Abstract
Nitrate (NO3−) and ammonium (NH4+) are the main forms of nitrogen available in the soil for plants. Excessive NH4+ accumulation in tissues is toxic for plants and exclusive NH4+-based nutrition enhances this effect. Ammonium toxicity syndrome commonly includes growth impairment, ion imbalance and chlorosis among others. In this work, we observed high intraspecific variability in chlorophyll content in 47 Arabidopsis thaliana natural accessions grown under 1 mM NH4+ or 1 mM NO3− as N-source. Interestingly, chlorophyll content increased in every accession upon ammonium nutrition. Moreover, this increase was independent of ammonium tolerance capacity. Thus, chlorosis seems to be an exclusive effect of severe ammonium toxicity while mild ammonium stress induces chlorophyll accumulation.
Keywords: ammonium, Arabidopsis thaliana, chlorophyll, natural variation, nitrogen nutrition, SPAD
Abbreviations
- Chl
chlorophyll
- N
nitrogen
- NH4+
ammonium
- NO3−
nitrate
Nitrogen (N), constituent of essential molecules like amino acids and nucleic acids, is a crucial element for plant growth. Most non-legume plants require 20–50 g of N taken up by their roots to produce 1 kg of dry biomass. Thus, natural supply of soil N usually limits crops productivity and requires the supplementation of N compounds.1 Nitrate (NO3−) and ammonium (NH4+) are the main forms of N available for plants. Amino acids may also represent an important N source under particular conditions of soil decomposition. The preferred form in which N is taken up depends on plant adaptation to soil environment. Generally, plants adapted to alkaline and oxygenated soils prefer NO3− while plants adapted to acidic and reducing conditions preferentially uptake NH4+.2 The intensive use of fertilizers has become agriculture a major contributor to global environmental threats.3,4 For instance, excessive NO3− addition provokes its leaking leading to water eutrophication. Interestingly, the use of ammonium-based fertilizers together with nitrification inhibitors has proven useful to both reduce nitrate leaching and the emission of greenhouse gases (N2O, NOx) associated to N-fertilization.5 Nevertheless, NH4+ accumulation is toxic to most plants, although toxicity thresholds greatly depend on the ammonium dose in function of the species.6 In fact, NH4+ in high concentrations is also toxic to fungi and animals.7 Even when some plants grow almost equally well on NO3− and NH4+, they may differ in many aspects from their metabolic activities and ion composition to morphological aspects.8 In general, high intraspecific variation exists in ammonium tolerance in different species including Arabidopsis.9,10
Ammonium accumulation in soils might come not only from its direct application as fertilizer, but from NH4+/NH3 deposition from the atmosphere and this accumulation is especially relevant in soils with low nitrification rates.7 Common ammonium toxicity symptoms include biomass reduction, reactive oxygen species overproduction, pH deregulation or ion imbalance. However the concept of “ammonium toxicity” is not completely clear, since ammonium nutrition can lead to biomass reduction, but to an increase in the quality of the final product.11
Chlorosis is also a well-established symptom of ammonium toxicity.12 Indeed, leaf chlorosis has been used for the screening of Arabidopsis mutants hypersensitive to ammonium.13 In this sense, the aim of our work was to determine chlorophyll (Chl) content in a collection of A. thaliana accessions with contrasted tolerance toward ammonium nutrition10 to check the possible relationship between Chl content and Arabidopsis intra-specific variability in ammonium tolerance. Thus, we evaluated chlorophyll (Chl) content, using a SPAD-502 meter, in a collection of 47 Arabidopsis world natural accessions (http://publiclines.versailles.inra.fr/naturalAccession/index) grown under limiting N-supply conditions (1 mM) with NH4+ or NO3− as N source (specific growth conditions are described in Sarasketa et al.10).
