Abstract
Plants need to adapt to various stress factors originating from the environment. Signal transduction pathways connecting the recognition of environmental cues and the initiation of appropriate downstream responses in plants often involve intracellular Ca2+ concentration changes. These changes must be deciphered into specific cellular signals. Calmodulin-like proteins, CMLs, act as Ca2+ sensors in plants and are known to be involved in various stress reactions. Here, we show that in Arabidopsis 2 different CMLs, AtCML37 and AtCML42 are antagonistically involved in drought stress response. Whereas a CML37 knock-out line, cml37, was highly susceptible to drought stress, CML42 knockout line, cml42, showed no obvious effect compared to wild type (WT) plants. Accordingly, the analysis of the phytohormone abscisic acid (ABA) revealed a significant reduction of ABA upon drought stress in cml37 plants, while in cml42 plants an increase of ABA was detected. Summarizing, our results show that both CML37 and CML42 are involved in drought stress response but show antagonistic effects.
Keywords: Arabidopsis thaliana, abscisic acid, CML37, CML42, calmodulin-like proteins, drought stress
During their life, plants are challenged by a multitude of different stresses. Thus, in order to survive, plants have to deal with and adapt to all forms of stress, biotic as well as abiotic. The signal transduction pathway connecting the recognition of such environmental cues and the downstream responses are still poorly understood. However, it is well accepted that Ca2+ is one of the major second messengers involved in many different signaling pathways.1 Changes in intracellular Ca2+ concentrations due to different stimuli are described as calcium signatures.2 However, Ca2+ signatures are not sufficient to explain specificity of the particular responses. Therefore, signaling components that can decipher and transduce the Ca2+ signal to further downstream signaling components are necessary. Such candidates for Ca2+-binding proteins are the so-called calmodulin-like proteins, CMLs, which act as Ca2+ sensors.3 CMLs are unique to plants with 50 members in Arabidopsis thaliana and they do not exhibit enzymatic activities but undergo conformational changes upon Ca2+ binding, which enables subseque8t interaction with target proteins that, upon binding, will be modulated in their activities.4
CMLs are involved in plant development and responses to various stress factors from the abiotic and the biotic environment. For instance, CML24 can cause changes in abscisic acid (ABA) level during ion stress as well as changes in flowering time; it also regulates pollen tube growth by modulating the actin cytoskeleton and controlling the cytosolic Ca2+ concentration.5-7
CML39 functions during early seedling establishment and activates defense responses.8 CML9 gene is induced by P. syringae infection, flg22 elicitor and salicylic acid (SA) and it alters plant responses to ABA and abiotic stress;9,10 CML8 is also induced by SA and salt stress.11 Transcripts of CML37, CML38 and CML39 are regulated by salt- and drought stress, some phytohormones (jasmonate and ABA), and biotic stress (phytopathogenic Pseudomonas syringae).12 Recently, several CMLs were identified that are regulated by herbivory or herbivore-related signals present in insect-derived oral secretions.13-15 For 2 particular calmodulin-like proteins, CML37 and CML42, it was demonstrated that they have antagonistic effects. CML42 represents a negative regulator of insect herbivory-induced defense, while CML37 is quite the opposite, a positive regulator of insect herbivory-induced defense.13,15 CML37 was shown to connect Ca2+ and jasmonate signaling by affecting the synthesis of jasmonic acid-isoleucine conjugate.15 All those examples demonstrate that almost all CML genes studied so far are involved in more than one stress-responsive pathway, including biotic and abiotic stress, as well as developmental stimuli. These findings make them ideal candidates in plants to study cross talk between different pathways on the one hand and specificity in the activation of pathways on the other hand.
