Abstract
Aripiprazole is a third-generation atypical antipsychotic and a dopamine D2 receptor partial agonist. In the present study, we investigated whether a single administration of aripiprazole to mice, either as a pretreatment or as a posttreatment, would affect stereotypy induced by methamphetamine (METH). Pretreatment of male ICR mice with aripiprazole (1 or 10 mg/kg, i.p.) attenuated the incidence of METH-induced stereotypical behavior in a dose-dependent manner. Pretreatment of mice with 1 mg/kg aripiprazole produced an increase in the locomotor activity in mice treated with METH compared with mice treated with vehicle plus METH and with 10 mg/kg aripiprazole plus METH. This increase in locomotion is indicative of a rightward shift in the dose–response curve for METH, consistent with a shift in the type of stereotypical behavior observed from biting to sniffing. Aripiprazole posttreatment, after METH-induced stereotypical behavior, was fully expressed and also significantly attenuated overall stereotypy in an aripiprazole dose-dependent manner. These data suggest that the antagonism of METH effects by aripiprazole should be investigated as a potential treatment for acute METH overdose.
Keywords: methamphetamine, stereotyped behavior, dopamine receptor, aripiprazole
Introduction
Methamphetamine (METH) is an amphetamine (AMPH) analog that is one of the most powerful addictive psychostimulant drugs abused worldwide.1 In addition to addiction and dependence, METH is also associated with the development of psychosis.2,3 METH-induced psychosis does not occur in all individuals and may be particularly associated with individuals with a predisposition to psychosis,4,5 which may have a genetic basis.6 The acute administration of rodents with high doses of AMPH/METH produces hyperlocomotion and repetitive, compulsive behavior called stereotypy.7–9 Stereotypy is considered to reflect the actions of high-dose METH that may model aspects of drug-induced psychoses, as well as psychoses in general.2,3,10,11 Although studies of the adverse effects associated with high-dose METH use have been reported using these animal models, successful treatments for METH dependence and METH-induced psychosis remain elusive.12,13 It is important to identify medications for METH-induced pathologies related to the acute and chronic consequences of METH use.
Aripiprazole (7-(4-(4-(2,3-dichlorophenyl)-1-piperazinyl) butyloxy)-3,4-dihydro-2(1H)-quinolinone) is a commonly used third-generation antipsychotic drug used to treat schizophrenia in humans.14 It has the pharmacological properties of a dopamine D2 receptor partial agonist,15–19 a serotonin 5-HT1A receptor agonist, and a 5-HT2 receptor antagonist (as reviewed by Wood and Reavill).20 Aripiprazole is also effective in the treatment of tics (ie, involuntary intermittent movements or vocalizations) in Tourette’s syndrome.21 These types of movements share underlying commonalities with stimulant-induced stereotypies.22,23 In addition aripiprazole inhibits apomorphine-induced stereotypy in rodents24,25 and reduces the locomotor-stimulant effects of cocaine.26
Apomorphine acts as a direct dopamine D1/D2 receptor agonist.27,28 On the other hand, METH, as a structural analog of AMPH, acts as an indirect dopaminergic agonist through actions at the monoamine transporters including dopamine transporter (DAT), as well as norepinephrine transporter (NET) and serotonin transporter (SERT), and the vesicular monoamine transporter 2, including the reversal of dopamine transport via DAT, resulting in nonvesicular efflux of dopamine from presynaptic terminals into extracellular space.29–33 Apomorphine and METH induce stereotyped behavior through an activation of dopaminergic neurotransmission,9,34–37 effects that are mediated largely by the dorsal striatum,38,39 although certainly specific behavioral processes appear to be mediated by different corticostriatal circuits. Aripiprazole has been suggested to inhibit apomorphine-induced stereotypy through antagonist actions at the dopamine D2 receptor.40 The mechanisms involved in the expression of stereotypy induced by direct and indirect dopamine agonists differ in certain respects, particularly with regard to the interplay between dopamine receptor subtypes.41 In line with these observations, there is a possibility that aripiprazole may inhibit METH-induced stereotypy, although there are few reports regarding the effects of aripiprazole on actions of high doses of METH, including stereotyped behavior. It is of interest to examine whether aripiprazole attenuates METH-induced stereotypy, similar to the case of apomorphine-induced stereotypy. In the present study, we investigated whether a single administration of mice with aripiprazole, administered either as a pretreatment or posttreatment, affected METH-induced stereotypy in mice.
Materials and Methods
Subjects
Male ICR mice (9–10 weeks old; Japan SLC, Shizuoka, Japan) were housed in groups of eight (cage size, 37 × 22 × 15 cm; with fresh wood chips) in a temperature-controlled (22 ± 2°C) and humidity-controlled (50 ± 10%) environment under a 12-hour light/dark cycle (lights on at 07:00) with food and water available ad libitum, except during testing. Observation of stereotypies was made by trained observers. Animal handling and care were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (8th edition, Institute of Laboratory Animal Resources-National Research Council, National Academy Press, 2011), and all experiments were reviewed and approved by the Institutional Animal Research Committee of Hyogo College of Medicine. The mice were only used once (n = 76, 11–13 weeks old, 38–54 g) after at least 1-week habituation in the facility.
Reagents
METH hydrochloride was purchased from Dainippon Sumitomo Pharma Co., Ltd.. Aripiprazole was purchased from Tokyo Chemical Industry Co., Ltd.. Doses of drugs refer to the weight of the salt. METH was dissolved in sterile saline and the injection dose was 10 mg/kg.42,43 METH-induced stereotypy is dose-dependent; lower doses stimulate locomotion but do not produce stereotypical behavior. For instance, about only 30% of mice exhibited maximal expression levels of stereotypy when 5 mg/kg of METH was administered.43 Since the intention of this study was to examine individual components of METH-induced stereotypy, a higher (ie, 10 mg/kg) dose was used. Aripiprazole was dissolved in vehicle solution, ie, sterile saline containing 2% Tween 80 (SigmaUltra grade)/0.5% carboxymethyl cellulose sodium salt (Sigma-Aldrich, St. Louis, MO, USA). Drug solutions were prepared in such a way that the necessary dose could be injected in a volume of 0.1 mL/10 g of body weight by an i.p. route.
Treatment protocol
Effect of aripiprazole pretreatment on METH-induced stereotypical behavior and locomotion
Mice (n = 44) were weighed and randomly divided into six groups (n = 7–8 per group): vehicle/saline (n = 8), 1 mg/kg aripiprazole/saline (n = 7), 10 mg/kg aripiprazole/saline (n = 7), vehicle/METH (n = 8), 1 mg/kg aripiprazole/METH (n = 7), and 10 mg/kg aripiprazole/METH (n = 7). Vehicle or aripiprazole was administered 30 minutes prior to saline or METH and immediately placed in the testing chamber to assess stereotypy and locomotion for 1 hour as described below (see Rating of stereotypy and Measurement of locomotor activity).
