Abstract
An index based on the initial absolute lymphocyte and monocyte counts may provide prognostic information regarding outcome beyond that of the International Prognostic Factors Index in management of patients with untreated diffuse large cell lymphoma who are receiving R-CHOP chemotherapy.
Background
The baseline absolute monocyte count and absolute lymphocyte count were used to generate a prognostic index (the AMLPI) for survival in diffuse large B-cell lymphoma (DLBCL).
Methods
Data from 245 patients with DLBCL who were treated with standard R-CHOP (rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, prednisone) were reviewed. By using the values previously reported for the AMLPI, its prognostic value was examined in our population.
Results
After a median follow-up of 22 months for censored observations, the 3-year progression-free survival (PFS) rates for the international prognostic index (IPI) 0–2 and 3–5 risk groups were 73% and 58%, respectively (P = .0004); comparable overall survival (OS) rates were 88% and 68%, respectively (P < .0001). For patients with IPI scores of 0–2, 1-year PFS rates for AMLPI low-, intermediate-, and high-risk groups were 92%, 89%, and 80%, respectively (P = .022); comparable 1-year OS rates were 96%, 95%, and 80%, respectively (P = .049). By multivariate analysis, with the adjustment of IPI in the model, AMLPI effects (low- vs. high-risk groups) on PFS and OS rates were significant, with P = .046 (hazard ratio [HR] 0.402 [95% CI, 0.164–0.986] and P = .052 (HR 0.325 [95% CI, 0.104–1.011]), respectively.
Conclusions
The absolute monocyte and lymphocyte counts prognostic index (the AMLPI) may add prognostic value beyond that of the IPI for patients with DLBCL who receive R-CHOP.
Keywords: International prognostic factors index, Large cell lymphoma, Prognostic factors, R-CHOP chemotherapy
Introduction
The combination of values of baseline absolute monocyte count (AMC) and absolute lymphocyte count (ALC) in diffuse large B-cell lymphoma (DLBCL) was prognostic for survival in DLBCL.1 However, this needed validation in other patient cohorts. Therefore, we sought to examine this score in our patients at M. D. Anderson Cancer Center (MDACC).
Patients and Methods
