Abstract
Fc effector functions such as antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cell-mediated phagocytosis (ADCP) are crucial to the efficacy of many antibody therapeutics. In addition to IgG, antibodies of the IgA isotype can also promote cell killing through engagement of myeloid lineage cells via interactions between the IgA-Fc and FcαRI (CD89). Herein, we describe a unique, tandem IgG1/IgA2 antibody format in the context of a trastuzumab variable domain that exhibits enhanced ADCC and ADCP capabilities. The IgG1/IgA2 tandem Fc format retains IgG1 FcγR binding as well as FcRn-mediated serum persistence, yet is augmented with myeloid cell-mediated effector functions via FcαRI/IgA Fc interactions. In this work, we demonstrate anti-human epidermal growth factor receptor-2 antibodies with the unique tandem IgG1/IgA2 Fc can better recruit and engage cytotoxic polymorphonuclear (PMN) cells than either the parental IgG1 or IgA2. Pharmacokinetics of IgG1/IgA2 in BALB/c mice are similar to the parental IgG, and far surpass the poor serum persistence of IgA2. The IgG1/IgA2 format is expressed at similar levels and with similar thermal stability to IgG1, and can be purified via standard protein A chromatography. The tandem IgG1/IgA2 format could potentially augment IgG-based immunotherapeutics with enhanced PMN-mediated cytotoxicity while avoiding many of the problems associated with developing IgAs.
Keywords: monoclonal antibody, ADCC, CD89, FcαRI, tandem, neutrophil, macrophage, trastuzumab, IgA
Abbreviations
- IgG
immunoglobulin G
- IgA
immunoglobulin A
- ADCC
antibody-dependent cell-mediated cytotoxicity
- ADCP
antibody-dependent cell-mediated phagocytosis
- CDC
complement dependent cytotoxicity
- TAA
tumor associated antigens
- FcRn
neonatal Fc receptor
- FcγR
Fc gamma receptor
- HER2
human epithelial receptor two
- SPR
surface plasmon resonance
- PMN
polymorphonuclear
- NK
natural killer
- PBMC
peripheral blood mononuclear cell
- E:T ratio
effector to target ratio
- FCM
flow cytometry
- LDH
lactate dehydrogenase
- MΦ
macrophage
- DSC
differential scanning calorimetry
- PK
pharmacokinetics
- AUC
area under the curve
- CL
clearance rate
- T½
half-life
- Vss
central compartment volume of distribution
- Cmax
maximum serum concentration
Introduction
Many of the current monoclonal antibody based immunotherapeutics depend on the recruitment and activation of immune effector cells for efficacy.1 These antibody immunotherapeutics (exclusively of the IgG isotype) utilize Fc-mediated mechanisms such as antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cell-mediated phagocytosis (ADCP) to kill cancer cells via targeting tumor-associated antigens (TAAs)2 or immunosuppressive cells via targeting cell surface receptors such as OX40.3,4
Considerable effort has been put into technologies that can enhance antibody effector functions. The main technologies used to improve target cell killing focus on improving the affinity between IgG1-Fc and FcγRIIIa, expressed on natural killer (NK) cells. Afucosylation of the N297 Fc glycan is a well-known method for enhancing NK-mediated ADCC.5,6 Concurrently, IgG variants have also been generated to enhance ADCP and complement-dependent cytotoxicity (CDC) by increasing the Fc domain's affinity to FcγRIIa or C1q respectively.7-9 These technologies are similar in that they attempt to enhance existing interactions between the IgG-Fc and immune receptors rather than introduce novel effector functions to IgG.
Serum IgA, the second most abundant immunoglobulin present in blood, can effectively engage polymorphonuclear (PMN) cells via the interaction with the FcαRI receptor (CD89).10 Ligation of FcαRI by IgA-containing immune complexes can trigger ADCC by neutrophils, degranulation of eosinophils, and phagocytosis by monocytes, and macrophages (MΦ).11 Other myeloid-derived cells such as mast cells and basophils do not express CD89.12-14 FcαRI engagement has long been proposed as a way to enhance the well-recognized anti-tumor ability of neutrophils.15-18 Dechant et al.19 and others have shown that replacing the IgG Fc of antibodies targeting tumor antigens with a human IgA Fc greatly improves ADCC by PMN cells and can promote neutrophil migration.20,21 In addition to neutrophils, both monocytes and MΦ have been shown to elicit FcαRI-mediated cell killing.22 Recently, the antitumor effect of IgA antibodies has been demonstrated in vivo for the first time in transgenic human CD89-expressing mice.23 Despite encouraging reports demonstrating the potential for IgA antibodies as cancer therapeutics, the utilization of the IgA isotype as a viable therapeutic has drawbacks. These include the inability of IgA to engage NK-mediated ADCC as well as faster serum clearance of IgA compared to IgG.24 The development and production of IgA therapeutics may also prove challenging due to complex O-linked glycosylation in the IgA1 subclass, multiple N-linked glycosylation sites in constant regions, and a cysteine-rich C-terminal tailpiece that can induce dimerization.22,25
Multiple constructs have previously been described that can potentially engage both CD89-expressing PMN cells while targeting TAAs (e.g., anti-CD89 bispecifics,20 and IgA antibodies19). IgG/IgA hybrids or fusions26,27 have also been described, including an engineered IgG-IgA “cross-isotype” construct by Kelton et al.28 that combines CD89 engagement with CDC activity on a single Fc domain. Herein, we describe a unique IgG1/IgA2 tandem Fc format that combines the potent NK-mediated cytotoxicity and FcRn-mediated long serum half-life of IgG1 with the effective PMN cell engagement of IgA2. We demonstrate that an anti-human epidermal growth factor receptor-2 (Her2) trastuzumab IgG1/IgA2 antibody has superior ADCC and ADCP activities compared to either their IgG1 or IgA2 counterparts. The IgG1/IgA2 tandem Fc format described in this report differs from many IgA-containing formats in that it can a) confer CD89 binding while retaining IgG like antigen binding, b) retain FcRn and protein A binding, and c) provide a modular platform to assess possible synergistic benefits to engaging both FcγRs and FcαRI within one molecule. This format also addresses many developmental liabilities present in other IgA-Fc-containing antibodies. Our results demonstrate the potential of IgG1/IgA2 tandem Fc as an alternative to well-established NK-mediated ADCC enhancement technologies by facilitating the recruitment of additional types of cytotoxic immune cells.
Results
Expression and purification of IgG1/IgA2 tandem and IgA2-Fc-containing antibodies
The IgA2(m1) Fc was utilized to endow IgG with CD89 binding. Models of the trastuzumab IgG1/IgA2 (Her2-IgG1/IgA2) tandem Fc and the trastuzumab IgA2 (Her2-IgA2) antibody formats described in this paper are presented in Figure 1. For Her2-IgG1/IgA2, amino acids 221 to 441 of IgA2-Fc are genetically fused to the IgG1 C-terminus with the IgA2 hinge (221–229) serving as a linker between the 2 Fc domains (Fig. S1A). To prevent dimerization, the cysteine tailpiece of IgA was not included in either the Her2-IgG1/IgA2 or Her2-IgA2 construct by introducing a stop codon after K441 in the IgA2 Fc. For Her2-IgA2, the same IgA2-Fc and hinge replaces the IgG1-Fc domain and hinge region. Expression levels for Her2-IgG1/IgA2 transiently expressed in HEK 293FT cells were comparable to that of the IgG parent at ˜ 200 mg/L (Table 1). Her2-IgG1/IgA2 and Her2-IgA2 retained binding to the Her2/neu antigen similarly to that of the parental IgG1 antibody (Fig. S1B, Table 1), and differential scanning calorimetry (DSC) analysis confirmed that addition or replacement with the IgA2-Fc had no negative impact on overall thermal stability (Fig. S1C, Table 1). Her2- IgG1/IgA2 monomer content (91.1%) determined by SEC after protein A purification was lower than Her2-IgG1 (97.6%) and similar to Her2-IgA2 (91.0%) (Fig. S1, Table 1).
