies, cat.no. 10082) and 1 mM sodium pyruvate (Life Technologies, cat.no. 11360) in U-shaped 96 well plates, centrifuged for 2 min at 200 g and incubated at 37°C. After 2 h, plates were centrifuged at 200 g for 5 min, and 20 μl of supernatant were mixed with 200 μl DELPHIA Europium-based reagent (PerkinElmer, cat.no. C135–100). For the MDA-MB-231 cells, ADCC assays were performed in a similar manner, but anti-CD142 mAbs were employed and the effector to target ratio was 25:1. For each assay, Relative Fluorescence Units were measured using Envision 2101 Multilabel Reader (Perkin Elmer). Percent lysis was calculated as (Sample Release - Spontaneous Release)/(Maximal release (from Triton-100 lysis) - Spontaneous Release) x 100%. Data were log-transformed and fitted to a sigmoidal dose-response curve using GraphPad Prism v5.
CDC assays
For WIL2-S CDC assays, cells were plated in triplicate 96-well U-bottom plates at 0.08 × 106 cells/well in RPMI + 10% FBS. Cells were incubated at room temperature with increasing concentrations of anti-CD20 IgG1 or anti-CD20 F(ab’)2 fragments generated with IdeS, MMP-3 or GluV8 in the presence or absence of a fixed concentration of 5 μg/ml anti-hinge mAb 2095–2 (100 μl total volume). After 1 h, 50 μl of a 10% rabbit complement (Invitrogen, cat.no. 31038100) solution was added, mixed by pipetting up and down, and then incubated at 37 °C. After 30 min, plates were centrifuged at 200 g for 5 min, and 50 μl of supernatant were mixed with 50 μl of lactate dehydrogenase detection kit (Roche, cat.no. 11 644 793 001) for 15 min at room temperature in the dark. Plates were read on a SpectraMax plus 384 (Molecular Devices) at 490 nm. Percent lysis was calculated as (Sample Release – WIL2-S in complement solution alone)/(Maximal release (from Triton-100 lysis) - WIL2-S in complement solution alone) × 100%. Data were log-transformed and fitted to a sigmoidal dose-response curve using GraphPad Prism v5.
Canine platelet depletion
Circulating blood platelets present a convenient and accessible system for the study of antibody-mediated cell clearance.26,27 Accordingly, we employed the anti-platelet mAb, c7E3, which also cross reacts with the corresponding epitope on canine and rat platelets.29 Two preparations of c7E3 were used: the intact murine-human IgG1 chimeric antibody, and a F(ab’)2 fragment prepared using the bacterial enzyme IdeS.31 Animal studies (3 animals per group) were conducted at Covance Research Laboratories (Denver, PA).
The c7E3 IgG and F(ab’)2 fragment were infused IV at the indicated doses (see Results) over a 20 min period with the goal of achieving equivalent coating of the entire circulating platelet pool. The PBS control solution and the mAb 2095–2 (alone at 0.5 mg/kg) were similarly infused. The additional combined treatment group received c7E3 F(ab’)2 and, after a 10 min delay, a 20 min infusion of 2095–2 (0.5 mg/kg). Platelet counts were determined in EDTA-anti-coagulated blood by standard automated analysis (Quest Diagnostics, Wyomissing PA). The average of the platelet count replicates (n = 5) at each time were normalized to the pre-dose number for each animal and expressed as percent of baseline. The average value for each treatment group of three animals was expressed and plotted relative to baseline (+/− SEM).
Rat platelet depletion
The dose-response profile of the combination regimen of c7E3 F(ab’)2 and 2095–2 was investigated in the more accessible rat model. The doses required for c7E3 IgG-mediated platelet clearance (see Results) reflected the lower affinity of the mAb for rat platelets compared with the canine system above.29 The protocol conformed to the study in canines with several exceptions. In particular, due to volume constraints imposed by retro-orbital blood collection in rats, baseline counts were not obtained for all test groups. Therefore, results were not normalized to each individual group's baseline; rather the absolute platelet counts were reported starting at the initial 1 h time point.
The test articles were injected IV via slow bolus for 30 s, using a 1 ml constant dose volume based on individual animal weights. The 2095–2 mAb doses were also infused intravenously at an ∼60 min interval after the F(ab’)2. Platelet counts were determined in EDTA-anti-coagulated blood samples using an ADVIA® 2120 hematology analyzer (Siemens). The average platelet counts for each group were plotted using GraphPad Prism v5 (+/− SEM)
Disclosures
All authors are employees of Janssen Research and Development, LLC.