Baseline AMC and ALC were retrospectively examined for a cohort of 245 consecutive patients with untreated DLBCL who were receiving standard R-CHOP (rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, prednisone) and who had been enrolled into the MDACC lymphoma database. Both AMC and ALC were dichotomized into high and low groups by using predefined cutoff points (610/µL for AMC and 1000/µL for ALC). An absolute monocyte and lymphocyte counts prognostic index (AMLPI) was generated, which stratified patients into 3 risk groups: low risk (AMC <610/µL and ALC >1000/µL), intermediate risk (AMC ≥610/µL or ALC ≤1000/µL), and high risk (AMC ≥610/µL and ALC ≤1000/µL).1 The prognostic effect of the AMLPI and the international prognostic index (IPI) on PFS and overall survival (OS) were examined by multivariate analysis.
Results and Discussion
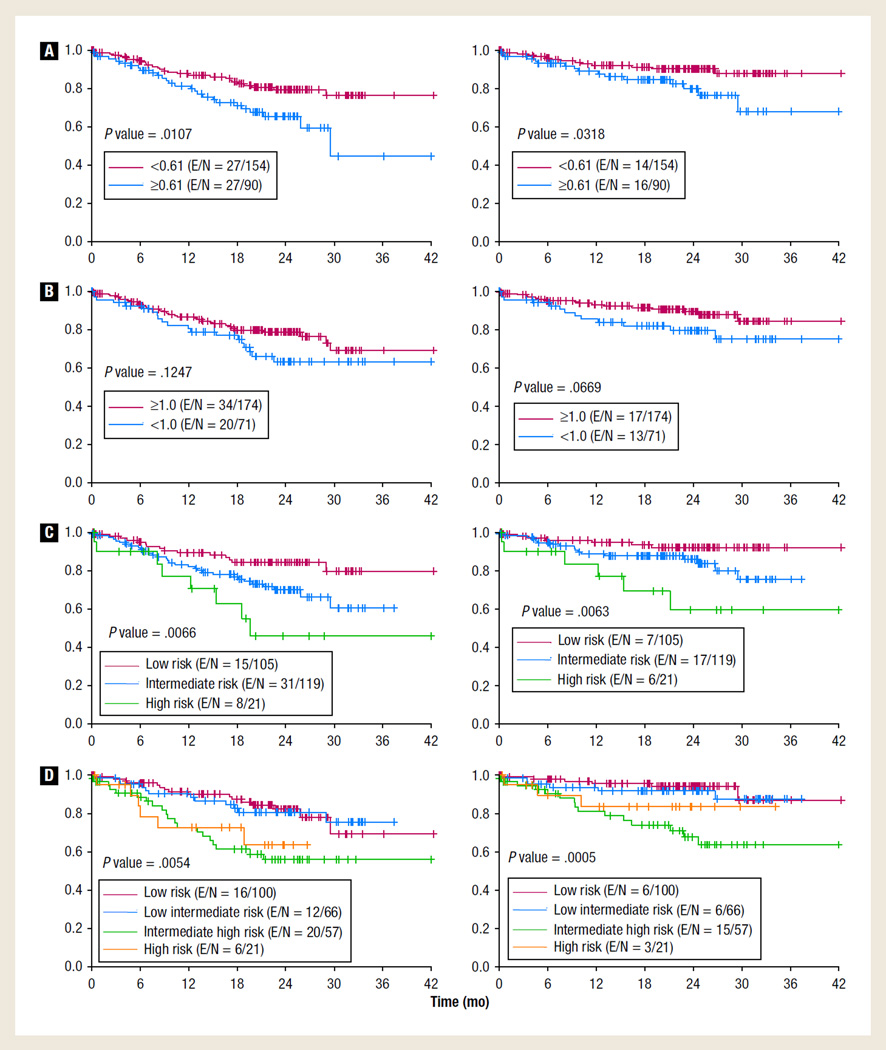
Two hundred forty-five patient records were evaluated; however, only 244 had full information for IPI calculations. The median age at diagnosis was 60 years (range, 19–92 years). Baseline characteristics of these patients are displayed in Table 1. At diagnosis, the median AMC was 500/µL (range, 60–1710/µL) and the median ALC was 133/µL (range, 0–1178/µL). All the patients were initially treated with standard R-CHOP. The IPI was calculated for 244 patients. Ninety (37%) patients had high AMC, and 71 (29%) had low ALC, respectively. AMLPI risk groups included 105 (43%) patients in the low-, 119 (48%) in the intermediate-, and 21 (9%) in the high-risk groups; with a median follow-up of 22 months (range, 0.03–42 months) for censored observations, 3-year PFS rates for these risk groups were 80%, 61%, and 46% (P = .007) and 3-year OS rates were 92%, 76%, and 60% (P = .006), respectively. Three-year PFS rates for 166 patients with IPI 0–2 and 78 patients with IPI 3–5 were 73% and 58%, respectively (P = .0004); comparable OS rates were 88% and 68%, respectively (P < .0001). By univariate analysis, a high AMC was associated with inferior PFS (P = .01) and OS (P = .03) (Figure 1A). Low ALC was not statistically associated with PFS or OS rates (Figure 1B). However, both IPI and AMLPI had significant effects on PFS and OS (Figure 1C, D). For patients with IPI 0–2; 1-year PFS rates for AMLPI low-, intermediate-, and high-risk groups were 92%, 89%, and 80%, respectively (P = .022); comparable 1-year OS rates were 96%, 95%, and 80%, respectively (P = .049). By multivariate analysis, the AMLPI effect (low- vs. high-risk groups) on the PFS rate was significant (P = .046) (HR 0.402 [95% CI, 0.164–0.986]) as was the IPI effect (3–5 vs. 0–2, P = .005) (HR 2.218 [95% CI, 1.266–3.884]); similar results were observed for OS (P = .052) (HR 0.325 [95% CI, 0.104–1.011]) and P = .003 (HR 3.245 [95% CI, 1.510–6.972]), respectively. However, the AMLPI effect was not significant in intermediate-risk disease (Table 2).
Table 1.