Figure 1.
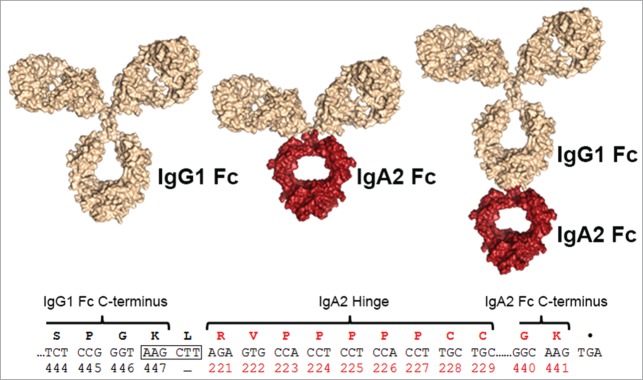
Design of the IgG1/IgA2 tandem Fc fusion. Structural models of antibody formats generated for this study are shown, with IgG1- (left), an IgA2-Fc and hinge domain with IgG Fab domains (middle) and the tandem IgG1/IgA2-Fc format with an IgA2-Fc and hinge fused to an IgG-Fc (right). Structures were generated in Pymol (Schrödinger) using a human IgG1 pdb model41 and an IgA Fc structure (PDB ID: 1OW0).42 The DNA and encoded protein sequence of the IgG1/IgA2-Fc linkage is shown with an introduced HindIII site (boxed) highlighted.
Table 1.
Expression, antigen binding, and thermal stability of analyzed antibodies
| Expression (mg/L) | Monomer content# after affinity column | Her2 binding EC50 (nM) | Lowest Tm (°C) | Fab Tm (°C) | |
|---|---|---|---|---|---|
| Her2-IgG1 | 180 | 97.6% | 2.4 | 72.1 | 82.5 |
| Her2- IgG1/IgA2 | 195 | 91.0% | 1.5 | 72.4 | 82.0 |
| Her2-IgA2 | N.D.* | 91.1% | 2.1 | 73** | 82.3 |
Determined by size-exclusion chromatography.
Her2-IgA2 expression levels were determined to be ≥ 50 mg/L by quantification from Peptide M agarose resin, but exact expression was not quantified.
Approximate value, determined by deconvolution of Fab Tm shoulder (see Fig. S1).
Receptor binding to antibody variants
Surface plasmon resonance (SPR) was used to measure the affinity for various Fcγ receptors, FcαRI, C1q and both human and murine FcRn to Her2-IgG1, Her2-IgA2, Her2- IgG1/IgA2 and Her2-IgG1-afuc (lacking fucosylation at the N297 glycan) (Table 2). Her2-IgG1 and Her2-IgG1/IgA2 binding to human and murine FcRn, C1q, FcγRI, FcγRIIa, FcγRIIb, and FcγRIIIa (158V and 158F allotypes) were similar to what has been previously reported for IgG antibodies.29 Her2-IgG1-afuc showed improved FcγRIIIa binding as expected. Neither Her2-IgG1 nor Her2-IgG1-afuc had any detectable binding to FcαRI, and Her2-IgA2 had no detectable binding to any of the Fcγ receptors, C1q or FcRn. Her2-IgA2 bound to soluble human FcαRI with an affinity of 122 nM. Her2-IgG1/IgA2 bound to FcαRI at an affinity similar to that of Her2-IgA2 while maintaining FcγRs and C1q binding characteristics similarly to that of Her2-IgG1 (Table 2).
Table 2.
FcγR, FcRn, C1q and FcαRI binding of antibodies determined by surface plasmon resonance
| KD values (nM) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| FcαRI | FcγRI | FcγRIIIa 158F | FcγRIIIa 158V | FcγRIIa | FcγRIIb | hFcRn | mFcRn | C1q | |
| Her2-IgG1 | N.B. | 6.05 | 1,770 | 409 | 638 | 4,980 | 1,210 | 97.5 | 222 |
| Her2-IgG1/IgA2 | 128 | 7.61 | 1,810 | 401 | 771 | 5,900 | 1,790 | 140 | 677 |
| Her2-IgA2 | 122 | N.B. | N.B. | N.B. | N.B. | N.B. | N.B. | N.B. | N.B. |
| Her2-IgG1-afuc | N.B. | 6.27 | 312 | 132 | 1,220 | 4,240 | 1,250 | 97.9 | 266 |
N.B. indicates no detectable binding.
ADCC activity of Her2-IgG1/IgA2 with different effector populations
We evaluated the ability of Her2-IgG1/IgA2 to promote ADCC using different effector cell populations. To assess FcγRIIIa-mediated cytotoxicity, the NK-derived cell line KC1333 stably expressing FcγRIIIa was used as effector cells (Fig. 2A). Her2-IgG1 and Her2-IgG1/IgA2 both induced BT474 target cell killing, indicating NK-mediated ADCC activity is retained in Her2-IgG1/IgA2. Her2-IgG1-afuc displayed superior cytotoxicity in this assay as expected. Her2-IgA2 and a non-Her2 binding control IgG did not induce NK- mediated ADCC (Fig. 2A).
Figure 2.