Effects of aripiprazole posttreatment on METH-induced stereotypical behavior and locomotion
Mice (n = 32) were weighed, and all mice were treated with 10 mg/kg of METH (i.p.). After the METH injection, all mice were placed in the testing chamber for measurements of stereotypical behavior and locomotion. In line with our previous observations, the frequency of stereotypy induced by 10 mg/kg METH reaches a plateau level 20–25 minutes after the METH injection.42,44 Thus, 25 minutes after the METH injection, when these stable levels had been reached, behavioral observations were ceased and mice were given posttreatment injections. Mice were randomly divided into four groups (n = 8 per group) and briefly removed from the testing chamber for injection with aripiprazole (0.1, 1.0, or 10.0 mg/kg, or 2% Tween 80/0.5% carboxymethyl cellulose as vehicle). Immediately after the aripiprazole (or vehicle) injections, mice were returned to the testing chamber. In line with our previous observations, the effects of handling and injection habituate within 20 minutes.45 Thus, 20 minutes later (ie, 45 minutes after the METH injection), behavioral measures (ie, rating of stereotyped behavior and measurement of locomotor activity) were continued for an additional 40 minutes (ie, until 85 minutes after the METH injection). Our previous study indicates that mice continue to exhibit almost maximal frequencies of stereotypical behavior 85 minutes after an METH (10 mg/kg) injection alone.44
Rating of stereotypy
Test subjects were placed in a transparent acrylic box (30 × 30 × 35 cm) with approximately 25 g of fresh wood chips spread on the floor of the chamber and observed for stereotypy for 60 minutes (aripiprazole pretreatment) or for 85 minutes with a break (25–45 minutes) of rating (aripiprazole posttreatment) following METH administration by observers unaware of the treatments. METH-induced stereotypy lasts for about 170 minutes after a 10-mg/kg i.p. injection.44 The expression frequencies of each component of stereotypical behavior observed for 2 hours are the same as the expression frequencies observed for 1 hour (for instance, 2-hour observations46 versus 1-hour observations).42 Therefore, we chose the period of 1 hour for our observations. Behavior was assessed at 30-second intervals, and the predominant behavior observed during each interval was recorded. Since individual stereotypical behaviors were unchanged for long periods (>30 seconds) after drug treatment, it was possible to record the observations by hand. The behaviors scored were inactive (awake and inactive, or sleeping), ambulation, rearing, persistent locomotion, head bobbing (up-and-down movements of the head), continuous sniffing, circling, and continuous nail and/or wood chip biting or licking, according to a method described previously.43 Ambulation, rearing, and persistent locomotion were considered locomotor and exploratory behaviors, and the last four categories were considered stereotypies. Persistent locomotion was not classified as stereotypy because the mice scored as having “persistent locomotion” showed horizontal locomotor activity less than or equal to that displayed by mice showing “hyperlocomotion” induced by 1 mg/kg METH (which is not generally defined as a stereotypy) measured by Animex Auto.47 The cumulative number of intervals within each 5-minute period in which stereotypies were observed is shown as a time course (maximal value = 10). Stereotypical cage climbing48 was not observed in our experimental system because of the use of an acrylic test chamber without a stainless steel grid top. Horizontal locomotor activity was simultaneously measured at the same time as described below.
Measurement of locomotor activity
Locomotor activity was measured in a transparent acrylic test box (30 × 30 × 35 cm) mentioned above using an Animex Auto apparatus (System MK-110 with a four-channel Interval Recorder model USBIR-4; Muromachi Kikai Co.) in a quiet room as described previously.47 The apparatus detects changes in electrical capacitance (oscillation frequency) in an LC (ie, inductance–capacitance) oscillator circuit system under the floor of the apparatus as an animal moves horizontally in an electric field. In this set of experiments, the sensitivity parameter was set at 580. The sensitivity parameter indicates an electric signal amplification factor and the maximal value is 1,000, which means 100.0%. Under this criterion, the count of oscillation frequency parallels the degree of horizontal locomotion. The acrylic test boxes were cleaned with 10% ethanol and wiped dry between sessions for each animal. All experiments were conducted between 9:00 and 16:00. There was the discrepancy in sample sizes between stereotypy and locomotor activity measures in a set of aripiprazole pretreatment experiments because of the loss of the locomotion data due to computer problems (see figure legends).
Statistics
Data are presented as mean ± the standard error of the mean (SEM). Statistical analysis was performed using mixed-factor analysis of variance (ANOVA), with or without repeated measures as appropriate, followed by Bonferroni/Dunn or Fisher’s PLSD post hoc tests (Statview 5.0 for Apple Macintosh, SAS Institute, Inc.). Statistical significance was set at P < 0.05.
Results
Effect of aripiprazole pretreatment on METH-induced stereotypical behavior and locomotion
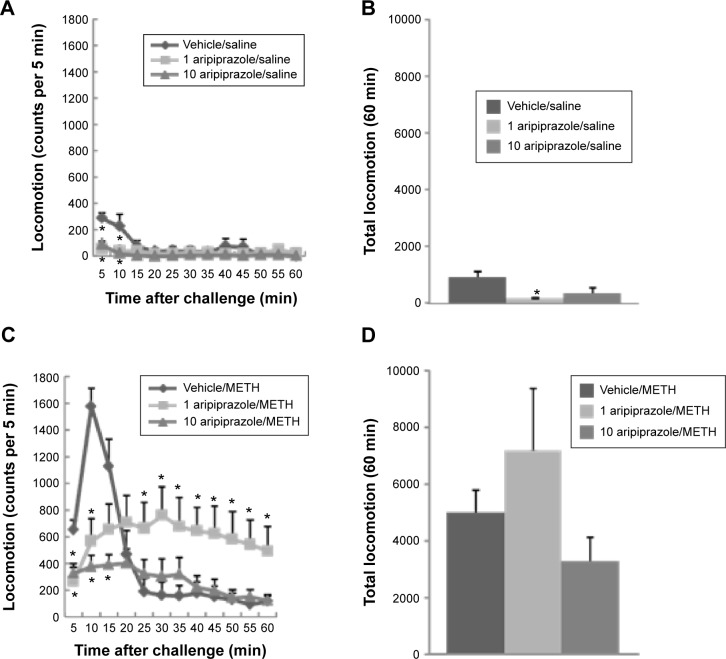
Mice were treated with 1 or 10 mg/kg aripiprazole i.p. (or saline containing 2% Tween 80/0.5% carboxymethyl cellulose as vehicle; see Reagents) immediately followed by an injection of 10 mg/kg METH (or saline). Figure 1 shows the time course of the frequency of all types of stereotypical behavior observed in mice immediately after saline (Fig. 1A) or METH (Fig. 1B) administration. Stereotypy was not observed after saline treatment alone, and treatment of mice with aripiprazole did not affect the overall frequency of stereotypical behavior in mice given saline (Fig. 1A). There was a large increase in stereotypy in mice after METH administration, beginning at 10 minutes postinjection, reaching a maximum at 25 minutes postinjection, and continuing unabated for the duration of the test session (Fig. 1B). By contrast, aripiprazole substantially attenuated the stereotypy induced by METH in a dose-dependent manner (Fig. 1B). A repeated-measures ANOVA (pretreatment × time) applied to the saline data represented in Figure 1A yielded no significant main effects of aripiprazole pretreatment (F(2,19) = 3.3, P = 0.06) or time (F(12,247) = 1.7, P = 0.07), nor a significant aripiprazole pretreatment × time interaction (F(24,247) = 1.4, P = 0.12). ANOVA (pretreatment × time) applied to the data represented in Figure 1B yielded significant main effects of aripiprazole pretreatment (F(2,19) = 76.6, P < 0.0001) and time (F(12,247) = 93.8, P < 0.0001) and also a significant aripiprazole pretreatment × time interaction (F(24,247) = 11.6, P < 0.0001). Post hoc pairwise comparisons identified significant differences at each time point between 15 and 60 minutes, demonstrating the differences in the time course of the response to METH between vehicle and aripiprazole pretreatment groups (Bonferroni/Dunn test, P < 0.05). The high dose of aripiprazole almost completely eliminated METH-induced stereotypy.