Baseline Characteristics
| Characteristics | |
|---|---|
| Median (range) Age, y | 60 (19–92) |
| Men, No. (%) | 132 (54) |
| Ann Arbor Stage, No. (%) | |
| I | 50 (20.4) |
| II | 46 (18.8) |
| III | 28 (11.4) |
| IV | 121 (49.4) |
| LDH, No. (%) | |
| >Normal | 114 (46.5) |
| ≤Normal | 131 (53.5) |
| Extranodal Sites of Disease, No. (%) | |
| >1 | 73 (29.8) |
| ≤1 | 172 (70.2) |
| ECOG PS, No. (%) | |
| >1 | 12 (4.9) |
| ≤1 | 233 (95.1) |
| IPI, No. (%) | |
| 0–1 | 100 (41.0) |
| 2 | 66 (27.0) |
| 3 | 57 (23.4) |
| 4 | 21 (8.6) |
| Radiation Treatment, No. (%) | 63 (26.7) |
| Absolute Monocyte Count, Median (IQR) | 500/µL (60–1710/µL) |
| Absolute Lymphocyte Count, Median (IQR) | 1330/µL (0–1178/µL) |
Abbreviations: ECOG PS = Eastern Cooperative Oncology Group Performance Status; IPI = international prognostic index; IQR = interquartile range; LDH = lactic dehydrogenase.
Figure 1. PFS and OS. (A) R-CHOP for DLBCL: PFS (left) and OS (right) by AMC; (B) R-CHOP for DLBCL: PFS (left) and OS (right) by ALC; (C) R-CHOP for DLBCL: PFS (left) and OS (right) by AMLPI; (D) R-CHOP for DLBCL: PFS (left) and OS (right) by IPI.
Abbreviations: ALC = absolute lymphocyte count; AMC = absolute monocyte count; AMLPI = absolute monocyte and lymphocyte prognostic index; DLBCL = diffuse large B-cell lymphoma; E = number of events; IPI = international prognostic index; N = total number of patients; OS = overall survival; PFS = progression-free survival; R-CHOP = rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone.
Table 2.
Multivariate Analyses of AMLPI and IPI for PFS and OS
| Variable | P Value | HR | 95% CI |
|---|---|---|---|
| OS | |||
| IPI | |||
| 3–5 vs. 1–2 | .0026 | 3.245 | 1.510–6.972 |
| AMLPI | |||
| Low vs. high | .0522 | 0.325 | 0.104–1.011 |
| Intermediate vs. high | .3740 | 0.647 | 0.247–1.691 |
| PFS | |||
| IPI | |||
| 3–5 vs. 1–2 | .0054 | 2.218 | 1.266–3.884 |
| AMLPI | |||
| Low vs. high | .0464 | 0.402 | 0.164–0.986 |
| Intermediate vs. high | .4871 | 0.752 | 0.336–1.682 |
Abbreviations: AMLPI = absolute monocyte and lymphocyte counts prognostic index; HR = hazard ratio; IPI = international prognostic index; OS = overall survival; PFS = progression free survival.
Contrary to conventional prognostic indexes, the AMLPI does not incorporate patient and tumor characteristics, and contributes to the simplicity of this index, because it instead is formed by laboratory values related to a patient’s adaptive immune response. Recent studies of gene-expression signatures support this concept and have demonstrated that cells that are invading the tumor microenvironment may provide invaluable prognostic information when compared with tumor characteristics alone.2,3 The AMC and ALC were combined to generate a score that was shown to be prognostic for survival in DLBCL.1 Because this needed further validation, we sought to examine this score in our cohort at MDACC.