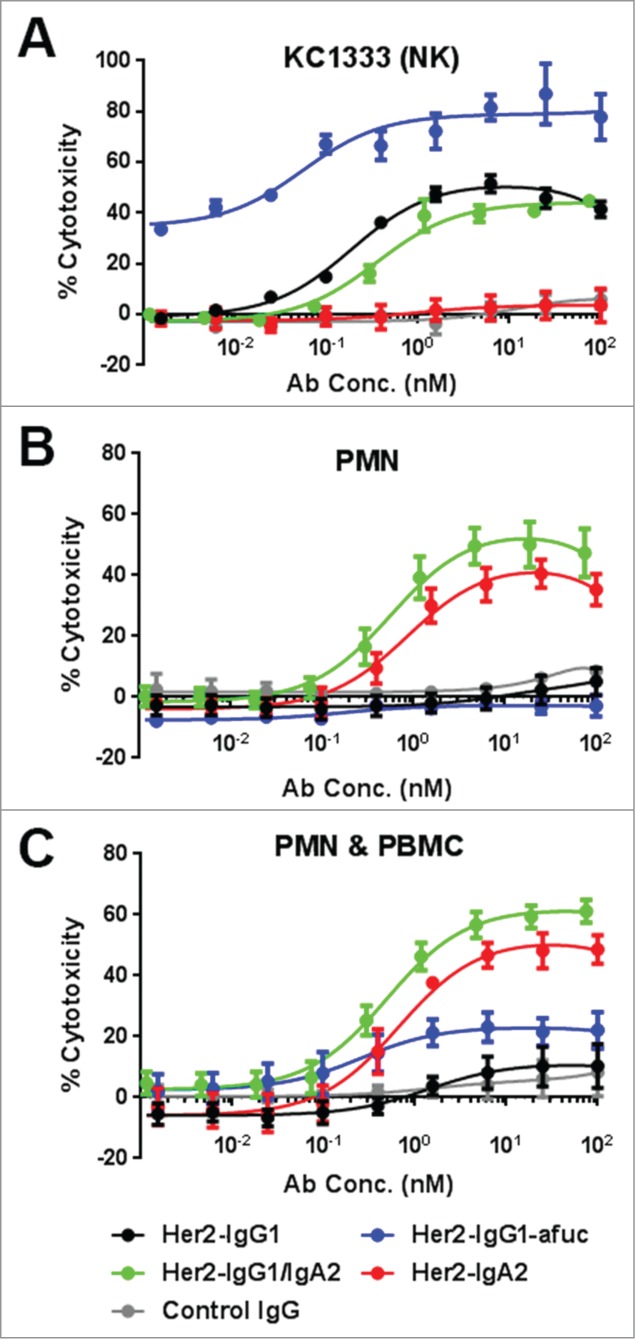
ADCC with different effector cell types. ADCC results are shown using BT474 cells as targets and either NK cell line KC1333 (A), the freshly isolated PMN cells (B) or both the PMN and PBMC cell fractions combined (C). For PMN and PMN plus PBMC experiments, data are the average of 5 different experiments with 5 separate donors. KC1333 data is combined from 3 separate experiments. E:T ratios were 2.5:1 when KC1333 cells were used as effector cells, and 20:1 with PMN and PMN plus PBMC effector cells.
ADCC was then evaluated using freshly isolated PMN cells (consisting of ∼85% neutrophils) as effector cells (Fig. 2B). At an E:T ratio of 20:1, both Her2- IgG1/IgA2 and Her2-IgA2 effectively promoted ADCC. Neither Her2-IgG1 nor Her2-IgG1-afuc elicited ADCC under these conditions. ADCC was further interrogated using combined, freshly isolated PMN and peripheral blood mononuclear cells (PBMCs), reconstituting an effector population representative of immune cell ratios found in blood. This effector cell population contains multiple cell types capable of eliciting cytotoxicity, including neutrophils and monocytes from the PMN fraction and NK cells present in the PBMC fraction. The two IgA2-containing constructs, Her2-IgA2 and Her2-IgG1/IgA2, elicited effective cytotoxicity from this effector cell population (Fig. 2C). Her2-IgG1-afuc, despite having enhanced NK-mediated ADCC, elicited much lower cytotoxicity than either 2 IgA2-Fc-containing antibodies, whereas Her2-IgG1 elicited very low levels of ADCC at this effector ratio with this combined population (Fig. 2C).
The PMN-mediated cytotoxicity by IgA2-Fc-containing antibodies was visualized using fluorescence microscopy at early (3 hr) and late (18 hr) time points (Fig. 3A). Wells incubated with either control IgG or Her2-IgG1 did not show PMN adherence to BT474 cells at either time point. Additionally, live BT474 cells could be observed at 18 hours. In contrast, at 3 hours PMN cells could be seen clumping near BT474 cells in both Her2-IgG1/IgA2 and Her2-IgA2-containing wells (Fig. 3A). Few intact BT474 cells could be visualized in these fields containing Her2-IgA2 or Her2-IgG1/IgA2 at the 18 hour time point. Those that were discernable were usually surrounded by multiple neutrophils and staining appeared weak, suggesting on-going cell lysis.
Figure 3.
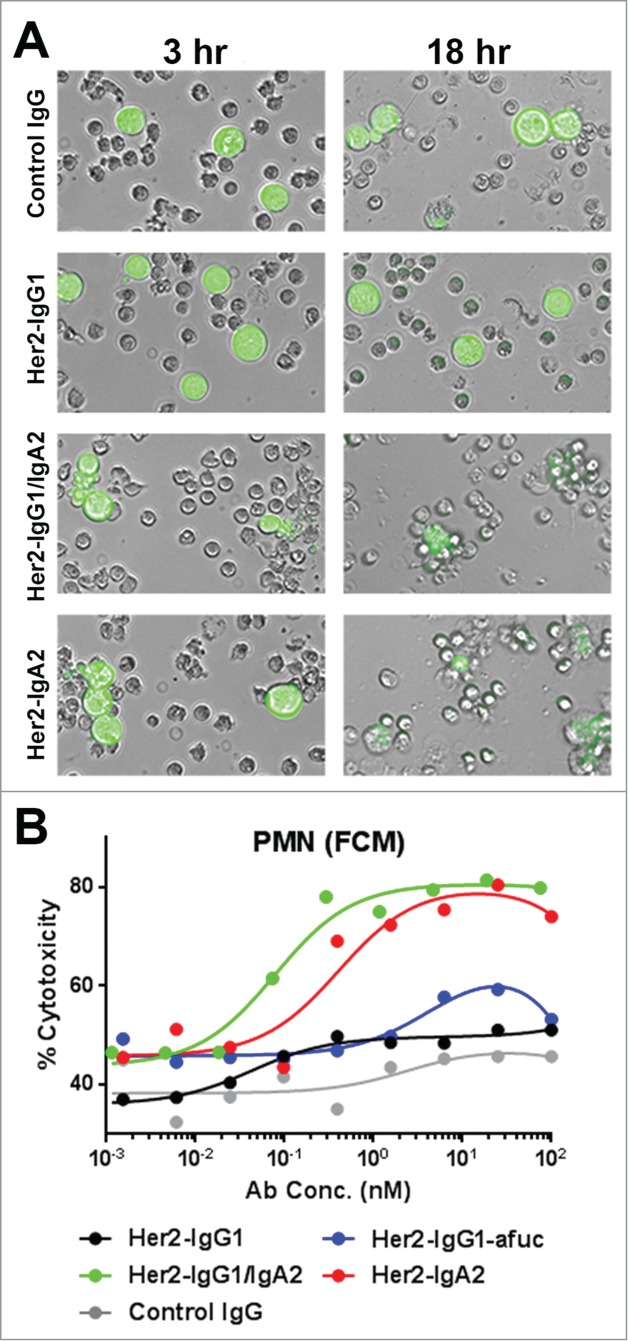
Visualizing PMN-mediated ADCC of mAb variants via microscopy and FCM. (A) Freshly isolated PMN cells (unlabeled) were incubated with Her2+++ BT474 cells (green) at an E:T ratio of 10:1 with either IgG isotype control, Her2-IgG1, Her2-IgG1/IgA2 or Her2-IgA2 at a concentration of 6 nM and visualized via fluorescence microscopy. PMN mediated ADCC (B) was assessed using an FCM based 3 color assay to measure target cell killing. An E:T ratio of 10:1 PMN:BT474 cells was used. Data are representative from one donor of 3 tested.