Figure 1.
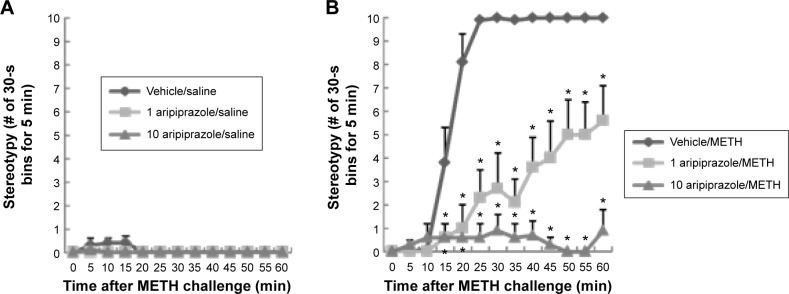
Frequencies of stereotypy after a single administration of saline (A) or 10 mg/kg METH (B) in mice pretreated with aripiprazole (1 or 10 mg/kg) or vehicle (ie, 2% Tween 80/0.5% carboxymethyl cellulose). Values are shown as the mean ± SEM (n = 7–8). *P < 0.05, compared with vehicle-pretreated mice (post hoc Bonferroni/Dunn test).
Figure 2 shows the time course of locomotor activity (Fig. 2A or C) and summed locomotor activity scores (Fig. 2B or D) observed in mice immediately after METH (Fig. 2C and D) or saline (Fig. 2A and B) administration. There was little locomotion after saline administration alone (Fig. 2A), and this occurred primarily in the first few minutes after the injections, locomotion quickly habituating to low levels after-ward. By contrast there was a large, but transient increase in locomotor activity after METH administration alone. This burst of METH-induced locomotion peaked at 10 minutes postinjection, followed by an abrupt decrease in locomotor activity (Fig. 2C) to levels similar to saline-treated groups (Fig. 2A). Of course, as is obvious from the preceding analysis, the behavior of METH-treated mice during this later time period was quite different from saline-treated mice despite apparently equivalent levels of locomotion. These mice were still quite behaviorally active, in sharp contrast to saline-treated mice; the reductions in locomotion were associated with increases in stereotypical behavior. Pretreatment of mice with aripiprazole alone (1 or 10 mg/kg) reduced the initial locomotion observed in mice after saline administration, during the first 5–10 minutes after the injection (Fig. 2A). Pretreatment of mice with either dose of aripiprazole largely blocked the pronounced elevation in locomotion observed in METH-treated mice during the first 20 minutes of the testing period. However, at the same time, METH/aripiprazole-treated mice continued to have elevated levels of locomotor behavior compared to saline-treated subjects throughout the test (Fig. 2C). Moreover, METH-treated subjects given the low dose of aripiprazole had higher levels of locomotor activity throughout the later portion of the test (25–60 minutes) than subjects treated with METH alone. The elevation of locomotor activity was blocked by the higher dose of aripiprazole. The suppression of locomotion by aripiprazole in saline-treated mice is apparent in the summed locomotor activity scores (Fig. 2B), but the effect of aripiprazole on locomotion in METH-treated mice is not (Fig. 2D), although there was a trend for the high dose to lower locomotor activity overall. This is not surprising given that the primary effect of the drug was upon the time course of locomotor activity and different effects were observed early and late in the locomotor activity test. A repeated-measures ANOVA (pretreatment × time) applied to the saline data represented in Figure 2A yielded significant main effects of aripiprazole pretreatment (F(2,17) = 4.6, P < 0.05) and time (F(11,204) = 6.8, P < 0.0001) and also yielded a significant aripiprazole pretreatment × time interaction (F(22,204) = 3.1, P < 0.0001). Post hoc pairwise comparisons showed significant differences of time course between saline and aripiprazole (1 or 10 mg/kg) pretreatment groups at each time point between 5 and 10 minutes (Bonferroni/Dunn test, P < 0.05). ANOVA (pretreatment) applied to the summed locomotor data represented in Figure 2B also yielded a significant main effect of aripiprazole pretreatment (F(2,17) = 4.6, P < 0.05). Post hoc Fisher’s PLSD test demonstrated a significant decrease in total locomotion in mice pretreated with 1 mg/kg aripiprazole compared with the vehicle group (Fig. 2B). ANOVA (pretreatment × time) applied to the data represented in Figure 2C yielded a significant main effect of time (F(11,204) = 21.3, P < 0.0001), but no significant main effect of aripiprazole pretreatment (F(2,17) = 1.6, P = 0.22), but a significant aripiprazole pretreatment × time interaction (F(22,204) = 16.7, P < 0.0001), indicative of the primary effect of the drug being on the time course of locomotion rather than locomotion overall. Post hoc pairwise comparisons showed significant differences between saline and 1 mg/kg aripiprazole pretreatment groups at time points of 5 and 10 minutes and at each time point between 25 and 60 minutes and significant differences between saline and 10 mg/kg aripiprazole pretreatment groups at each time point between 5 and 15 minutes (Bonferroni/Dunn test, P < 0.05). ANOVA (pretreatment) applied to the summed locomotor data represented in Figure 2D yielded no significant main effect of aripiprazole pretreatment (F(2,17) = 1.6, P = 0.22).
Figure 2.
Horizontal locomotor activity after a single administration of saline (A and B) or 10 mg/kg METH (C and D) in mice pretreated with aripiprazole (1 or 10 mg/kg) or vehicle. Values are shown as the mean ± SEM (n = 6–7). There was the discrepancy in sample sizes between Figure 1 and Figure 2 because of the loss of the locomotion data due to computer problems. *P < 0.05, compared with vehicle-pretreated mice (post hoc Bonferroni/Dunn test).