Monocytes promote tumorigenesis and angiogenesis,4 and suppress the host immune response to cancer, which may explain why elevated monocyte (and neutrophil) counts in solid tumors confer a negative prognosis.5,6 Monocytosis is thought to be prognostic in DLBCL because of the induction of a host immunosuppressive state.7 In addition, monocytes within the tumor microenvironment inducibly express the T-cell co-inhibitory ligand B7-H1 (PD-L1).7 The latter impairs the expansion of effector cells and leads to the expansion of suppressive regulatory T cells. Certain myeloid-lineage cells also have a supporting role in tumor angiogenesis, particularly the subpopulation of monocytes that express the angiopoietin-2 receptor Tie2.8
Monocytes in the circulation represent the pool of macrophages1 and are an important source of soluble mediators,9 which may help support the evolution of malignant cells.10,11 On a pathophysiological level, the Activated B-Cell (ABC) type of DLBCL depends on the STAT3 and nuclear factor-kB pathways, which regulate the enrollment of monocytes.1 In the clinic, the immunomodulatory agent lenalidomide demonstrates the potential to increase the antitumor effects of rituximab against DLBCL, especially for those with an ABC phenotype, which is presumed due to the recruitment of natural killer cells to the lymphoma sites in preclinical models.12 Therefore, the cell of origin of DLBCL subtypes may be responsible for outcomes according to AMC, although others have found no differences in AMC results among DLBCL subtypes. In fact, a recent work showed that the AMC/ALC score was independent of immunohistochemically determined cell of origin and it added to its ability to identify patients with high-risk disease in DLBCL.13
Lymphopenia is a surrogate marker of host immunologic incompetence.14 Lymphocytes also act as mediators of antibody-dependent cell-mediated cytotoxicity15 and may be why lymphopenia is an adverse prognostic feature for indolent and aggressive lymphomas, particularly DLBCL.16 Other investigators have also reported that the AMC may predict OS rates for patients with follicular lymphoma and in chronic lymphocytic leukemia in the accelerated phase.17,18 Studies of the values of AMC and ALC could lead to novel therapeutic strategies in treating DLBCL, including depletion of tumorigenic myeloid-lineage cells or reversal of lymphopenia.1 Such approaches are being explored in solid tumors.19 Likewise, genetically engineered lymphocyte therapy in refractory lymphoma to chemotherapy is the subject of a pilot study (NCT01029366).
In the post-rituximab era, AMLPI is a simple tool that could be used as a prognostic model for patients with DLBCL and that should be compared with other more complicated models, such as gene-expression profiling or other indexes developed in the prerituximab era, and should also be compared with the value of other more expensive tests, including positron emission tomography scans.20,21 In our study, when compared with the IPI, AMLPI could further stratify patients with IPI 0–2, consistent with published literature.1 These results should be validated in prospective trials.
Clinical Practice Points.
The International Prognostic Factors Index is an important determinant in therapy of large cell lymphoma.
A study of additional features at the time of diagnosis might provide better prognostic information for therapy of these patients.
Investigators have suggested that the number of lymphocytes and monocytes in the blood at the time of diagnosis might provide information regarding outcomes of therapy for diffuse large cell lymphoma.
In this study, an index based upon absolute levels of lymphocytes and monocytes could segregate these patients into three groups with different survival rates, including those with good, intermediate, and poor survival rates.
This new index appeared to be as valuable as the International Prognostic factors Index, and may provide additional prognostic value when combined with the IPI for patients with this disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors have stated that they have no conflicts of interest.
References
- 1.Wilcox RA, Ristow K, Habermann TM, et al. The absolute monocyte and lymphocyte prognostic score predicts survival and identifies high-risk patients in diffuse large-B-cell lymphoma. Leukemia. 2011;25:1502–1509. doi: 10.1038/leu.2011.112. [DOI] [PubMed] [Google Scholar]
- 2.Lenz G, Wright G, Dave SS, et al. Lymphoma/Leukemia Molecular Profiling Project. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med. 2008;22:2313. doi: 10.1056/NEJMoa0802885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ansell SM, Stenson M, Habermann TM, et al. CD4+ T-cell immune response to large B-cell non-Hodgkin’s lymphoma predicts patient outcome. J Clin Oncol. 2001;19:720–726. doi: 10.1200/JCO.2001.19.3.720. [DOI] [PubMed] [Google Scholar]