To further evaluate and confirm the PMN-mediated cytotoxicity of Her2-IgG1/IgA2, a 3-color flow cytometry (FCM) assay was employed. PMN cells (labeled with Calcien AM Violet) and BT474 cells (labeled with Calcein AM) were incubated with antibodies at an E:T ratio of 10:1 for 18 hrs. Afterwards, cells were stained with a near-IR live/dead stain to assess membrane integrity. Populations of PMN and BT474 cells were differentiated by their fluorescent label and the percent cytotoxicity of the target cell population was determined (Fig. 3B). The results mirrored those of the PMN-mediated ADCC assays using LDH release as the read out. Again, only the IgA2-Fc-containing formats, Her2-IgA2 and Her2-IgG1/IgA2, had improved cytotoxicity compared to Her2-IgG1 or Her2-IgG1-afuc (Fig. 3B).
Macrophage mediated ADCP of antibody variants
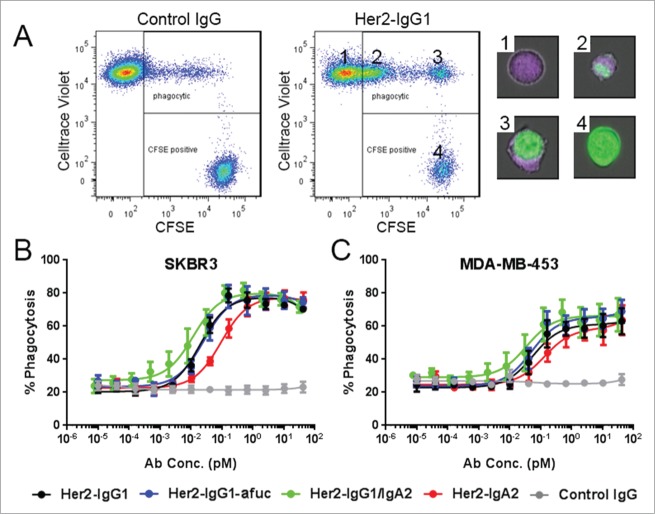
Macrophage are an additional myeloid-derived cell population expressing both FcγRs and FcαRI capable of tumor cell killing, mainly by ADCP.30 We evaluated the ability of our antibodies to facilitate ADCP using monocyte derived MΦ by FCM. MΦ were labeled with celltrace violet and target cells were labeled with CFSE. In the presence of antibodies to the target cells, phagocytosis could be observed as an increase in the number of double positive cells (Fig. 4A). Phagocytosis of both a Her2+++ (SK-BR-3) and a lower expressing Her2++ cell line (MDA-MB-453) were assessed with the panel of anti-Her2 antibodies (Fig. 4B). SK-BR-3 and MDA-MB-453 target cells were both phagocytosed in the presence of all anti-Her2 antibodies tested. Phagocytosis of the Her2-IgG1/IgA2 was slightly improved compared to Her2-IgG1 or Her2-IgG1-afuc in both cell lines, and was further improved compared to Her2-IgA2.
Figure 4.
ADCP of SK-BR-3 and MDA-MB-453 cells with mAb variants. Human monocyte-derived MФ were labeled with celltrace violet and co-cultured with CFSE-labeled Her2 expressing cells at an 8:1 effector: target ratio for 2 h in the presence of the indicated antibodies. Cells were collected and stained with live dead far red, and analyzed by FCM. Dot plots (A) are of live cells in SK-BR-3 cell / MΦ co-cultures in the presence of 650pM IgG isotype control or Her2-IgG1. Images of MФ (purple), SK-BR-3 (green) and double positive cells from the indicated regions of the dot plot were captured using an imaging cytometer. Phagocytosis of SK-BR-3 cells (B) and MDA-MB-453 cells (C) was quantified as the percent double positive cells of CFSE positive cells. Data are mean ± SEM. of experiments performed with MΦ from n ≥ 4 independent donors.
Pharmacokinetics of antibody variants in Balb/C mice
The serum persistence of Her2-IgG1/IgA2 was determined in mice. As Her2-IgG1/IgA2 contains Fcs from 2 different isotypes, we sought to confirm that the antibody could still be recycled efficiently by FcRn, resulting in IgG1-like serum persistence. Serum concentration-time profiles of Her2-IgG1, Her2-IgA2, and Her2-IgG1/IgA2 in Balb/C mice are shown in Figure 5, and the relevant pharmacokinetic (PK) parameters were summarized in Table 3. Her2-IgG1 has a half-life of 5.2 days whereas Her2-IgA2 exhibits rapid clearance in comparison (half-life of 9 hrs). Her2- IgG1/IgA2 exhibits clearance at a rate similar to that of the parental IgG1 molecule, suggesting that FcRn recycling is unimpaired in the tandem IgG1/IgA2-Fc format.
Figure 5.
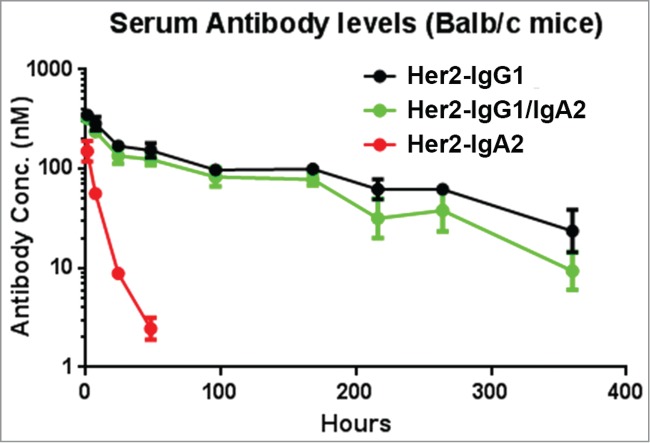
Pharmacokinetic profiles of mAb variants in BALB/c mice. Serum levels for Her2-IgG1 (black), Her2-IgG1/IgA2 (green), and Her2-IgA2 (red) in BALB/c mice were quantified by ELISA detecting human Fab. Mice were injected with a single IV bolus dose of 2.5 mg/kg. Each data point represents the average serum concentration of the mAb in 4 mice for up to 14 days. Error bars represent standard deviation. The data was fit to a 2 compartment model for the calculation of pharmacokinetic parameters (Table 3).
Table 3.
Pharmacokinetic parameters for antibody variants in Balb/c mice
| CL (mL/day/kg) | T ½ (days) | Vss (ml/kg) | AUCinf (day*ug/ml) | Cmax (ug/ml) | |
|---|---|---|---|---|---|
| Her2-IgG1 | 10.7 ± 1.7 | 5.2 ± 1.6 | 76 ± 15 | 239 ± 34 | 52.4 ± 5.2 |
| Her2- IgG1/IgA2 | 11.9 ± 2.5 | 4.0 ± 1.3 | 67 ± 8.7 | 219 ± 56 | 60.4 ± 8.7 |
| Her2-IgA2 | 308 ± 52 | 0.38 ± 0.027 | 134 ± 30 | 8.29 ± 1.3 | 22.7 ± 6.0 |
Parameters are estimated from mean data calculated from 4 mice at each time point per group. AUC stands for area under curve; CL, clearance rate; T½, half-life; Vss, central compartment volume of distribution.
Discussion
The potential clinical benefits of utilizing the IgA isotype as a cancer immunotherapeutic to engage PMN cells have been recognized for some time.10 Nevertheless, multiple obstacles have slowed validation of this approach. Foremost, the dissimilarity between IgA systems in human and rodents and the lack of FcαRI in mice have slowed the in vivo investigation of therapeutic IgAs in oncology until recently.23 Additionally, IgA immunotherapies face challenges related to the relatively complex structure of IgA and the shorter serum half-life compared to IgG.