Effect of aripiprazole pretreatment on METH-induced categories of stereotypical behaviors
Four individual categories of stereotypical behaviors were observed, stereotypical head-bobbing, circling, sniffing, and biting. The frequency of each behavior, as well as the summed incidence of stereotypy, is presented in Table 1. METH challenge and aripiprazole pretreatment affected the incidence of each behavior and altered the distribution of behavioral output. METH did not induce stereotypical head-bobbing or circling, but did induce stereotypical sniffing and biting. The type of stereotypical behavior induced by METH alone was biting. The low dose of aripiprazole almost completely eliminated biting, while stereotypical sniffing increased. This represents a shift in the dose–response for METH-induced stereotypy as sniffing is observed at lower doses than biting.49 The highest dose of aripiprazole almost completely eliminated both types of stereotypical behavior. The overall pattern is also present in the summed stereotypy scores, which show a dose-dependent reduction. To analyze the effects of METH on individual components of stereotypy and total stereotypy statistically, two-way ANOVA (pretreatment × challenge) was applied separately for each pretreatment (ie, vehicle or 1 and 10 mg/kg aripiprazole) and challenge (METH and saline) as shown in Table 1. In mice pretreated with vehicle, ANOVA demonstrated significant main effects of METH challenge for stereotypical sniffing (F(1,43) = 12.0, P < 0.01), biting (F(1,43) = 52.2, P < 0.001), and total stereotypy (F(1,43) = 187.7, P < 0.001) but not for stereotypical head-bobbing (F(1,43) = 2.8, P = 0.10) or circling (F(1,43) = 1.1, P = 0.30). ANOVA also yielded significant main effects of aripiprazole pretreatment for stereotypical biting (F(2,43) = 55.3, P < 0.001) and total stereotypy (F(2,43) = 72.1, P < 0.001) but not stereotypical sniffing (F(2,43) = 2.8, P = 0.07), head-bobbing (F(2,43) = 2.9, P = 0.07), or circling (F(2,43) = 1.1, P = 0.3). ANOVA also produced significant aripiprazole pretreatment × METH challenge interactions for stereotypical biting (F(2,43) = 54.0, P < 0.001) and total stereotypy (F(1,43) = 68.7, P < 0.001). Stereotypical sniffing was significantly increased by METH compared with corresponding saline-challenged mice (Fisher’s PLSD test, P < 0.05), and stereotypical sniffing was significantly increased by pretreatment with 1 but not 10 mg/kg aripiprazole compared with vehicle-pretreated mice (Fisher’s PLSD test, P < 0.05). Stereotypical biting was significantly increased by METH compared with saline-challenged mice pretreated with vehicle (Fisher’s PLSD test, P < 0.05), but not in mice pretreated with aripiprazole (1 or 10 mg/kg). Total stereotypy was significantly increased by METH compared with saline-challenged mice pretreated with vehicle or 1 (but not 10) mg/kg aripiprazole (Fisher’s PLSD test, P < 0.05).
Table 1.
Effect of aripiprazole pretreatment on METH-induced stereotypy in mice.
| HEAD-BOBBING | CIRCLING | SNIFFING | BITING | TOTAL STEREOTYPY | |
|---|---|---|---|---|---|
| Challenge: saline | |||||
| Vehicle pretreatment (n = 8) | N.D. | N.D. | 0.5 ± 0.3 | 0.5 ± 0.3 | 1.0 ± 0.5 |
| 1 mg/kg aripiprazole (n = 7) | N.D. | 0.1 ± 0.1 | N.D. | N.D. | 0.1 ± 0.1 |
| 10 mg/kg aripiprazole (n = 7) | N.D. | N.D. | N.D. | N.D. | N.D. |
| Challenge: 10 mg/kg METH | |||||
| Vehicle pretreatment (n = 8) | 1.1 ± 0.6 | N.D. | 10.6 ± 9.2* | 80.0 ± 10.0* | 91.8 ± 2.6* |
| 1 mg/kg aripiprazole (n = 7) | N.D. | N.D. | 30.9 ± 8.2*,† | N.D.† | 30.9 ± 8.2*,† |
| 10 mg/kg aripiprazole (n = 7) | N.D. | N.D. | 5.6 ± 4.3*,‡ | 0.3 ± 0.2† | 5.9 ± 4.3†,‡ |
Notes: Expression pattern of stereotypical behavior after METH injection (frequency of observation for 30-second bins over 60 minutes). Mice were injected with 10 mg/kg of METH or saline 30 minutes after pretreatment with aripiprazole (1 or 10 mg/kg) or vehicle. Values are expressed as number of 30-second bins in which each behavior was the predominant behavior for 60 minutes after METH injection (mean ± SEM). N.D., not detected (ie, 0.0 ± 0.0).
P < 0.05, compared with corresponding group challenged with saline (one-way ANOVA followed by a post hoc Fisher’s PLSD test).
P < 0.05, compared with corresponding group pretreated with vehicle (one-way ANOVA followed by a post hoc Fisher’s PLSD test).
P < 0.05, compared with corresponding group pretreated with 1 mg/kg aripiprazole (one-way ANOVA followed by a post hoc Fisher’s PLSD test).
Effect of aripiprazole posttreatment on METH-induced stereotypical behavior and locomotion
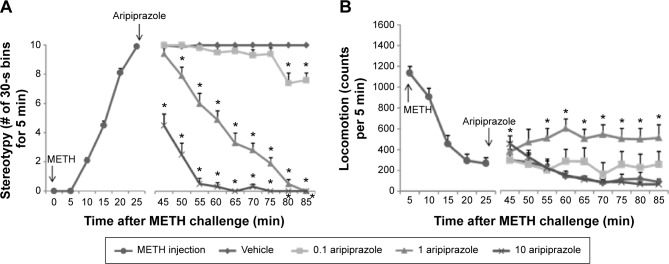
Figure 3 shows the time course of the frequency of all types of stereotypical behavior (A) and locomotion (B) observed in mice beginning immediately after METH administration. There was an increase in overall stereotypy in mice after METH administration, beginning at 10 minutes postinjection, reaching a maximum at 25 minutes postinjection, which was maintained for the duration of the experiment (Fig. 3A), which is the same pattern observed in the previous experiment. Posttreatment of mice with vehicle did not affect the overall frequency of stereotypical behaviors in mice given METH, but the stereotypy induced by METH was attenuated by posttreatment with aripiprazole in a dose-dependent manner (Fig. 3A). A repeated-measures ANOVA (posttreatment × time) applied to the posttreatment data (45–85 minutes) represented in Figure 3A yielded significant main effects of aripiprazole posttreatment (F(3,28) = 358, P < 0.0001) and time (F(8,252) = 65.9, P < 0.0001) and also yielded a significant aripiprazole posttreatment × time interaction (F(24,252) = 20.1, P < 0.0001). Post hoc pairwise comparisons showed significant differences of time course between vehicle and aripiprazole (0.1 mg/kg) posttreatment groups at time points of 80 and 85 minutes, vehicle and aripiprazole (1 mg/kg) posttreatment groups at each time point between 50 and 85 minutes, and vehicle and aripiprazole (10 mg/kg) posttreatment groups at each time point between 45 and 85 minutes (Bonferroni/Dunn test, P < 0.05).
Figure 3.
Effect of aripiprazole posttreatment on METH-induced stereotypy (A) and horizontal locomotion (B) in mice. Mice were injected with 10 mg/kg of METH for 25 minutes followed by treatment of aripiprazole (0.1, 1, or 10 mg/kg; down arrow) or vehicle for additional 1 hour (total METH treatment period = 85 minutes). Values are expressed as number of 30-second bins for 5 minutes (A) or counts per 5 minutes (mean ± SEM, n = 8). Behavioral measures were ceased counting for 20 minutes just after aripiprazole (or vehicle) treatment and restarted for additional 60 minutes. *P < 0.05, compared with vehicle-treated mice (post hoc Bonferroni/Dunn test).