SPAD-502 is a handy apparatus that measures the transmittance of red and infrared radiation and calculates a value that corresponds to the amount of Chl present in the sample leaf. For Arabidopsis the calibration equations to convert SPAD values to total Chl content have been calculated per unit of leaf area and per fresh weight with R2 values of 0.99 and 0.98 respectively, thus validating the use of SPAD-502 for Chl measurement in Arabidopsis with a ca. 6% deviation on average.14
In a previous study, we showed that 1 mM NH4+ nutrition provoked a significant reduction in shoot biomass in 24 out of the 47 Arabidopsis natural accessions analyzed.10 However, despite growth inhibition, we observed Chl content increase in every accession (Fig. 1). Chl content might increase in response to several environmental changes. For instance, low irradiance is commonly accepted to enhance Chl content thus resulting in foliar nitrogen investment for light harvesting.15 However, plant growth stage, leaf age, soil water and nutrients deficiency other than N can affect these measurements.16
Figure 1.
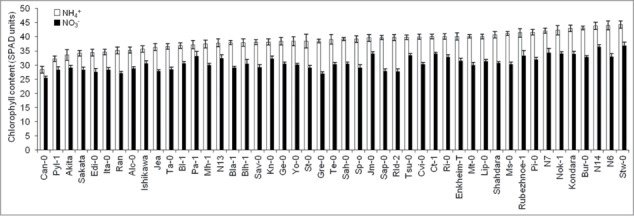
Natural variation in 47 Arabidopsis thaliana accessions chlorophyll content (SPAD units) under nitrate or ammonium as N-source. Values represent (mean ± SE for n = 8-12). Chlorophyll content for each individual plant was obtained by calculating the mean of 6 independent measurements with SPAD-502 device. Significant differences were found for every accession under ammonium compared with nitrate (P < 0.05).
In this study, we observed high intraspecific variation in Chl content in Arabidopsis (Fig. 1 and 2). However, there was no correlation between Chl content and shoot biomass both under ammonium (r2 = 0.011, P = 0.942) and nitrate nutrition (r2 = 0.061, P = 0.685). Thus, in our conditions of growth under low-NH4+ supply, Chl content does not seem to be related with Arabidopsis ammonium tolerance capacity. Similarly, we found no correlation between Chl content and leaves ammonium concentration (r2 = 0.154, P = 0.301 under ammonium nutrition and r2 = 0.084, P = 0.573 under nitrate nutrition).
Figure 2.
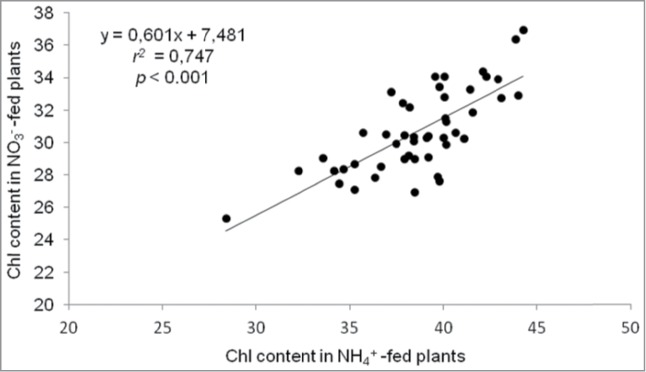
Scatter plot of chlorophyll content (SPAD units) of NO3−-fed plants (vertical axis) versus NH4+-fed plants (horizontal axis). Linear regression and Pearson correlation coefficient (r2) are given.
Studies based in ammonium stress in Arabidopsis have generally described Chl content reduction as a toxicity symptom.17,18 However, Chl content increase has been also reported.19 Interestingly, Li et al.20 showed that under mixed nutrition (5 mM NO3− supplemented with increasing concentrations of NH4+) Arabidopsis cv. Col-0 plants exposed to low ammonium stress experienced a ca. 25% increase in Chl content while under high ammonium stress experienced severe chlorosis. Generally, Chl content increase under mild-ammonium toxicity could be simply due to a greater effect of the stress in leaf expansion than in Chl biosynthesis or to a side-effect due to overall accumulation of N-compounds commonly observed under ammonium nutrition.7,10,13,21 Besides, it has been shown that enhanced photosynthetic rates induced by high irradiance favor ammonium tolerance by providing more carbon skeletons for NH4+ assimilation.21,22 Thus, Chl content increase could be a strategy to somehow try to increase CO2 assimilation and thus mitigate NH4+ accumulation.