Here, we decided to further investigate properties of the antagonists CML37 (At5g42380) and CM42 (At4g20780). Based on an experiment that suggested an involvement of CML42 in drought stress,13 we chose this particular stress factor again for our investigation. We observed that cml37 plants were much more susceptible for drought stress than WT and cml42 plants (Fig. 1). While cml37 plants were already completely dried out, cml42 and WT plants survived the treatment. In plants, drought stress is always combined with osmotic stress, which regulates ABA biosynthesis.16 Thus, the level of ABA was determined in WT, cml37 and cml42 plants after one week of drought stress and after a second cycle of drought stress, again for one week (Fig. 2). The content of ABA in cml37 plants exposed to dryness was significantly lower when compared to WT (Fig. 2). While WT plants contained an average of 200 ng ABA (g FW)−1 after one week, and 100 ng ABA (g FW)−1 after 2 weeks of drought stress, cml37 plants could only reach level of about 40 ng ABA (g FW)−1 at both time points. In contrast, cml42 plants accumulated about 250 ng ABA (g FW)−1 already after the first week and, in contrast to the WT, retained this high level in the second week.
Figure 1.
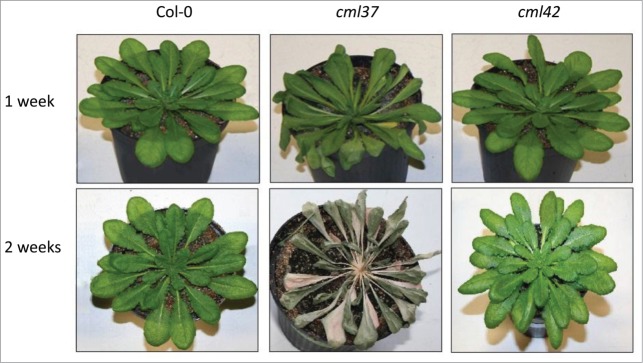
Drought phenotype of Arabidopsis WT (Col-0), cml37 and cml42 knockout plants. Drought phenotype of WT Col-0 (left), cml37 (middle) and cml42 plants each after 1 (top line) or 2 weeks (bottom line) of drought treatment. The plants shown represent the typical phenotype observed in multiple replicates.
Figure 2.
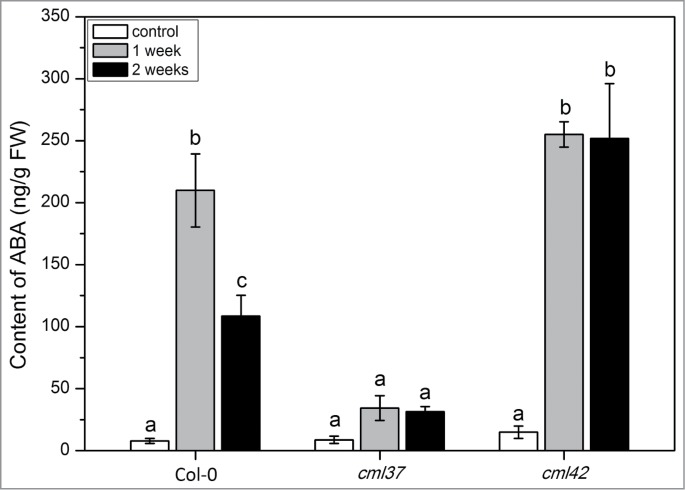
ABA level in Arabidopsis WT (Col-0), cml37 and cml42 knock-out plants upon drought treatment. Mean content (± SE, n = 6) of ABA in Col-0, cml37 and cml42 plants. ABA content was analyzed in untreated plants (white), and plants subjected to 1 week (gray) and 2 weeks of drought treatment (black). ABA was measured from the whole plant rosette. Statistically significant differences between treatments were analyzed by One Way ANOVA (P < 0.05, SNK test).
The accumulation of ABA plays an important role in drought and osmotic stress response. ABA promotes the closure of stomata and affects drought-related gene expression which mediates a higher drought tolerance and resistance.17 Thus, the phenotypical results shown in Figure 1 can be directly explained by a much lower accumulation of ABA in the cml37 mutant.