A different pattern of effects was observed in locomotion measures compared to those observed in rating of stereotypy. The effects were very similar to those observed for aripiprazole pretreatments. There was an increase in locomotor activity in mice after METH injection alone at 5 minutes postinjection, followed by an abrupt reduction in locomotion (Fig. 3B) to levels similar to the saline-treated groups (Fig. 2C). However, as discussed in the previous section, the behavior of METH-treated mice was distinctly different from saline-treated mice, and the reduction in locomotion coincided with the onset of other forms of stereotypical behavior. Posttreatment of mice with 0.1 or 10 mg/kg aripiprazole did not affect locomotion in mice given METH, but persistent locomotion was observed in mice given METH followed by 1 mg/kg aripiprazole 30 minutes later (Fig. 3B). As discussed for the previous experiment, this would appear to represent a rightward shift in the dose–response curve for METH and is consistent with the larger reduction in overall stereotypy observed at the highest dose of aripiprazole. A repeated-measures ANOVA (posttreatment × time) applied to the posttreatment data (45–85 minutes) represented in Figure 3B yielded significant main effects of aripiprazole posttreatment (F(3,28) = 5.0, P < 0.01) and time (F(8,252) = 3.9, P < 0.001) and also yielded a significant aripiprazole posttreatment × time interaction (F(24,252) = 2.8, P < 0.0001). Post hoc pairwise comparisons showed significant differences of time course between the vehicle and aripiprazole (10 mg/kg) posttreatment group at a time point of 45 minutes and between vehicle and the aripiprazole (1 mg/kg) posttreatment group at each time point between 55 and 85 minutes (Bonferroni/Dunn test, P < 0.05).
Effect of aripiprazole posttreatment on METH-induced categories of stereotypical behaviors
Four individual categories of METH-induced stereotypical behaviors were observed, and the frequency of each behavior observed as the predominant behavior in each 30-second bin and the sum of all stereotypy measures are presented in Table 2. At time 0, all mice (n = 32) were injected with 10 mg/kg METH and the stereotypical behavior was observed. The most frequently expressed component of behavior was stereotypical biting, with a lesser incidence of stereotypical sniffing, in time period between 0 and 25 minutes after METH injection (Table 2). As in the previous experiment, little head-bobbing or circling was observed. Aripiprazole posttreatment affected the incidence of sniffing and biting behavior and altered the distribution of behavioral output. To analyze the effects of METH on individual components of stereotypy, as well as total stereotypy, one-way ANOVA (aripiprazole posttreatment) was applied separately for each posttreatment (ie, vehicle or 0.1, 1, and 10 mg/kg aripiprazole). Stereotypical head-bobbing and circling were not analyzed because these behaviors were not observed at all in the time period between 45 and 85 minutes after METH injection (Table 2). ANOVA identified significant main effects of aripiprazole posttreatment on stereotypical sniffing (F(3,28) = 8.4, P < 0.001), biting (F(3,28) = 110.7, P < 0.001), and total stereotypy (F(3,28) = 376.4, P < 0.001). Stereotypical sniffing was significantly increased by METH in mice treated with 0.1 and 1 (but not 10) mg/kg aripiprazole compared with mice treated with vehicle (Fisher’s PLSD test, P < 0.05). Stereotypical biting was significantly decreased by METH in mice treated with 1 and 10 (but not 0.1) mg/kg aripiprazole compared with mice treated with vehicle (Fisher’s PLSD test, P < 0.05). Total stereotypy induced by MET was significantly and dose-dependently decreased in mice treated with 1 and 10 (but not 0.1) mg/kg aripiprazole compared with mice treated with vehicle (Fisher’s PLSD test, P < 0.05).
Table 2.
Effect of aripiprazole posttreatment on METH-induced stereotypy in mice.
| HEAD-BOBBING | CIRCLING | SNIFFING | BITING | TOTAL STEREOTYPY | |
|---|---|---|---|---|---|
| 0–25 minutes after METH | |||||
| 10 mg/kg METH (n = 32) | 0.9 ± 0.2 | 0.9 ± 0.2 | 6.3 ± 0.5 | 16.4 ± 0.8 | 24.5 ± 0.6 |
| 45–85 minutes after METH | |||||
| Vehicle (n = 8) | N.D. | N.D. | 4.8 ± 1.4 | 75.1 ± 1.4 | 79.9 ± 0.1 |
| 0.1 mg/kg aripiprazole (n = 8) | N.D. | N.D. | 15.5 ± 5.6* | 57.0 ± 6.6 | 72.6 ± 0.2 |
| 1 mg/kg aripiprazole (n = 8) | N.D. | N.D. | 21.5 ± 1.9* | 5.3 ± 2.1*,† | 26.8 ± 2.9*,† |
| 10 mg/kg aripiprazole (n = 8) | N.D. | N.D. | 2.9 ± 1.1†,‡ | 0.5 ± 0.3*,† | 3.4 ± 1.4*,†,‡ |
Notes: Expression pattern of stereotypical behavior after METH injection (frequency of observation for 30-second bins over 60 minutes). Mice were injected with 10 mg/kg of METH for 25 minutes of behavioral measures followed by treatment of aripiprazole (0.1, 1, or 10 mg/kg) or vehicle. Twenty minutes after aripiprazole (or vehicle) treatment, behavioral measures were assessed again. Values are expressed as number of 30-second bins in which each behavior was the predominant behavior for 0–25 and 45–85 minutes after METH injection (mean ± SEM). N.D., not detected (ie, 0.0 ± 0.0).
P < 0.05, compared with group treated with vehicle (one-way ANOVA followed by a post hoc Fisher’s PLSD test).
P < 0.05, compared with group treated with 0.1 mg/kg aripiprazole (one-way ANOVA followed by a post hoc Fisher’s PLSD test).
P < 0.05, compared with group treated with 1 mg/kg aripiprazole (one-way ANOVA followed by a post hoc Fisher’s PLSD test).
Discussion
In the present study, we demonstrated that aripiprazole effectively attenuated METH-induced stereotypy in a dose-dependent manner, after either pretreatment (Fig. 1B) or posttreatment (Fig. 3A). There is a ceiling effect in the rating of stereotypy following METH, which suggests a possibility that the magnitude of aripiprazole influence is underestimated. Aripiprazole given in combination with saline treatment leads to a nearly (but no) significant main effect of pretreatment and this result could be a floor effect which may be attributed to stereotypy rating. In fact, aripiprazole alone induced no stereotypical behavior (Fig. 1A), although it did have motor-decreasing effects in saline-treated mice (Fig. 2A and B). Reductions in spontaneous behavior are associated with dopamine D2 receptor antagonism produced by antipsychotic drugs.50 It is likely that the motor-decreasing effect of aripiprazole is induced by dopamine D2 receptor antagonist properties of a major aripiprazole metabolite found in rodents (called DM-1451 or hydroxyl-aripiprazole).51 Importantly, those authors found that this metabolite is not found in humans (where it is called OPC-14857 or dehydro-aripiprazole), so that in humans aripiprazole should have more mixed agonist/antagonists properties. In humans, aripiprazole is effective in the treatment of schizophrenia with a low incidence of extrapyramidal and other motor side effects.52 Stereotypical behavior induced by high doses of acute METH is difficult to ameliorate by the administration of exogenous agents after initial induction.53 Aripiprazole, by contrast, was effective even after the induction of METH-induced stereotypy (Fig. 3A). This observation increases interest in this compound as a potential candidate for treatment of METH overdose in the clinic, as this situation would be more applicable to its clinical use.