Acknowledgments
The authors would like to thank William Strohl, David M Knight, A Simon Lynch, Tomas Malia, Gary Gilliland, Raymond Sweet, Ningyan Zhang, and Zhiqiang An for thoughtful comments and conversations, and Rangappa Ramachandra of Covance for conducting the canine platelet depletion study.
References
- 1. Brezski RJ, Knight DM, Jordan RE. The origins, specificity, and potential biological relevance of human anti-IgG hinge autoantibodies. ScientificWorldJournal 2011; 11:1153-67; PMID:21623461; http://dx.doi.org/ 10.1100/tsw.2011.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brezski RJ, Luongo JL, Petrone D, Ryan MH, Zhong D, Tam SH, Schmidt AP, Kruszynski M, Whitaker BP, Knight DM, et al. Human anti-IgG1 hinge autoantibodies reconstitute the effector functions of proteolytically inactivated IgGs. J Immunol 2008; 181:3183-92; PMID:18713989; http://dx.doi.org/ 10.4049/jimmunol.181.5.3183 [DOI] [PubMed] [Google Scholar]
- 3. Brezski RJ, Jordan RE. Cleavage of IgGs by proteases associated with invasive diseases: an evasion tactic against host immunity? MAbs 2010; 2:212-20; PMID:20400859; http://dx.doi.org/ 10.4161/mabs.2.3.11780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. von Pawel-Rammingen U. Streptococcal IdeS and its impact on immune response and inflammation. J Innate Immun 2012; 4:132-40; PMID:22248585; http://dx.doi.org/ 10.1159/000332940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brezski RJ, Oberholtzer A, Strake B, Jordan RE. The in vitro resistance of IgG2 to proteolytic attack concurs with a comparative paucity of autoantibodies against peptide analogs of the IgG2 hinge. MAbs 2011; 3:558-67; PMID:22123056; http://dx.doi.org/ 10.4161/mabs.3.6.18119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schmidt A, DeRiggi D, Jordan RE, Whitaker B, Heavner GA, Kruszynski M. A synthetic peptide approach for elucidating the points of natural auto-antibody reactivity to proteolytic fragments of human IgG. Adv Exp Med Biol 2009; 611:411-2; PMID:19400244; http://dx.doi.org/ 10.1007/978-0-387-73657-0_177 [DOI] [PubMed] [Google Scholar]
- 7. Jefferis R. Antibody therapeutics: isotype and glycoform selection. Expert Opin Biol Ther 2007; 7:1401-13; PMID:17727329; http://dx.doi.org/ 10.1517/14712598.7.9.1401 [DOI] [PubMed] [Google Scholar]
- 8. Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol 2008; 8:34-47; PMID:18064051; http://dx.doi.org/ 10.1038/nri2206 [DOI] [PubMed] [Google Scholar]
- 9. Brezski RJ, Vafa O, Petrone D, Tam SH, Powers G, Ryan MH, Luongo JL, Oberholtzer A, Knight DM, Jordan RE. Tumor-associated and microbial proteases compromise host IgG effector functions by a single cleavage proximal to the hinge. Proc Natl Acad Sci U S A 2009; 106:17864-9; PMID:19815504; http://dx.doi.org/ 10.1073/pnas.0904174106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fan X, Brezski RJ, Fa M, Deng H, Oberholtzer A, Gonzalez A, Dubinsky WP, Strohl WR, Jordan RE, Zhang N, et al. A single proteolytic cleavage within the lower hinge of trastuzumab reduces immune effector function and in vivo efficacy. Breast Cancer Res 2012; 14:R116; PMID:22873525; http://dx.doi.org/ 10.1186/bcr3240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yano S, Kaku S, Suzuki K, Terazaki C, Sakayori T, Kawasaki T, Kawamura K, Sugita Y, Hoshino K, Masuho Y. Natural antibodies against the immunoglobulin F(ab’)2 fragment cause elimination of antigens recognized by the F(ab’)2 from the circulation. Eur J Immunol 1995; 25:3128-33; PMID:7489753; http://dx.doi.org/ 10.1002/eji.1830251121 [DOI] [PubMed] [Google Scholar]