We developed the tandem IgG1/IgA2-Fc format to augment IgG with an improved ability to engage abundant cytotoxic PMN cells in addition to NK-cells. By linking IgG and IgA Fcs in tandem, binding to IgG Fcγ receptors, C1q, FcRn, and Protein A is retained while FcαRI binding is endowed to the antibody. We demonstrated, using the Her2-IgG1/IgA2 as a test case, that this antibody format has IgG1-like expression levels and comparable thermal stability (Table 1, Fig. S1). Her2-IgG1/IgA2 soluble aggregate levels were higher than Her2-IgG1 (Table 1, Fig. S1), however, we expect that monomeric content could be improved upon optimization of elution and neutralization conditions.
A critical advantage of the tandem IgG1/IgA2 format is its improved developability compared to other IgA isotypes. IgA2-Fc was utilized rather than IgA1 because IgA1 has a long, protease-susceptible, heavily O-glycosylated hinge region31 that would likely pose problems for downstream development. Additionally, IgA2 has been shown to have a more potent ADCC response than IgA1.19,32 Notably, by fusing the Fc of IgA2(m1) to the C-terminus of IgG1-Fc, we eliminate the reported instability of heavy and light chain pairing observed for the IgA2 allotype.22 The IgA tailpiece was deleted in IgG1/IgA2 to avoid undesirable intermolecular interactions or protein aggregation. Another advantage of the IgG1/IgA2 tandem Fc format is its modularity. The preserved binding to Fcγ receptors in the tandem format (Table 2) suggests that IgG1/IgA2-Fc is likely compatible with IgG-Fc modifications33 that have been shown to improve NK-mediated ADCC in the IgG-Fc.
Our work shows that IgG1/IgA2-Fc antibody can have superior ADCC or ADCP activities over either parental (IgG1 or IgA2) formats (Figs. 2–4). This format compares favorably to other engineered hybrid Fc approaches that do not retain the full effector receptor binding repertoire of both IgG1 and IgA or lack of FcRn mediated long serum persistence.26–28 The IgG1/IgA2 tandem Fc also retains C1q binding (Table 2), and exhibits CDC activity with an anti-CD20 IgG1/IgA2 antibody against CD20 expressing cell lines (data not shown). Future work using relevant in vivo models (such as human CD89 transgenic mice) will ultimately be necessary to validate IgG1/IgA2 (and other IgA based approaches) as viable cancer immunotherapies. As different types of malignancies will vary in both susceptibility to CD89 mediated killing by myeloid cells and levels of PMN infiltrate within tumor tissues, it will be important to match immunotherapies targeting CD89 with tumors sensitive to such therapy.
IgG1/IgA2-Fc not only has the ability to effectively engage multiple cell types, it can likely engage both Fcγ and Fcα receptors on individual cells co-expressing those receptors. One concern when engaging both receptor classes on an individual PMN cell is that the levels of intracellular FcR γ chain (used for signaling by both FcγRI and FcαRI receptors) could be a limiting factor. A previously study by van Egmond et al.34 has shown that with dual engagement of these receptors, the common signaling FcR γ chain used by the FcγRI, FcγRII and FcαRI does not limit the activities of the respective receptors on PMN cells. Indeed, simultaneous engagement of these receptors on PMN cells was shown to lead to enhanced ADCC beyond that seen with individual engagement.34 This may explain the small but consistently observed increases in PMN-mediated ADCC with Her2-IgG1/IgA2 compared with Her2-IgA2 (Fig. 2). Such improved ADCC activity is particularly significant when compared with Her2-IgG1, which had only limited activity when both PBMC and PMN are used as effector cells, despite Her2-IgG1 being able to engage FcγRIIa on neutrophils. The low level of ADCC induced by Her2-IgG1 in this case is likely due to the small number of NK cells present, and would probably rise if effector cell concentrations were increased.
In addition to their potential in cancer immunotherapy, IgA antibodies are an important isotype for fighting infectious diseases. Serum IgA has been shown to play a key role in clearance of pathogens via FcαRI expressing Kupffer cells in the liver or PMN cells in lung.35,36 It has recently been demonstrated that passive transfer of human IgA mAbs against a Mycobacterium tuberculosis antigen protects human CD89 transgenic mice (but not CD89-negative littermates) from infection.37 Additionally, IgA can clear bacteria by neutrophil NETosis, i.e., the formation of an extracellular fibril/chromatin matrix by neutrophils.38 In light of these studies, we foresee that the IgG1/IgA2 format described in this work could improve the efficacy of antibody-based immunotherapy against infectious disease in addition to cancer immunotherapy.
Methods
Antibody expression and purification
All constructs (except Her2-IgG1-afuc) were transiently expressed in HEK 293FT cells using 293fectin™ and Freestyle™ medium (Life Technologies). The culture medium was collected 10 days after transfection. IgG- and IgG1/IgA2-Fc antibodies were purified by standard protein A affinity chromatography. Her2-IgA2 was purified via Peptide M agarose resin (Invivogen). Monomer content after purification was determined by analytical size-exclusion chromatography. Preparative size-exclusion chromatography was used to remove soluble aggregate from Her2-IgG1/IgA2 and Her2-IgA2 and to >98% monomeric protein. The afucosylated antibody Her2-IgG1-afuc was generated by expressing Her2-IgG1 in a fucosyltransferase-deficient producer Chinese hamster ovary cell line (BioWa Potelligent Technology) and purified via standard Protein A chromatography.
Her2 ELISA
Human HER2/ErbB2 extracellular domain (Sino Biological) was coated on 96-well maxisorp plates (Nunc) overnight at 4°C (2 μg/ml in phosphate-buffered saline (PBS)). Plates were then blocked with 2% BSA in PBS for 2 hours at room temperature. After washing, titrated antibodies were added and allowed to incubate for 1 hour. After three washes in PBS, peroxidase-conjugated anti-Human Fd (Southern Biotech) was used for detection. The wells were washed 3 times, and TMB substrate solution was added. The reaction was stopped with 2 N H2SO4 and the absorbance at 450 nm was measured.
Differential scanning calorimetry
A Microcal VP-DSC scanning microcalorimeter (Microcal) was used for all DSC experiments. All solutions and samples used for DSC were filtered using a 0.22 μm filter prior to loading into the calorimeter. Samples were analyzed in PBS with PBS serving as the reference buffer for the DSC experiments. Prior to sample measurement, baseline measurements (buffer-versus-buffer) were obtained to be subtracted from the sample measurement. Dialyzed samples (at a concentration of 1 mg/mL) were added to the sample well and DSC measurements were performed at a 1°C/min scan rate. Data analysis was carried out using the Origin™ DSC software provided by Microcal.