In summary, it is clear that severe ammonium toxicity is associated with chlorosis and with net photosynthesis decline.7 However, this appears to be specific of a severe stress as Chl content does not seem to be a direct effect of ammonium toxicity; since, as shown for instance in this work, inhibition of growth10 is not accompanied by chlorosis. Similarly, we observed no correlation between Chl content variation and Arabidopsis intraspecific ammonium tolerance. Future works focused in NH4+ nutrition and its relationship with leaves pigment composition and biosynthesis will be helpful to understand how and why Chl content increases in relation to the N-source provided.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Anabel Robredo for technical assistance.
Funding
The research leading to these results has received funding from the Basque Government (IT526-10) and the People Program (Marie Curie Actions) of the European Union´sSeventh Framework Program (FP7/2007–2013) under REA grant agreement number 334019.
References
- 1.Robertson GP, Vitousek PM. Nitrogen in agriculture: balancing the cost of an essential resource. Ann Rev Environ Res 2009; 97-125; http://dx.doi.org/ 10.1146/annurev.environ.032108.105046 [DOI] [Google Scholar]
- 2.Masclaux-Daubresse C, Daniel-Vedele F, Dechorgnat J, Chardon F, Gaufichon L, Suzuki A. Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Ann Bot 2010; 105:1141-57; PMID:20299346; http://dx.doi.org/ 10.1093/aob/mcq028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tilman D, Balzer C, Hill J, Befort BL. Global food demand and the sustainable intensification of agriculture. Proc Natl Acad Sci U S A 2011; 108:20260-4; PMID:22106295; http://dx.doi.org/ 10.1073/pnas.1116437108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foley JA, Ramankutty N, Brauman KA, Cassidy ES, Gerber JS, Johnston M, Mueller ND, O´Connell C, Ray DK, West PC, et al.. Solutions for a cultivated planet. Nature 2011; 478:337-42; PMID:21993620; http://dx.doi.org/ 10.1038/nature10452 [DOI] [PubMed] [Google Scholar]
- 5.IPCC Mitigation of climate change In: Metz B, Davidson O, Bosch P, Dave R, Meter L, eds. Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel of Climate Change. 2007; Cambridge: Cambridge University Press. [Google Scholar]
- 6.Li BH, Li GJ, Kronzucker HJ, Baluska F, Shi WM. Ammonium stress in Arabidopsis: signaling, genetic loci, and physiological targets. Trends Plant Sci 2014; 19:107-14; PMID:24126103; http://dx.doi.org/ 10.5363/tits.19.10_7 [DOI] [PubMed] [Google Scholar]
- 7.Britto DT, Kronzucker HJ. NH4+ toxicity in higher plants: a critical review. J Plant Physiol 2002; 159:567-84; PMID:11925294; http://dx.doi.org/ 10.1078/0176-1617-077411925294 [DOI] [Google Scholar]
- 8.Kandlbinder A, da Cruz C, Kaiser WM. Response of primary plant metabolism to the N-source. Zeitschrift Fur Pflanzenernahrung Und Bodenkunde 1997; 160:269-74; http://dx.doi.org/ 10.1002/jpln.19971600221 [DOI] [Google Scholar]
- 9.Rauh BL, Basten C, Buckler ES. Quantitative trait loci analysis of growth response to varying nitrogen sources in Arabidopsis thaliana. Theor Appl Genet 2002; 104:743-50; PMID:12582633; http://dx.doi.org/ 10.1007/s00122-001-0815-y [DOI] [PubMed] [Google Scholar]
- 10.Sarasketa A, González-Moro MB, González-Murua C, Marino D. Exploring ammonium tolerance in a large panel of Arabidopsis thaliana natural accessions. J Exp Bot 2014; 65:6023-6033; http://dx.doi.org/ 10.1093/jxb/eru342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuertes-Mendizabal T, González-Torralba J, Arregui LM, González-Murua C, González-Moro MB, Estavillo JM. Ammonium as sole N source improves grain quality in wheat. J Sci Food Agric 2013; 93:2162-71; PMID:23339023; http://dx.doi.org/ 10.1002/jsfa.6022 [DOI] [PubMed] [Google Scholar]