Most other antipsychotic medications act as full dopamine D2 receptor antagonists, while aripiprazole acts as a dopamine D2 receptor partial agonist.15–19 Aripiprazole also acts as a serotonin 5-HT1A receptor partial agonist and a 5-HT2A receptor antagonist.20 Due to the unique mechanism of action of this drug, aripiprazole is associated with a broader spectrum of clinical efficacy compared with conventional antipsychotics.54 Given the effects of aripiprazole on individual components of stereotypical behavior, it will be of interest to investigate the effect of aripiprazole in other conditions that involve stereotyped or repetitive behavior. METH-induced stereotypical biting was eliminated by aripiprazole (1 and 10 mg/kg) pretreatment or posttreatment, while METH-induced stereotypical sniffing showed a biphasic response that was likely indicative of a leftward shift in the dose–response curve for METH-induced stereotypy (Tables 1 and 2). The neural mechanisms underlying the expression of individual components of stereotypical behavior associated with AMPH-like drugs have been anatomically dissociated with regard to striatal subregions. Stereotypical biting is associated with central and anterior portions of the caudate putamen, while stereotypical sniffing is associated with the nucleus accumbens.35 Other orofacial behaviors appear to involve the ventrolateral striatum.55 Both mesolimbic and nigrostriatal dopaminergic mechanisms have been proposed to play crucial roles in the expression of stereotypy after acute psychostimulant administration, depending on dose.11,56,57 Given the reductions in locomotion and all aspects of stereotypical behavior in the present experiments, depending on the dose administered, it would appear that aripiprazole affects both systems, although there is at least some evidence for selectively of some effects of aripiprazole for mesolimbic versus nigrostriatal systems.58
There is always the possibility that aripiprazole alters the brain concentration of METH or in some other way affects brain responses to METH. It has been reported that aripiprazole decreases the number of DAT-binding sites in the nucleus accumbens and the ventral tegmental area,58 but this is after subchronic or chronic treatments, so the relevance of this observation to the current findings are uncertain. In any case, it would be difficult to predict how this would impact upon stereotypical responses to METH. It might be thought that decreases in DAT-binding sites would increase the effective concentration of METH at DAT, or the extracellular increases in DAT concentration because of reductions in DA uptake. However, this does not accord with the present observations of reduced stereotypical biting (Tables 1 and 2). Furthermore, heterozygous DAT knockout mice have reduced locomotor-stimulant responses to METH.59 As mentioned in the introduction, METH acts on NET and SERT in addition to DAT and alters extracellular norepinephrine and serotonin in addition to dopamine,33 so that a possibility that aripiprazole may act through serotonin receptors to alter METH-induced stereotyped behavior could not be ruled out. Further studies are needed to clarify the possibility.
As described above, aripiprazole alone did not induce stereotypical behavior despite the dopamine D2 receptor partial agonist properties of this drug. It is likely that the partial antagonist properties of aripiprazole prevent such an outcome, as has been suggested in other circumstances where aripiprazole reduces AMPH self-administration.60 Moreover, it would appear that those same properties antagonize the behavioral effects of high METH doses, although part of those effects may result from the effects of an aripiprazole metabolite.51 On the basis of these findings, it is suggested that blockade of dopamine D2 receptors by dopamine D2 receptor partial agonists, such as aripiprazole, may be effective for treatment of METH overdose, with a lower potential for adverse effects than full antagonists.
Acknowledgments
We thank Ms A. Yoshioka of the Department of Pharmacology, Hyogo College of Medicine, for preparing the animal study proposal. We also thank Ms. C. Sakashita and Mr T. Nakajima of Joint-Use Research Facilities, Hyogo College of Medicine, for offering and operating computer technology resources and constructing the acrylic test boxes, respectively, and Mr K. Watabe of Technical Department, Muromachi Kikai Co., Ltd., for technical assistance.
Footnotes
ACADEMIC EDITOR: Lora Tally Watts, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1,772 words, excluding any confidential comments to the academic editor.
FUNDING: This research was supported, in part, by a Grant-in-Aid for Researchers, Hyogo College of Medicine (2015 to NK), and Japan Society for the Promotion of Science (JSPS) KAKENHI (grant number 15K08603 to JK). The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: NK, JK, FSH, MK, HS, and MT. Analyzed the data: NK, JK, FSH, MK, and HS. Wrote the first draft of the manuscript: NK, JK, FSH, and MT. Contributed to the writing of the manuscript: NK, JK, FSH, MK, HS, GRU, and MT. Agree with manuscript results and conclusions: NK, JK, FSH, MK, HS, GRU, and MT. Jointly developed the structure and arguments for the paper: FSH and GRU. Made critical revisions and approved final version: NK, JK, and FSH. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.National Institute on Drug Abuse NIDA Drug Facts: Methamphetamine. 2014. [Accessed February 5, 2014]. Available at: http://www.drugabuse.gov/sites/default/files/drugfactsmeth.pdf.
- 2.Ujike H, Sato M. Clinical features of sensitization to methamphetamine observed in patients with methamphetamine dependence and psychosis. Ann N Y Acad Sci. 2004;1025:279–287. doi: 10.1196/annals.1316.035. [DOI] [PubMed] [Google Scholar]
- 3.Yui K, Ikemoto S, Ishiguro T, Goto K. Studies of amphetamine or methamphetamine psychosis in Japan: relation of methamphetamine psychosis to schizophrenia. Ann N Y Acad Sci. 2000;914:1–12. doi: 10.1111/j.1749-6632.2000.tb05178.x. [DOI] [PubMed] [Google Scholar]
- 4.Bramness JG, Gundersen ØH, Guterstam J, et al. Amphetamine-induced psychosis—a separate diagnostic entity or primary psychosis triggered in the vulnerable? BMC Psychiatry. 2012;12:221. doi: 10.1186/1471-244X-12-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen CK, Lin SK, Sham PC, et al. Pre-morbid characteristics and co-morbidity of methamphetamine users with and without psychosis. Psychol Med. 2003;33(8):1407–1414. doi: 10.1017/s0033291703008353. [DOI] [PubMed] [Google Scholar]
- 6.Ikeda M, Okahisa Y, Aleksic B, et al. Evidence for shared genetic risk between methamphetamine-induced psychosis and schizophrenia. Neuropsychopharmacology. 2013;38(10):1864–1870. doi: 10.1038/npp.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Randrup A, Munkvad I. Stereotyped activities produced by amphetamine in several animal species and man. Psychopharmacologia. 1967;11(4):300–310. doi: 10.1007/BF00404607. [DOI] [PubMed] [Google Scholar]