Hence, we can state that CML37 is a positive, while CML42 is a negative regulator of ABA accumulation that is induced by drought stress. These results also reflect and confirm the striking antagonism found for these particular CMLs in insect herbivory resistance.13,15 Further experiments with over-expressing CML37 and CML42 lines, as well as double knock out lines will be carried out in the near future in order to understand how such an antagonistic interaction is realized on the cellular and molecular level.
Materials & Methods
Plant growth and drought treatment
Arabidopsis thaliana ecotype Columbia (Col-0) was used for all experiments and plants were grown as described before.13 CML knock-out lines cml37 (SALK_011488C) and cml42 (SALK_041400C) were obtained from the SALK Institute and selected for homozygosity.13,15,18 For all experiments 4 week old plants, grown under short-day conditions, were used. Drought treatment was applied for 1 or 2 weeks. In particular, plants were not watered for 1 week (normally daily) till substrate was completely dry, before plants were harvested for ABA analysis. In parallel, after 1 week of drought stress plants were fully watered (till the pot was fully soaked with water), and subjected to a second week of drought stress before ABA analysis was performed. To minimize experimental variation, WT and mutants were placed in the same tray.
Phytohormone analysis
For abscisic acid phytohormone extraction 250 mg of fresh plant material was used. Leaf material was weighed and frozen in liquid nitrogen. D6-abscisic acid (Santa Cruz Biotechnology, Heidelberg, Germany) was used as internal standard. Samples were homogenized for 1 minute at 1000 rpm in the Genogrinder 2010 (Spex Sample Prep, Stanmore, UK) and extracted and analyzed as described before using an Agilent 1200 HPLC system (Agilent Technologies, Böblingen, Germany) equipped with a Zorbax Eclipse XDB-C18 column (50 × 4.6 mm, 1.8 μm, Agilent) for chromatography and an API 3200 tandem mass spectrometer (Applied Biosystems, Darmstadt, Germany) equipped with a Turbospray ion source that was operated in the negative ionization mode for mass spectrometry.13
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank A. Lehr for excellent technical assistance, the greenhouse team for growing plants, and W. Boland for continuous support.
Funding
We thank the Max Planck Society for funding.
References
- 1. Dodd AN, Kudla J, Sanders D. The language of calcium signaling. Annu Rev Plant Biol 2010; 61:593-620; PMID:20192754; http://dx.doi.org/ 10.1146/annurev-arplant-070109-104628 [DOI] [PubMed] [Google Scholar]
- 2. Sanders D, Pelloux J, Brownlee C, Harper JF. Calcium at the crossroads of signaling. Plant Cell 2002; 14:S401-17; PMID:12045291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McCormack E, Tsai YC, Braam J. Handling calcium signaling: arabidopsis CaMs and CMLs. Trends Plant Sci 2005; 10:383-9; PMID:16023399; http://dx.doi.org/ 10.1016/j.tplants.2005.07.001 [DOI] [PubMed] [Google Scholar]
- 4. Perochon A, Aldon D, Galaud J-P, Ranty B. Calmodulin and calmodulin-like proteins in plant calcium signaling. Biochimie 2011; 93:2048-53; PMID:21798306; http://dx.doi.org/ 10.1016/j.biochi.2011.07.012 [DOI] [PubMed] [Google Scholar]
- 5. Yang X, Wang SS, Wang M, Qiao Z, Bao CC, Zhang W. Arabidopsis thaliana calmodulin-like protein CML24 regulates pollen tube growth by modulating the actin cytoskeleton and controlling the cytosolic Ca(2+) concentration. Plant Mol Biol 2014; 86:225-36; PMID:25139229; http://dx.doi.org/ 10.1007/s11103-014-0220-y [DOI] [PubMed] [Google Scholar]