- 12. Rispens T, de Vrieze H, de Groot E, Wouters D, Stapel S, Wolbink GJ, Aarden LA. Antibodies to constant domains of therapeutic monoclonal antibodies: anti-hinge antibodies in immunogenicity testing. J Immunol Methods 2012; 375:93-9; PMID:21986105; http://dx.doi.org/ 10.1016/j.jim.2011.09.011 [DOI] [PubMed] [Google Scholar]
- 13. Biancheri P, Di Sabatino A, Corazza GR, MacDonald TT. Proteases and the gut barrier. Cell Tissue Res 2013; 351:269-80; PMID:22427120; http://dx.doi.org/ 10.1007/s00441-012-1390-z [DOI] [PubMed] [Google Scholar]
- 14. van de Stadt LA, de Vrieze H, Derksen NI, Brouwer M, Wouters D, van Schaardenburg D, Wolbink G, Rispens T. Antibodies to IgG4 hinge can be found in Rheumatoid Arthritis patients during all stages of disease and may exacerbate chronic antibody-mediated inflammation. Arthritis Rheum 2013; In press; PMID:24375288 [DOI] [PubMed] [Google Scholar]
- 15. Terness P, Kirschfink M, Navolan D, Dufter C, Kohl I, Opelz G, Roelcke D. Striking inverse correlation between IgG anti-F(ab’)2 and autoantibody production in patients with cold agglutination. Blood 1995; 85:548-51; PMID:7812010 [PubMed] [Google Scholar]
- 16. Süsal C, Oberg HH, Daniel V, Dörr C, Terness P, Huth-Kühne A, Zimmermann R, Opelz G. Isotypes and IgG subclasses of anti-Fab antibodies in human immunodeficiency virus-infected hemophilia patients. Vox Sang 1994; 66:37-45; PMID:7908473; http://dx.doi.org/ 10.1111/j.1423-0410.1994.tb00274.x [DOI] [PubMed] [Google Scholar]
- 17. Williams RC, Jr., Malone CC, Huffman GR, Silvestris F, Croker BP, Ayoub EM, Massengill S. Active systemic lupus erythematosus is associated with depletion of the natural generic anti-idiotype (anti-F(ab’)2) system. J Rheumatol 1995; 22:1075-85; PMID:7674233 [PubMed] [Google Scholar]
- 18. Süsal C, Maier H, Lorenz K, Opelz G. Association of IgA-anti-Fab autoantibodies with disease stage in head-and-neck cancer. Int J Cancer 1994; 57:47-50; PMID:8150540; http://dx.doi.org/ 10.1002/ijc.2910570109 [DOI] [PubMed] [Google Scholar]
- 19. Terness PI, Navolan D, Dufter C, Welschof M, Opelz G. Immunosuppressive anti-immunoglobulin autoantibodies: specificity, gene structure and function in health and disease. Cell Mol Biol (Noisy-le-grand) 2002; 48:271-8; PMID:12030431 [PubMed] [Google Scholar]
- 20. Vincents B, von Pawel-Rammingen U, Björck L, Abrahamson M. Enzymatic characterization of the streptococcal endopeptidase, IdeS, reveals that it is a cysteine protease with strict specificity for IgG cleavage due to exosite binding. Biochemistry 2004; 43:15540-9; PMID:15581366; http://dx.doi.org/ 10.1021/bi048284d [DOI] [PubMed] [Google Scholar]
- 21. von Pawel-Rammingen U, Johansson BP, Björck L. IdeS, a novel streptococcal cysteine proteinase with unique specificity for immunoglobulin G. EMBO J 2002; 21:1607-15; PMID:11927545; http://dx.doi.org/ 10.1093/emboj/21.7.1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Edelman GM, Cunningham BA, Gall WE, Gottlieb PD, Rutishauser U, Waxdal MJ. The covalent structure of an entire gammaG immunoglobulin molecule. Proc Natl Acad Sci U S A 1969; 63:78-85; PMID:5257969; http://dx.doi.org/ 10.1073/pnas.63.1.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Richards JO, Karki S, Lazar GA, Chen H, Dang W, Desjarlais JR. Optimization of antibody binding to FcgammaRIIa enhances macrophage phagocytosis of tumor cells. Mol Cancer Ther 2008; 7:2517-27; PMID:18723496; http://dx.doi.org/ 10.1158/1535-7163.MCT-08-0201 [DOI] [PubMed] [Google Scholar]
- 24. Abdullah N, Greenman J, Pimenidou A, Topping KP, Monson JR. The role of monocytes and natural killer cells in mediating antibody-dependent lysis of colorectal tumour cells. Cancer Immunol Immunother 1999; 48:517-24; PMID:10602889; http://dx.doi.org/ 10.1007/s002620050600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, Daëron M. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood 2009; 113:3716-25; PMID:19018092; http://dx.doi.org/ 10.1182/blood-2008-09-179754 [DOI] [PubMed] [Google Scholar]