Surface plasmon resonance measurements
The binding of FcαRI (Sino biological), FcγRs, C1q (Quidel), and both murine (mFcRn) and human FcRn (hFcRn) to immobilized antibody variants was monitored by SPR with a ProteOn™ XPR36 (Bio-Rad). FcγRIIIa (158V and 158F), FcγRIIa, FcγRIIb, FcγRIa, hFcRn and mFcRn were all generated at Medimmune. Antibodies were first coupled to a GLC sensor chip according to manufacturer's instructions. Excess reactive groups were blocked with a 6-min injection of 1 M ethanolamine. Antibodies were immobilized at a surface density of ∼5000 RUs for equilibrium measurements and <200 RUs for kinetic binding experiments. FcRn was used at concentrations ranging from 0.45 nM to 3 μM at a flow rate of 25 μL/min for steady-state measurements and 75 μL/min for kinetic measurements. One channel was always left unmodified to provide a blank reference surface. Dilutions and binding experiments were carried out at 25°C in PBS pH 6.0 or pH 7.4, containing 0.05% Tween 20. Steady-state binding data were collected for 10 min. Antibody surfaces were regenerated with a 15-second injection of 5 mM HCl. Binding affinities were determined by using the ProteOn™ Manager software. Dissociation constants (KDs) were determined by fitting the corresponding binding isotherms for steady-state data or by fitting the kinetics for association and dissociation employing a 1:1 Langmuir or a 1:1 Langmuir Mass Transfer model.
Isolation of human effector cells and description of cell lines
Blood samples were obtained from healthy donors after obtaining informed consent. PMN and PBMCs were isolated from freshly drawn peripheral blood using a Polymorphprep™ (Axis-Shield) gradient following manufacturer's directions. PMN cells isolated using this method were found to have ∼85% neutrophil content as determined by Giemsa staining. The PMN & PBMC effector cell population is a mixture of the PMN and PBMC fractions isolated from the Polymorphoprep™ gradient. Residual RBCs in both effector cell populations were lysed with ACK lysing buffer. The KC1333 NK cell line (BioWa) expressing human CD16 was maintained in RPMI 1640 medium, supplemented with 10% FBS. BT474 (Her2+++), SK-BR-3 (Her2+++) and MDA-MB-453 (Her2++) human breast cancer cells were obtained from ATCC (Rockville, MD) and maintained in DMEM supplemented with 10% FBS. Her2 expression (and assignment of “+” scoring)39 was confirmed with HercepTest™ (Dako A/S, Glostrup, Denmark).
Antibody-dependent cell-mediated cytotoxicity assays
ADCC assays were performed at an effector to target (E:T) ratio of 20:1 PMN or PMN & PBMC and 2.5:1 using KC1333 cells. Cell killing was measured using the Cytotox 96® Non-Radioactive Cytotoxicity Assay (Promega). Briefly, cells (10,000 target cells/well in a U bottom 96-well plate) were incubated at 37°C for 20 hrs in RPMI supplemented with 2.5 mM glutamine, 10% FBS, and 1% Pen-Strep prior to removing supernatant for LDH release measurements. The percentage of cellular cytotoxicity was calculated using the formula: % cytotoxicity = (experimental value - spontaneous effector - spontaneous target) / (maximal target - spontaneous target) ×100. Data were graphed and analyzed using GraphPad Prism 6.0. Curves were fitted using a nonlinear regression model with a sigmoidal dose response.
ADCC FCM analysis and microscopy
ADCC experiments for flow cytometric analysis were performed as above; however PMN and BT474 cells (at a ratio of 10:1 PMN:BT474 cells) were labeled with cell-permeable Calcein-AM violet and Calcein-AM, respectively, prior to the experiment. After 20 hours at 37°C, cells were then labeled with LIVE/DEAD® Fixable Near-IR Dead Cell Stain (Life Technologies) according to manufacturer's directions. FCM data were collected via LSR II (BD bioscience) and analyzed in FlowJo v7.6.1 (Tree Star Inc.). No compensation was necessary for the 3-color experiment. Percent cytotoxicity was calculated as follows: % cytotoxicity = 100 × (count Calcein-AM+, LIVE/DEAD® Fixable Near-IR+ cells)/(total count Calcein-AM+ cells). For microscopy experiments BT474 cells were labeled with Calcein-AM, and PMN cells were unlabeled. An E:T ratio of 10:1 was used with antibodies added at a concentration of 6 nM. Fluorescence microscopy was performed using a FLoid Cell Imaging Station (Life Technologies).
ADCP FCM analysis
Preparation of human monocyte-derived MΦ and quantification of target cell phagocytosis was performed as described previously.40 Briefly, PBMC were purified from healthy donor blood, monocytes were enriched by adherence and differentiated over 6 days in RPMI with 100ng/mL human M-CSF (R & D Systems), 10% FBS and penicillin-streptomycin solution (Life Technologies). MΦ were detached with accutase (PAA laboratories) followed by scraping, labeled with celltrace violet (3 μM, Life Technologies) and seeded at 8 × 104 cells/well in flat-bottomed 96-well plates. After resting overnight, 1 × 104 CFSE-labeled SK-BR-3 or MDA-MB-453 (10 μM, Life Technologies) were added per well along with the indicated concentrations of antibodies. After 2 h, adherent cells were detached with accutase, combined with non-adherent cells, stained with live dead far red (Invitrogen), and fixed in 1% PFA. Phagocytosis was quantified by FCM (FACS Canto II with 405 nm, 488 nm and 633 nm lasers, BD Biosciences), with data analysis in FlowJo v7.6.1. Data were gated on single, live cells, and % phagocytosis was calculated as follows: % phagocytosis = 100 × (count CFSE+, celltrace violet+ cells)/(total count CFSE+ cells)
Data were graphed and analyzed using GraphPad Prism 6.0. Curves were fitted using a nonlinear regression model with a sigmoidal dose response. Images of different cell populations in MΦ/tumor cell co-cultures were captured with ImagestreamX imaging cytometer and analyzed with IDEAS software (Amnis Corporation).
In vivo pharmacokinetics in BALB/C Mice
Female (8 weeks old) BALB/c mice were given a bolus intravenous (IV) dose of 2.5 mg/kg antibody on day 0. Eight mice were used per antibody tested, with 2 groups of 4 (A group or B group) bled at alternate time points. Blood samples were obtained from the retro-orbital plexus using capillary pipettes at different time points throughout the 25 day study. A quantitative ELISA was used to monitor the serum concentrations of the tested antibodies. Briefly, 96-well plates were coated with 2 μg/ml of AffiPure Goat anti-human F(ab’)2 fragment-specific antibody (Jackson ImmunoResearch). Plates were blocked with 3% BSA in PBS for an hour, and then incubated with appropriately diluted serum samples (1:300 for earlier time points and 1:50 or 1:100 for later time points). Goat anti-human Fd-specific HRP-conjugated antibody (Southern Biotechnology Associates) was used to detect the human antibody (dilution 1:10000). Absorbance at 450 nm was measured after development with TMB substrate (KPL) according to manufacturer's directions. Standard curves were generated for each antibody variant diluted into 1:100 pre-bleed mouse serum (taken at day -3). The linear portions of standard curves generated in GraphPad Prism 6.0 were then used to quantify human IgG in the serum samples.
Disclosure of Potential Conflicts of Interest
The authors have no conflict of interest and financial interest to disclose.