- 6. Delk NA, Johnson KA, Chowdhury NI, Braam J. CML24, regulated in expression by diverse stimuli, encodes a potential Ca2+ sensor that functions in responses to abscisic acid, daylength, and ion stress. Plant Physiol 2005; 139:240-53; PMID:16113225; http://dx.doi.org/ 10.1104/pp.105.062612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hubbard K, Hotta C, Gardner M, Braam J, Webb A. The Arabidopsis thaliana Calmodulin-like protein CML24 is a regulator of rhythmic Ca^2^+ signalling and flowering time. Comp Biochem Physiol A Mol Integr Physiol 2008; 150:0-; http://dx.doi.org/ 10.1016/j.cbpa.2008.04.395 [DOI] [Google Scholar]
- 8. Bender KW, Rosenbaum DM, Vanderbeld B, Ubaid M, Snedden WA. The Arabidopsis calmodulin-like protein, CML39, functions during early seedling establishment. Plant J 2013; 76:634-47; PMID:24033804; http://dx.doi.org/ 10.1111/tpj.12323 [DOI] [PubMed] [Google Scholar]
- 9. Leba LJ, Cheval C, Ortiz-Martin I, Ranty B, Beuzon CR, Galaud JP, Aldon D. CML9, an Arabidopsis calmodulin-like protein, contributes to plant innate immunity through a flagellin-dependent signalling pathway. Plant J 2012; 71:976-89; PMID:22563930; http://dx.doi.org/ 10.1111/j.1365-313X.2012.05045.x [DOI] [PubMed] [Google Scholar]
- 10. Magnan F, Ranty B, Charpenteau M, Sotta B, Galaud JP, Aldon D. Mutations in AtCML9, a calmodulin-like protein from Arabidopsis thaliana, alter plant responses to abiotic stress and abscisic acid. Plant J 2008; 56:575-89; PMID:18643966; http://dx.doi.org/ 10.1111/j.1365-313X.2008.03622.x [DOI] [PubMed] [Google Scholar]
- 11. Park HC, Park CY, Koo SC, Cheong MS, Kim KE, Kim MC, Lim CO, Lee SY, Yun DJ, Chung WS. AtCML8, a calmodulin-like protein, differentially activating CaM-dependent enzymes in Arabidopsis thaliana. Plant Cell Rep 2010; 29:1297-304; PMID:20820784; http://dx.doi.org/ 10.1007/s00299-010-0916-7 [DOI] [PubMed] [Google Scholar]
- 12. Vanderbeld B, Snedden WA. Developmental and stimulus-induced expression patterns of Arabidopsis calmodulin-like genes CML37, CML38 and CML39. Plant Mol Biol 2007; 64:683-97; PMID:17579812; http://dx.doi.org/ 10.1007/s11103-007-9189-0 [DOI] [PubMed] [Google Scholar]
- 13. Vadassery J, Reichelt M, Hause B, Gershenzon J, Boland W, Mithöfer A. CML42-mediated calcium signaling coordinates responses to Spodoptera herbivory and abiotic stresses in Arabidopsis. Plant Physiol 2012; 159:1159-75; PMID:22570470; http://dx.doi.org/ 10.1104/pp.112.198150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vadassery J, Scholz SS, Mithöfer A. Multiple calmodulin-like proteins in Arabidopsis are induced by insect-derived (Spodoptera littoralis) oral secretion. Plant Signal Behav 2012; 7:1277-80; PMID:22902684; http://dx.doi.org/ 10.4161/psb.21664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scholz SS, Vadassery J, Heyer M, Reichelt M, Bender KW, Snedden WA, Boland W, Mithöfer A. Mutation of the Arabidopsis calmodulin-like protein CML37 deregulates the jasmonate pathway and enhances susceptibility to herbivory. Mol Plant 2014; 7:1712-26; PMID:25267731 [DOI] [PubMed] [Google Scholar]
- 16. Zhu JK. Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 2002; 53:247-73; PMID:12221975; http://dx.doi.org/ 10.1146/annurev.arplant.53.091401.143329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Finkelstein RR, Gampala SSL, Rock CD. Abscisic acid signaling in seeds and seedlings. Plant Cell 2002; 14:S15-S45; PMID:12045268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen HM, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 2003; 301:653-7; PMID:12893945; http://dx.doi.org/ 10.1126/science.1086391 [DOI] [PubMed] [Google Scholar]