- 8.Groves PM, Rebec GV. Biochemistry and behavior: some central actions of amphetamine and antipsychotic drugs. Annu Rev Psychol. 1976;27:91–127. doi: 10.1146/annurev.ps.27.020176.000515. [DOI] [PubMed] [Google Scholar]
- 9.Nishikawa T, Mataga N, Takashima M, Toru M. Behavioral sensitization and relative hyperresponsiveness of striatal and limbic dopaminergic neurons after repeated methamphetamine treatment. Eur J Pharmacol. 1983;88(2–3):195–203. doi: 10.1016/0014-2999(83)90006-7. [DOI] [PubMed] [Google Scholar]
- 10.Hsieh JH, Stein DJ, Howells FM. The neurobiology of methamphetamine induced psychosis. Front Hum Neurosci. 2014;8:537. doi: 10.3389/fnhum.2014.00537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396(2):157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- 12.Karila L, Weinstein A, Aubin HJ, Benyamina A, Reynaud M, Batki SL. Pharmacological approaches to methamphetamine dependence: a focused review. Br J Clin Pharmacol. 2010;69(6):578–592. doi: 10.1111/j.1365-2125.2010.03639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cretzmeyer M, Sarrazin MV, Huber DL, Block RI, Hall JA. Treatment of methamphetamine abuse: research findings and clinical directions. J Subst Abuse Treat. 2003;24(3):267–277. doi: 10.1016/s0740-5472(03)00028-x. [DOI] [PubMed] [Google Scholar]
- 14.DeLeon A, Patel NC, Crismon ML. Aripiprazole: a comprehensive review of its pharmacology, clinical efficacy, and tolerability. Clin Ther. 2004;26(5):649–666. doi: 10.1016/s0149-2918(04)90066-5. [DOI] [PubMed] [Google Scholar]
- 15.Koener B, Focant MC, Bosier B, Maloteaux JM, Hermans E. Increasing the density of the D2L receptor and manipulating the receptor environment are required to evidence the partial agonist properties of aripiprazole. Prog Neuropsychopharmacol Biol Psychiatry. 2012;36(1):60–70. doi: 10.1016/j.pnpbp.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Urban JD, Vargas GA, von Zastrow M, Mailman RB. Aripiprazole has functionally selective actions at dopamine D2 receptor-mediated signaling pathways. Neuropsychopharmacology. 2007;32(1):67–77. doi: 10.1038/sj.npp.1301071. [DOI] [PubMed] [Google Scholar]
- 17.Ozdemir V, Fourie J, Ozdener F. Aripiprazole (Otsuka Pharmaceutical Co) Curr Opin Investig Drugs. 2002;3(1):113–120. [PubMed] [Google Scholar]
- 18.Lawler CP, Prioleau C, Lewis MM, et al. Interactions of the novel antipsychotic aripiprazole (OPC-14597) with dopamine and serotonin receptor subtypes. Neuropsychopharmacology. 1999;20(6):612–627. doi: 10.1016/S0893-133X(98)00099-2. [DOI] [PubMed] [Google Scholar]
- 19.Shapiro DA, Renock S, Arrington E, et al. Aripiprazole, a novel atypical anti-psychotic drug with a unique and robust pharmacology. Neuropsychopharmacology. 2003;28(8):1400–1411. doi: 10.1038/sj.npp.1300203. [DOI] [PubMed] [Google Scholar]
- 20.Wood M, Reavill C. Aripiprazole acts as a selective dopamine D2 receptor partial agonist. Expert Opin Investig Drugs. 2007;16(6):771–775. doi: 10.1517/13543784.16.6.771. [DOI] [PubMed] [Google Scholar]
- 21.Wenzel C, Kleimann A, Bokemeyer S, Muller-Vahl KR. Aripiprazole for the treatment of Tourette syndrome: a case series of 100 patients. J Clin Psychopharmacol. 2012;32(4):548–550. doi: 10.1097/JCP.0b013e31825ac2cb. [DOI] [PubMed] [Google Scholar]
- 22.Aliane V, Perez S, Bohren Y, Deniau JM, Kemel ML. Key role of striatal cholinergic interneurons in processes leading to arrest of motor stereotypies. Brain. 2011;134(pt 1):110–118. doi: 10.1093/brain/awq285. [DOI] [PubMed] [Google Scholar]
- 23.Hallett JJ, Harling-Berg CJ, Knopf PM, Stopa EG, Kiessling LS. Anti-striatal antibodies in Tourette syndrome cause neuronal dysfunction. J Neuroimmunol. 2000;111(1–2):195–202. doi: 10.1016/s0165-5728(00)00320-9. [DOI] [PubMed] [Google Scholar]
- 24.Kohnomi S, Suemaru K, Kawasaki H, Araki H. Effect of aripiprazole on 5-HT2 receptor-mediated wet-dog shake responses and disruption of prepulse inhibition in rats. J Pharmacol Sci. 2008;106(4):645–650. doi: 10.1254/jphs.fp0071924. [DOI] [PubMed] [Google Scholar]
- 25.Koener B, Goursaud S, Van De Stadt M, et al. Pharmacological blockade of dopamine D2 receptors by aripiprazole is not associated with striatal sensitization. Naunyn Schmiedebergs Arch Pharmacol. 2011;383(1):65–77. doi: 10.1007/s00210-010-0577-7. [DOI] [PubMed] [Google Scholar]
- 26.Leite JV, Guimaraes FS, Moreira FA. Aripiprazole, an atypical antipsychotic, prevents the motor hyperactivity induced by psychotomimetics and psychostimulants in mice. Eur J Pharmacol. 2008;578(2–3):222–227. doi: 10.1016/j.ejphar.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 27.Chipkin RE, McQuade RD, Iorio LC. D1 and D2 dopamine binding site up-regulation and apomorphine-induced stereotypy. Pharmacol Biochem Behav. 1987;28(4):477–482. doi: 10.1016/0091-3057(87)90509-0. [DOI] [PubMed] [Google Scholar]
- 28.Schechter MD, Greer NL. Evidence that the stimulus properties of apomorphine are mediated by both D1 and D2 receptor activation. Life Sci. 1987;40(25):2461–2471. doi: 10.1016/0024-3205(87)90762-4. [DOI] [PubMed] [Google Scholar]
- 29.Khoshbouei H, Wang H, Lechleiter JD, Javitch JA, Galli A. Amphetamine-induced dopamine efflux. A voltage-sensitive and intracellular Na+-dependent mechanism. J Biol Chem. 2003;278(14):12070–12077. doi: 10.1074/jbc.M212815200. [DOI] [PubMed] [Google Scholar]
- 30.Sulzer D, Chen TK, Lau YY, Kristensen H, Rayport S, Ewing A. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J Neurosci. 1995;15(5 pt 2):4102–4108. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones SR, Gainetdinov RR, Wightman RM, Caron MG. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J Neurosci. 1998;18(6):1979–1986. doi: 10.1523/JNEUROSCI.18-06-01979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fleckenstein AE, Volz TJ, Hanson GR. Psychostimulant-induced alterations in vesicular monoamine transporter-2 function: neurotoxic and therapeutic implications. Neuropharmacology. 2009;56(suppl 1):133–138. doi: 10.1016/j.neuropharm.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cruickshank CC, Dyer KR. A review of the clinical pharmacology of methamphetamine. Addiction (Abingdon, England) 2009;104(7):1085–1099. doi: 10.1111/j.1360-0443.2009.02564.x. [DOI] [PubMed] [Google Scholar]
- 34.Kuczenski R, Segal DS, Cho AK, Melega W. Hippocampus norepinephrine, caudate dopamine and serotonin, and behavioral responses to the stereoisomers of amphetamine and methamphetamine. J Neurosci. 1995;15(2):1308–1317. doi: 10.1523/JNEUROSCI.15-02-01308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Costall B, Marsden CD, Naylor RJ, Pycock CJ. Stereotyped behaviour patterns and hyperactivity induced by amphetamine and apomorphine after discrete 6-hydroxydopamine lesions of extrapyramidal and mesolimbic nuclei. Brain Res. 1977;123(1):89–111. doi: 10.1016/0006-8993(77)90645-x. [DOI] [PubMed] [Google Scholar]