Acknowledgments
We would like to acknowledge Xiang Qing Yu for assistance with PK analysis and Samuel Perry and John Li for assistance with ADCC assays.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website
References
- 1.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol 2010; 10:317-27; PMID:20414205; http://dx.doi.org/ 10.1038/nri2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iannello A, Ahmad A. Role of antibody-dependent cell-mediated cytotoxicity in the efficacy of therapeutic anti-cancer monoclonal antibodies. Cancer Metastasis Rev 2005; 24:487-99; PMID:16408158; http://dx.doi.org/ 10.1007/s10555-005-6192-2 [DOI] [PubMed] [Google Scholar]
- 3.Bulliard Y, Jolicoeur R, Zhang J, Dranoff G, Wilson NS, Brogdon JL. OX40 engagement depletes intratumoral Tregs via activating Fc[gamma]Rs, leading to antitumor efficacy. Immunol Cell Biol 2014; 92(6):475-80; PMID:24732076; http://dx.doi.org/ 10.1038/icb.2014.26 [DOI] [PubMed] [Google Scholar]
- 4.Smyth MJ, Ngiow SF, Teng MWL. Targeting regulatory T cells in tumor immunotherapy. Immunol Cell Biol 2014; 92(6):473-4; PMID:24777313; http://dx.doi.org/ 10.1038/icb.2014.33 [DOI] [PubMed] [Google Scholar]
- 5.Satoh M, Iida S, Shitara K. Non-fucosylated therapeutic antibodies as next-generation therapeutic antibodies. Expert Opin Biol Ther 2006; 6:1161-73; PMID:17049014; http://dx.doi.org/ 10.1517/14712598.6.11.1161 [DOI] [PubMed] [Google Scholar]
- 6.Umana P, Jean-Mairet J, Moudry R, Amstutz H, Bailey JE. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat Biotechnol 1999; 17:176-80; PMID:10052355; http://dx.doi.org/ 10.1038/6179 [DOI] [PubMed] [Google Scholar]
- 7.Jung ST, Kelton W, Kang TH, Ng DTW, Andersen JT, Sandlie I, Sarkar CA, Georgiou G. Effective phagocytosis of low her2 tumor cell lines with engineered, aglycosylated igG displaying high Fc gamma RIIa affinity and selectivity. ACS Chem Biol 2013; 8:368-75; PMID:23030766; http://dx.doi.org/ 10.1021/cb300455f [DOI] [PubMed] [Google Scholar]
- 8.Moore GL, Chen H, Karki S, Lazar GA. Engineered Fc variant antibodies with enhanced ability to recruit complement and mediate effector functions. mAbs 2010; 2:181-9; PMID:20150767; http://dx.doi.org/ 10.4161/mabs.2.2.11158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richards JO, Karki S, Lazar GA, Chen H, Dang W, Desjarlais JR. Optimization of antibody binding to Fc gamma RIIa enhances macrophage phagocytosis of tumor cells. Mol Cancer Ther 2008; 7:2517-27; PMID:18723496; http://dx.doi.org/ 10.1158/1535-7163.MCT-08-0201 [DOI] [PubMed] [Google Scholar]
- 10.Dechant M, Valerius T. IgA antibodies for cancer therapy. Crit Rev Oncol/Hematol 2001; 39:69-77; PMID:11418303; http://dx.doi.org/ 10.1016/S1040-8428(01)00105-6 [DOI] [PubMed] [Google Scholar]
- 11.Snoeck V, Peters IR, Cox E. The IgA system: a comparison of structure and function in different species. Vet Res 2006; 37:455-67; PMID:16611558; http://dx.doi.org/ 10.1051/vetres:2006010 [DOI] [PubMed] [Google Scholar]
- 12.Fureder W, Agis H, Sperr WR, Lechner K, Valent P. The surface-membrane antigen phenotype of human blood basophils. Allergy 1994; 49:861-5; PMID:7709996; http://dx.doi.org/ 10.1111/j.1398-9995.1994.tb00788.x [DOI] [PubMed] [Google Scholar]
- 13.Fureder W, Bankl HC, Toth J, Walchshofer S, Sperr W, Agis H, Semper H, Sillaber C, Lechner K, Valent P. Immunophenotypic and functional characterization of human tonsillar mast cells. J Leukoc Biol 1997; 61:592-9; PMID:9129208 [DOI] [PubMed] [Google Scholar]
- 14.Monteiro RC, Kubagawa H, Cooper MD. Cellular-distribution, regulation, and biochemical nature of an fc-alpha receptor in humans. J Exp Med 1990; 171:597-613; PMID:2137852; http://dx.doi.org/ 10.1084/jem.171.3.597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albanesi M, Mancardi DA, Jonsson F, Iannascoli B, Fiette L, Di Santo JP, Lowell CA, Bruhns P. Neutrophils mediate antibody-induced antitumor effects in mice. Blood 2013; 122:3160-4; PMID:23980063; http://dx.doi.org/ 10.1182/blood-2013-04-497446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Carlo E, Forni G, Lollini P, Colombo MP, Modesti A, Musiani P. The intriguing role of polymorphonuclear neutrophils in antitumor reactions. Blood 2001; 97:339-45; PMID:11154206; http://dx.doi.org/ 10.1182/blood.V97.2.339 [DOI] [PubMed] [Google Scholar]
- 17.Souto JC, Vila L, Bru A. Polymorphonuclear neutrophils and cancer: Intense and sustained neutrophilia as a treatment against solid tumors. Med Res Rev 2011; 31:311-63; PMID:19967776; http://dx.doi.org/ 10.1002/med.20185 [DOI] [PubMed] [Google Scholar]
- 18.van Egmond M. Neutrophils in antibody-based immunotherapy of cancer. Expert Opin Biol Ther 2008; 8:83-94; PMID:18081538; http://dx.doi.org/ 10.1517/14712598.8.1.83 [DOI] [PubMed] [Google Scholar]
- 19.Dechant M, Vidarsson G, Stockmeyer B, Repp R, Glennie MJ, Gramatzki M, van de Winkel JGJ, Valerius T. Chimeric IgA antibodies against HLA class II effectively trigger lymphoma cell killing. Blood 2002; 100:4574-80; PMID:12393717; http://dx.doi.org/ 10.1182/blood-2002-03-0687 [DOI] [PubMed] [Google Scholar]
- 20.Valerius T, Stockmeyer B, vanSpriel AB, Graziano RF, vandenHerikOudijk IE, Repp R, Deo YM, Lund J, Kalden JR, Gramatzki M, et al.. Fc alpha RI (CD89) as a novel trigger molecule for bispecific antibody therapy. Blood 1997; 90:4485-92; PMID:9373259 [PubMed] [Google Scholar]
- 21.van der Steen L, Tuk CW, Bakema JE, Kooij G, Reijerkerk A, Vidarsson G, Bouma G, Kraal G, de Vries HE, Beelen RHJ, et al.. Immunoglobulin A: Fc alpha RI interactions induce neutrophil migration through release of leukotriene B4. Gastroenterology 2009; 137:2018-29; PMID:19555692; http://dx.doi.org/ 10.1053/j.gastro.2009.06.047 [DOI] [PubMed] [Google Scholar]
- 22.Lohse S, Brunke C, Derer S, Peipp M, Boross P, Kellner C, Beyer T, Dechant M, van der Winkel JG, Leusen JH, et al.. Characterization of a mutated IgA2 antibody of the m(1) allotype against the epidermal growth factor receptor for the recruitment of monocytes and macrophages. J Biol Chem 2012; 287:25139-50; PMID:22679018; http://dx.doi.org/ 10.1074/jbc.M112.353060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boross P, Lohse S, Nederend M, Jansen JHM, van Tetering G, Dechant M, Peipp M, Royle L, Liew LP, Boon L, et al.. IgA EGFR antibodies mediate tumour killing in vivo. EMBO Mol Med 2013; 5:1213-26; PMID:23918228; http://dx.doi.org/ 10.1002/emmm.201201929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morell A, Skvaril F, Noseda G, Barandun S. Metabolic properties of human IgA subclasses. Clin Exp Immunol 1973; 13:521-8; PMID:4717094 [PMC free article] [PubMed] [Google Scholar]
- 25.Brunke C, Lohse S, Derer S, Peipp M, Boross P, Kellner C, Beyer T, Dechant M, Royle L, Liew LP, et al.. Effect of a tail piece cysteine deletion on biochemical and functional properties of an epidermal growth factor receptor-directed IgA2 m(1) antibody. mAbs 2013; 5:936-45; PMID:24492345; http://dx.doi.org/ 10.4161/mabs.26396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chintalacharuvu KR, Vuong LUC, Loi LA, Larrick JW, Morrison SL. Hybrid IgA2/IgG1 antibodies with tailor-made effector functions. Clin Immunol 2001; 101:21-31; PMID:11580223; http://dx.doi.org/ 10.1006/clim.2001.5083 [DOI] [PubMed] [Google Scholar]
- 27.Lazar GA, inventor; Xencor Inc., assignee . Fc Polypeptides with Novel Fc Ligand Binding Sites. United States Patent Application No. WO/2005/077981 A2 2005, August 25.