- 4.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;3:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donskov F, von der Maase H. Impact of immune parameters on long-term survival in metastatic renal cell carcinoma. J Clin Oncol. 2006;24:1997–2005. doi: 10.1200/JCO.2005.03.9594. [DOI] [PubMed] [Google Scholar]
- 6.Takasaki Y, Iwanaga M, Tsukasaki K, et al. Impact of visceral involvements and blood cell count abnormalities on survival in adult T-cell leukemia/lymphoma (ATLL) Leuk Res. 2007;31:751–757. doi: 10.1016/j.leukres.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Wilcox RA, Feldman AL, Wada DA, et al. B7-H1 (PD-L1, CD274) suppresses host immunity in T-cell lymphoproliferative disorders. Wilcox RA. Blood. 2009;10:2149. doi: 10.1182/blood-2009-04-216671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venneri MA, De Palma M, Ponzoni M, et al. Identification of proangiogenic TIE2-expressing monocytes (TEMs) in human peripheral blood and cancer. Blood. 2007;109:5276–5285. doi: 10.1182/blood-2006-10-053504. [DOI] [PubMed] [Google Scholar]
- 9.Novak AJ, Grote DM, Stenson M, et al. Expression of BLyS and its receptors in B-cell non-Hodgkin lymphoma: correlation with disease activity and patient outcome. Blood. 2004;104:2247–2253. doi: 10.1182/blood-2004-02-0762. [DOI] [PubMed] [Google Scholar]
- 10.Wilcox RA, Wada DA, Ziesmer SC, et al. Monocytes promote tumor cell survival in T-cell lymphoproliferative disorders and are impaired in their ability to differentiate into mature dendritic cells. Blood. 2009;114:2936–2944. doi: 10.1182/blood-2009-05-220111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seiffert M, Schulz A, Ohl S, et al. Soluble CD14 is a novel monocyte-derived survival factor for chronic lymphocytic leukemia cells, which is induced by CLL cells in vitro and present at abnormally high levels in vivo. Blood. 2010;116:4223–4230. doi: 10.1182/blood-2010-05-284505. [DOI] [PubMed] [Google Scholar]
- 12.Wilson WH, Hernandez-Ilizaliturri FJ, Dunleavy K, et al. Novel disease targets and management approaches for diffuse large B-cell lymphoma. Leuk Lymphoma. 2010;51(suppl 1):1–10. doi: 10.3109/10428194.2010.500045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porrata LF, Ristow K, Habermann TM, et al. Absolute monocyte/lymphocyte count prognostic score is independent of immunohistochemically determined cell of origin in predicting survival in diffuse large B-cell lymphoma. Leuk Lymphoma. 2012;53:2159–21651. doi: 10.3109/10428194.2012.690605. [DOI] [PubMed] [Google Scholar]
- 14.Shivakumar L, Ansell S. Targeting B-lymphocyte stimulator/B-cell activating factor and a proliferation-inducing ligand in hematologic malignancies. Clin Lymph Myeloma. 2006;7:106–108. doi: 10.3816/CLM.2006.n.046. [DOI] [PubMed] [Google Scholar]
- 15.Weiner GJ. Rituximab: mechanism of action. Semin Hematol. 2010;47:115–123. doi: 10.1053/j.seminhematol.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song MK, Chung JS, Seol YM, et al. Influence of low absolute lymphocyte count of patients with nongerminal center type diffuse large B-cell lymphoma with R-CHOP therapy. Ann Oncol. 2010;21:140–144. doi: 10.1093/annonc/mdp505. [DOI] [PubMed] [Google Scholar]
- 17.Wilcox RA, Ristow K, Habermann TM, et al. The absolute monocyte count is associated with overall survival in patients newly diagnosed with follicular lymphoma. Leuk Lymphoma. 2012;53:575–580. doi: 10.3109/10428194.2011.637211. [DOI] [PubMed] [Google Scholar]
- 18.Wilcox RA, Neil Kay N, Timothy G, et al. In patients newly diagnosed with chronic lymphocytic leukemia the absolute monocyte count at presentation is directly associated with disease progression independently from Rai staging or cytogenetics. ASH Annual Meeting Abstracts. 2011;118:2835. 2835. [Google Scholar]
- 19.Ko JS, Bukowski RM, Fincke JH. Myeloid-derived suppressor cells: a novel therapeutic target. Curr Oncol Rep. 2009;11:87–93. doi: 10.1007/s11912-009-0014-6. [DOI] [PubMed] [Google Scholar]
- 20.Mounier N, Briere J, Gisselbrecht C, et al. Rituximab plus CHOP (R-CHOP) overcomes bcl-2-associated resistance to chemotherapy in elderly patients with diffuse large B-cell lymphoma (DLBCL) Blood. 2003;11:4279. doi: 10.1182/blood-2002-11-3442. [DOI] [PubMed] [Google Scholar]
- 21.Lenz G, Wright G, Dave SS, et al. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med. 2008;359:2313–2323. doi: 10.1056/NEJMoa0802885. [DOI] [PMC free article] [PubMed] [Google Scholar]