- 36.Costall B, Naylor RJ. The role of telencephalic dopaminergic systems in the mediation of apomorphine-stereotyped behaviour. Eur J Pharmacol. 1973;24(1):8–24. doi: 10.1016/0014-2999(73)90108-8. [DOI] [PubMed] [Google Scholar]
- 37.Lal S, Feldmuller F. Effect of amphetamine and apomorphine on brain monoamines and behaviour in the immature and young adult rat. Arch Int Pharmacodyn Ther. 1975;218(2):239–251. [PubMed] [Google Scholar]
- 38.Cameron DL, Crocker AD. Localization of striatal dopamine receptor function by central injection of an irreversible receptor antagonist. Neuroscience. 1989;32(3):769–778. doi: 10.1016/0306-4522(89)90297-2. [DOI] [PubMed] [Google Scholar]
- 39.Kelly PH, Seviour PW, Iversen SD. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975;94(3):507–522. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- 40.Hirose T, Uwahodo Y, Yamada S, et al. Mechanism of action of aripiprazole predicts clinical efficacy and a favourable side-effect profile. J Psychopharmacol(Oxford, England) 2004;18(3):375–383. doi: 10.1177/026988110401800308. [DOI] [PubMed] [Google Scholar]
- 41.Fetsko LA, Xu R, Wang Y. Alterations in D1/D2 synergism may account for enhanced stereotypy and reduced climbing in mice lacking dopamine D2L receptor. Brain Res. 2003;967(1–2):191–200. doi: 10.1016/s0006-8993(02)04277-4. [DOI] [PubMed] [Google Scholar]
- 42.Kitanaka J, Kitanaka N, Tatsuta T, et al. Sigma1 receptor antagonists determine the behavioral pattern of the methamphetamine-induced stereotypy in mice. Psychopharmacology. 2009;203(4):781–792. doi: 10.1007/s00213-008-1425-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kitanaka J, Kitanaka N, Hall FS, et al. Pretreatment with nomifensine or nomifensine analogue 4-phenyl-1,2,3,4-tetrahydroisoquinoline augments methamphetamine-induced stereotypical behavior in mice. Brain Res. 2012;1439:15–26. doi: 10.1016/j.brainres.2011.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tatsuta T, Kitanaka N, Kitanaka J, Morita Y, Takemura M. Lobeline attenuates methamphetamine-induced stereotypy in adolescent mice. Neurochem Res. 2006;31(11):1359–1369. doi: 10.1007/s11064-006-9180-1. [DOI] [PubMed] [Google Scholar]
- 45.Kitanaka N, Kitanaka J, Takemura M. Behavioral sensitization and alteration in monoamine metabolism in mice after single versus repeated methamphetamine administration. Eur J Pharmacol. 2003;474(1):63–70. doi: 10.1016/s0014-2999(03)02015-6. [DOI] [PubMed] [Google Scholar]
- 46.Tatsuta T, Kitanaka N, Kitanaka J, Morita Y, Takemura M. Lack of effect of anti-convulsant topiramate on methamphetamine-induced stereotypy and rewarding property in mice. Pharmacol Biochem Behav. 2007;87(1):48–55. doi: 10.1016/j.pbb.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 47.Kitanaka N, Kitanaka J, Takemura M. Inhibition of methamphetamine-induced hyperlocomotion in mice by clorgyline, a monoamine oxidase-a inhibitor, through alteration of the 5-hydroxytryptamine turnover in the striatum. Neuroscience. 2005;130(2):295–308. doi: 10.1016/j.neuroscience.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 48.Gianutsos G, Morrow G, Light S, Sweeney MJ. Dopaminergic properties of nomifensine. Pharmacol Biochem Behav. 1982;17(5):951–954. doi: 10.1016/0091-3057(82)90478-6. [DOI] [PubMed] [Google Scholar]
- 49.Kelly PH, Iversen SD. Selective 6OHDA-induced destruction of mesolimbic dopamine neurons: abolition of psychostimulant-induced locomotor activity in rats. Eur J Pharmacol. 1976;40(1):45–56. doi: 10.1016/0014-2999(76)90352-6. [DOI] [PubMed] [Google Scholar]
- 50.Schwartz JT, Brotman AW. A clinical guide to antipsychotic drugs. Drugs. 1992;44(6):981–992. doi: 10.2165/00003495-199244060-00007. [DOI] [PubMed] [Google Scholar]
- 51.Wood MD, Scott C, Clarke K, et al. Aripiprazole and its human metabolite are partial agonists at the human dopamine D2 receptor, but the rodent metabolite displays antagonist properties. Eur J Pharmacol. 2006;546(1–3):88–94. doi: 10.1016/j.ejphar.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 52.Naber D, Lambert M. Aripiprazole: a new atypical antipsychotic with a different pharmacological mechanism. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(8):1213–1219. doi: 10.1016/j.pnpbp.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 53.Kitanaka J, Kitanaka N, Tatsuta T, Morita Y, Kinoshita H, Takemura M. Regulating the expression patterns of bizarre behavior: a therapeutic option for amphetamine-type drug-induced stereotypy? In: Columbus AM, editor. Advances in Psychology Research. Vol. 60. New York: Nova Science Publishers Inc; 2009. pp. 143–154. [Google Scholar]
- 54.Aman MG, McDougle CJ, Scahill L, et al. Research Units on Pediatric Psychopharmacology Autism Network. Medication and parent training in children with pervasive developmental disorders and serious behavior problems: results from a randomized clinical trial. J Am Acad Child Adolesc Psychiatry. 2009;48(12):1143–1154. doi: 10.1097/CHI.0b013e3181bfd669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dickson PR, Lang CG, Hinton SC, Kelley AE. Oral stereotypy induced by amphetamine microinjection into striatum: an anatomical mapping study. Neuroscience. 1994;61(1):81–91. doi: 10.1016/0306-4522(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 56.Lieberman JA, Kinon BJ, Loebel AD. Dopaminergic mechanisms in idiopathic and drug-induced psychoses. Schizophr Bull. 1990;16(1):97–110. doi: 10.1093/schbul/16.1.97. [DOI] [PubMed] [Google Scholar]
- 57.Budygin EA. Dopamine uptake inhibition is positively correlated with cocaine-induced stereotyped behavior. Neurosci Lett. 2007;429(1):55–58. doi: 10.1016/j.neulet.2007.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han M, Huang XF, Deng C. Aripiprazole differentially affects mesolimbic and nigrostriatal dopaminergic transmission: implications for long-term drug efficacy and low extrapyramidal side-effects. Int J Neuropsychopharmacol. 2009;12(7):941–952. doi: 10.1017/S1461145709009948. [DOI] [PubMed] [Google Scholar]
- 59.Fukushima S, Shen H, Hata H, et al. Methamphetamine-induced locomotor activity and sensitization in dopamine transporter and vesicular monoamine transporter 2 double mutant mice. Psychopharmacology. 2007;193(1):55–62. doi: 10.1007/s00213-007-0749-4. [DOI] [PubMed] [Google Scholar]
- 60.Izzo E, Orsini C, Koob GF, Pulvirenti L. A dopamine partial agonist and antagonist block amphetamine self-administration in a progressive ratio schedule. Pharmacol Biochem Behav. 2001;68(4):701–708. doi: 10.1016/s0091-3057(01)00472-5. [DOI] [PubMed] [Google Scholar]