- 28.Kelton W, Mehta N, Charab W, Lee J, Lee C-H, Kojima T, Kang TH, Georgiou G. IgGA: a “cross-isotype” engineered human Fc antibody domain that displays both IgG-like and IgA-like effector functions. Chem Biol 2014; 21:1603-9; PMID:25500223; http://dx.doi.org/ 10.1016/j.chembiol.2014.10.017 [DOI] [PubMed] [Google Scholar]
- 29.Bruckheimer EM, Fazenbaker CA, Gallagher S, Mulgrew K, Fuhrmann S, Coffman KT, Walsh W, Ready S, Cook K, Damschroder M, et al.. Antibody-dependent cell-mediated cytotoxicity effector-enhanced EphA2 agonist monoclonal antibody demonstrates potent activity against human tumors. Neoplasia 2009. Jun; 11(6):509-17, 2 p following 517 2010; PMID:19484140; http://dx.doi.org/ 10.1593/neo.81578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Egmond M, Hanneke van Vuuren AJ, van de Winkel JG. The human Fc receptor for IgA (Fc alpha RI, CD89) on transgenic peritoneal macrophages triggers phagocytosis and tumor cell lysis. Immunol Lett 1999; 68:83-7; PMID:10397160; http://dx.doi.org/ 10.1016/S0165-2478(99)00034-6 [DOI] [PubMed] [Google Scholar]
- 31.Mattu TS, Pleass RJ, Willis AC, Kilian M, Wormald MR, Lellouch AC, Rudd PM, Woof JM, Dwek RA. The glycosylation and structure of human serum IgA1, Fab, and Fc regions and the role of N-glycosylation on Fc alpha receptor interactions. J Biol Chem 1998; 273:2260-72; PMID:9442070; http://dx.doi.org/ 10.1074/jbc.273.4.2260 [DOI] [PubMed] [Google Scholar]
- 32.Dechant M, Beyer T, Schneider-Merck T, Weisner W, Peipp M, van de Winkel JGJ, Valerius T. Effector mechanisms of recombinant IgA antibodies against epidermal growth factor receptor. J Immunol 2007; 179:2936-43; PMID:17709508; http://dx.doi.org/ 10.4049/jimmunol.179.5.2936 [DOI] [PubMed] [Google Scholar]
- 33.Strohl WR. Optimization of Fc-mediated effector functions of monoclonal antibodies. Curr Opin Biotechnol 2009; 20:685-91; PMID:19896358; http://dx.doi.org/ 10.1016/j.copbio.2009.10.011 [DOI] [PubMed] [Google Scholar]
- 34.van Egmond M, van Spriel AB, Vermeulen H, Huls G, van Garderen E, van de Winkel JGJ. Enhancement of polymorphonuclear cell-mediated tumor cell killing on simultaneous engagement of Fc gamma RI (CD64) and Fc alpha RI (CD89). Cancer Res 2001; 61:4055-60; PMID:11358825 [PubMed] [Google Scholar]
- 35.Hellwig SMM, van Spriel AB, Schellekens JFP, Mooi FR, van de Winkel JGJ. Immunoglobulin A-mediated protection against Bordetella pertussis infection. Infection and Immunity 2001; 69:4846-50; PMID:11447159; http://dx.doi.org/ 10.1128/IAI.69.8.4846-4850.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Egmond M, van Garderen E, van Spriel AB, Damen CA, van Amersfoort ES, van Zandbergen G, van Hattum J, Kuiper J, van de Winkel JGJ. Fc alpha RI-positive liver Kupffer cells: reappraisal of the function of immunoglobulin A in immunity. Nat Med 2000; 6:680-5; PMID:10835685; http://dx.doi.org/ 10.1038/76261 [DOI] [PubMed] [Google Scholar]
- 37.Balu S, Reljic R, Lewis MJ, Pleass RJ, McIntosh R, van Kooten C, van Egmond M, Challacombe S, Woof JM, Ivanyi J. A novel human IgA monoclonal antibody protects against tuberculosis. J Immunol 2011; 186:3113-9; PMID:21257971; http://dx.doi.org/ 10.4049/jimmunol.1003189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aleyd E, van Hout MWM, Ganzevles SH, Hoeben KA, Everts V, Bakema JE, van Egmond M. Immunoglobulin A enhances NETosis and release of neutrophil extracellular traps by polymorphonuclear cells via Fc alpha RI. Eur J Clin Invest 2014; 44:21-2. [DOI] [PubMed] [Google Scholar]
- 39.Rhodes A, Jasani B, Couturier J, McKinley MJ, Morgan JM, Dodson AR, Navabi H, Miller KD, Balaton AJ. A formalin-fixed, paraffin-processed cell line standard for quality control of immunohistochemical assay of HER-2/neu expression in breast cancer. Am J Clin Pathol 2002; 117:81-9; PMID:11789735; http://dx.doi.org/ 10.1309/4NCM-QJ9W-QM0J-6QJE [DOI] [PubMed] [Google Scholar]
- 40.Luheshi N, Davies G, Poon E, Wiggins K, McCourt M, Legg J. Th1 cytokines are more effective than Th2 cytokines at licensing anti-tumour functions in CD40-activated human macrophages in vitro. Eur J Immunol 2014; 44:162-72; PMID:24114634; http://dx.doi.org/ 10.1002/eji.201343351 [DOI] [PubMed] [Google Scholar]
- 41.Clark M. IgG effector mechanisms. Chem Immunol 1997; 65:88-100; PMID:9018874; http://dx.doi.org/ 10.1159/000425634 [DOI] [PubMed] [Google Scholar]
- 42.Herr AB, Ballister ER, Bjorkman PJ. Insights into IgA-mediated immune responses from the crystal structures of human Fc alpha RI and its complex with IgA1-Fc. Nature 2003; 423:614-20; PMID:12768205; http://dx.doi.org/ 10.1038/nature01685 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.