- 12.Miller AJ, Cramer MD. Root nitrogen acquisition and assimilation. Plant Soil 2004; 274:1-36; http://dx.doi.org/ 10.1007/s11104-004-0965-1 [DOI] [Google Scholar]
- 13.Li GJ, Dong GQ, Li BH, Li Q, Kronzucker HJ, Shi WM. Isolation and characterization of a novel ammonium overly sensitive mutant, amos2, in Arabidopsis thaliana. Planta 2012; 235:239-52; PMID:21866344; http://dx.doi.org/ 10.1007/s00425-011-1504-y [DOI] [PubMed] [Google Scholar]
- 14.Ling QH, Huang WH, Jarvis P. Use of a SPAD-502 meter to measure leaf chlorophyll concentration in Arabidopsis thaliana. Photosynth Res 2011; 107:209-14; PMID:21188527; http://dx.doi.org/ 10.1007/s11120-010-9606-0 [DOI] [PubMed] [Google Scholar]
- 15.Niinemets U, Tenhunen JD. A model separating leaf structural and physiological effects on carbon gain along light gradients for the shade-tolerant species Acer saccharum. Plant Cell Environ 1997; 20:845-66; http://dx.doi.org/ 10.1046/j.1365-3040.1997.d01-133.x [DOI] [Google Scholar]
- 16.Muñoz-Huerta RF, Guevara-Gonzalez RG, Contreras-Medina LM, Torres-Pacheco I, Prado-Olivarez J, Ocampo-Velazquez RV. A review of methods for sensing the nitrogen status in plants: Advantages, disadvantages and recent advances. Sensors 2013; 13:10823-43; http://dx.doi.org/ 10.3390/s130810823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helali SM, Nebli H, Kaddour R, Mahmoudi H, Lachaal M, Ouerghi Z. Influence of nitrate-ammonium ratio on growth and nutrition of Arabidopsis thaliana. Plant Soil 2010; 336:65-74; http://dx.doi.org/ 10.1007/s11104-010-0445-8 [DOI] [Google Scholar]
- 18.Jadid N, Mialoundama AS, Heintz D, Ayoub D, Erhardt M, Mutterer J, Meyer J, Alioua A, Van Dorsselaer A, Rahier A, et al.. DOLICHOL PHOSPHATE MANNOSE SYNTHASE1 mediates the biogenesis of isoprenyl-linked glycans and influences development, stress response, and ammonium hypersensitivity in Arabidopsis. Plant Cell 2011; 23:1985-2005; PMID:21558543; http://dx.doi.org/ 10.1105/tpc.111.083634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hachiya T, Watanabe CK, Fujimoto M, Ishikawa T, Takahara K, Kawai-Yamada M, Uchimiya H, Uesono Y, Terashima I, Noguchi K. Nitrate addition alleviates ammonium toxicity without lessening ammonium accumulation, organic acid depletion and inorganic cation depletion in Arabidopsis thaliana shoots. Plant Cell Physiol 2012; 53:577-91; PMID:22318863; http://dx.doi.org/ 10.1093/pcp/pcs012 [DOI] [PubMed] [Google Scholar]
- 20.Li BH, Li Q, Xiong LM, Kronzucker HJ, Kramer U, Shi WM. Arabidopsis plastid AMOS1/EGY1 Integrates abscisic acid signaling to regulate global gene expression response to ammonium stress. Plant Physiol 2012; 160:2040-51; PMID:23064408; http://dx.doi.org/ 10.1104/pp.112.206508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Setien I, Fuertes-Mendizabal T, González A, Aparicio-Tejo PM, González-Murua C, González-Moro MB, Estavillo JM. High irradiance improves ammonium tolerance in wheat plants by increasing N assimilation. J Plant Physiol 2013; 170:758-771; PMID:23485260; http://dx.doi.org/ 10.1016/j.jplph.2012.12.015 [DOI] [PubMed] [Google Scholar]
- 22.Ariz I, Artola E, Asensio AC, Cruchaga S, Aparicio-Tejo PM, Moran JF. High irradiances increases NH4+ tolerance in Pisum sativum: Higher carbon and energy availability improve ion balance but not N assimilation. 2011; J Plant Physiol 2011; 168:1009-1015; PMID:21371777; http://dx.doi.org/ 10.1016/j.jplph.2010.11.022 [DOI] [PubMed] [Google Scholar]