- 26. Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science 2005; 310:1510-2; PMID:16322460; http://dx.doi.org/ 10.1126/science.1118948 [DOI] [PubMed] [Google Scholar]
- 27. Sazinsky SL, Ott RG, Silver NW, Tidor B, Ravetch JV, Wittrup KD. Aglycosylated immunoglobulin G1 variants productively engage activating Fc receptors. Proc Natl Acad Sci U S A 2008; 105:20167-72; PMID:19074274; http://dx.doi.org/ 10.1073/pnas.0809257105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Knight DM, Wagner C, Jordan R, McAleer MF, DeRita R, Fass DN, Coller BS, Weisman HF, Ghrayeb J. The immunogenicity of the 7E3 murine monoclonal Fab antibody fragment variable region is dramatically reduced in humans by substitution of human for murine constant regions. Mol Immunol 1995; 32:1271-81; PMID:8559151; http://dx.doi.org/ 10.1016/0161-5890(95)00085-2 [DOI] [PubMed] [Google Scholar]
- 29. Sassoli PM, Emmell EL, Tam SH, Trikha M, Zhou Z, Jordan RE, Nakada MT. 7E3 F(ab’)2, an effective antagonist of rat alphaIIbbeta3 and alphavbeta3, blocks in vivo thrombus formation and in vitro angiogenesis. Thromb Haemost 2001; 85:896-902; PMID:11372685 [PubMed] [Google Scholar]
- 30. Terness PI, Navolan D, Dufter C, Welschof M, Opelz G. Immunosuppressive anti-immunoglobulin autoantibodies: specificity, gene structure and function in health and disease. Cell Mol Biol (Noisy-le-grand) 2002; 48:271-8; PMID:12030431 [PubMed] [Google Scholar]
- 31. Ryan MH, Petrone D, Nemeth JF, Barnathan E, Björck L, Jordan RE. Proteolysis of purified IgGs by human and bacterial enzymes in vitro and the detection of specific proteolytic fragments of endogenous IgG in rheumatoid synovial fluid. Mol Immunol 2008; 45:1837-46; PMID:18157932; http://dx.doi.org/ 10.1016/j.molimm.2007.10.043 [DOI] [PubMed] [Google Scholar]
- 32. Lutz HU, Fumia S. Therapeutic cleavage of IgG is dangerous in humans. Trends Immunol 2008; 29:353-4, author reply 355-6; PMID:18602342; http://dx.doi.org/ 10.1016/j.it.2008.04.009 [DOI] [PubMed] [Google Scholar]
- 33. Malia TJ, Teplyakov A, Brezski RJ, Luo J, Kinder M, Sweet RW, Almagro JC, Jordan RE, Gilliland GL. Structure and specificity of an antibody targeting a proteolytically cleaved IgG hinge. Proteins 2014; In press; PMID:24638881; http://dx.doi.org/ 10.1002/prot.24545 [DOI] [PubMed] [Google Scholar]
- 34. Terness P, Kohl I, Hübener G, Battistutta R, Moroder L, Welschof M, Dufter C, Finger M, Hain C, Jung M, et al. The natural human IgG anti-F(ab’)2 antibody recognizes a conformational IgG1 hinge epitope. J Immunol 1995; 154:6446-52; PMID:7539020 [PubMed] [Google Scholar]
- 35. Kinder M, Greenplate AR, Grugan KD, Soring KL, Heeringa KA, McCarthy SG, Bannish G, Perpetua M, Lynch F, Jordan RE, et al. Engineered protease-resistant antibodies with selectable cell-killing functions. J Biol Chem 2013; 288:30843-54; PMID:23986451; http://dx.doi.org/ 10.1074/jbc.M113.486142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ngo CV, Picha K, McCabe F, Millar H, Tawadros R, Tam SH, Nakada MT, Anderson GM. CNTO 859, a humanized anti-tissue factor monoclonal antibody, is a potent inhibitor of breast cancer metastasis and tumor growth in xenograft models. Int J Cancer 2007; 120:1261-7; PMID:17192924; http://dx.doi.org/ 10.1002/ijc.22426 [DOI] [PubMed] [Google Scholar]
- 37. Huang Y, Gu B, Wu R, Zhang J, Li Y, Zhang M. Development of a rabbit monoclonal antibody group against Smads and immunocytochemical study of human and mouse embryonic stem cells. Hybridoma (Larchmt) 2007; 26:387-91; PMID:18158783; http://dx.doi.org/ 10.1089/hyb.2007.0517 [DOI] [PubMed] [Google Scholar]


