Abstract
Background
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder of the gastrointestinal tract that significantly impacts the health-related quality of life (HR-QOL). A decreased HR-QOL has been demonstrated in patients with active disease compared to patients in remission. In this cross-sectional study, we examined the role of depression and disease activity as independent factors in predicting patient's HR-QOL.
Methods
105 patients with either Crohn's disease (CD) or Ulcerative Colitis (UC) were enrolled. Disease activity was evaluated by the Crohn's disease activity index (CDAI) or Seo Activity Index (SAI). Depressive symptoms were evaluated by Beck's Depression Inventory II (BDI-II) and Primary Care (BDI-PC). HR-QOL was evaluated by the Short Inflammatory Bowel Disease Questionnaire (SIBDQ). Simple and multiple regressions were performed on quality of life score with multiple demographic and clinical variables as predictors.
Results
The prevalence of depression in our study population is 25%. In both CD and UC patients, depression is the most significant predictor to a poor HR-QOL (in CD, P=8.22×10-6; in UC, P=2.02×10-6). HR-QOL is weakly affected by disease activity (in CD, P=0.110; in UC, P=0.00492). In CD, Biologic use displays positive effect on HR-QOL (P=0.00780). In total, the proportion of variance explained by all predictors is 61% for CD and 53% for UC, while the depression alone explains 44% and 36%.
Conclusion
Our study demonstrates the importance of depression toward the quality of life in IBD patients. The diagnosis of depression should be actively sought out and treated in outpatient IBD practices.
Keywords: inflammatory bowel disease, depression, disease activity, health-related quality of life
Introduction
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder of the gastrointestinal tract, a consequence of a complex interplay between genetic, developmental and environmental factors. (1) With a peak incidence between the ages of 15 and 40 years, IBD often impacts patients' lives significantly starting in late adolescence and continuing throughout adulthood. Patients with IBD have a reduced quality of life (QOL) as a presumed consequence of the disabling symptoms of active disease as well as the side effects of medical treatments and surgical interventions. (2)
Psychiatric conditions are common in the community particularly in those with chronic medical conditions. (3-5) Psychiatric co-morbidities can impact both patient QOL and disease outcomes. (6, 7) Similar to other chronic illnesses, patients with IBD generally exhibit a higher prevalence of psychological disturbances than the general population. (8-10) However, the exact relationship between psychological disorders and IBD remains unclear. It has been suggested that there are reciprocal influential processes, such that the experience of the disease is sufficiently stressful to trigger or intensify an underlying psychiatric condition. Conversely, anxiety or depression may be sufficient to trigger or exacerbate the chronic health condition itself. (11-13)
Health-related quality of life (HR-QOL), defined as the functional effect of an illness and its treatment on a patient as perceived by the patient, are important clinical outcome in chronic diseases such as IBD. (14) Self-reported HR-QOL would be expected to be worsened during periods of active disease. However, some studies have shown a direct correlation between disease activity and HR-QOL while other studies have shown otherwise. (15-18) There is a growing interest in the role that psychosocial factors play in determining HR-QOL in IBD. Recent studies have begun to shed light on the association between psychological distress (such as depression and anxiety) and personality variables on the HR-QOL in IBD patients. (19, 20) Despite methodological differences, a consistent theme remains that disease activity and psychological distress are important determinants of poor HR-QOL, yet the details of this relationship have been poorly described.
We set forth in this cross-sectional study, using validated patient-centered instruments, to examine contributing factors to HR-QOL in patients with IBD. In addition to demographic and clinical factors, this study focused on disease activity as well as depression scores in determining the HR-QOL. The methods used in our study may be easily adaptable to busy clinical practice. The utilization of these methods could further improve the care that is provided to patients with IBD.
Methods
Patients cared for by the Yale University Inflammatory Bowel Disease Program were invited to participate during regularly scheduled outpatient clinic appointments between September 2008 and January 2010.
All patients had been previously diagnosed with Crohn's disease (CD) or ulcerative colitis (UC) on the basis of Lennard-Jones criteria. (21) Patients were excluded if they were younger than18 years, had unclassified IBD, were unable to complete the questionnaire or understand the consent form, were diagnosed with Crohn's disease and had an end ileostomy, or diagnosed with UC and had undergone total procto-colectomy with IPAA (Ileal Pouch Anal Anastomosis) formation. The physician obtained clinical and demographic data through direct questioning of the patient. Any unknown information was gathered from the patient's medical chart. The data assessed included: age, gender, disease type, disease location, disease phenotype (CD), disease duration, number of hospitalizations and surgeries, primary care physician (PCP) utilization and medications. (Table 1)
Table 1. Clinical and Demographic Characteristics.
| Measurement | CD | UC | ||||||
|---|---|---|---|---|---|---|---|---|
| Disease Activity | Grade | All | Remission | Active | All | Remission | Active | |
| Number | 55 | 44 | 11 | 50 | 16 | 34 | ||
| Age (years) | Median (SD) | 33 (12.9) | 32.5 (13.0) | 40.0 (12.7) | 38 (15.9) | 43.5 (16.7) | 38.0 (15.3) | |
| Male Gender | Number (Proportion) | 30 (54.5%) | 24 (54.5%) | 6 (54.5%) | 24 (48%) | 10 (62.5%) | 20 (58.8%) | |
| Disease Duration (years) | Median (SD) | 6 (6.87) | 5.5 (7.23) | 6.0 (5.33) | 9 (10.0) | 9 (9.46) | 9 (10.3) | |
| Hospitalizations(n) | Median (SD) | 2 (5.46) | 1.0 (5.62) | 4.0 (4.39) | 1 (2.08) | 1 (2.96) | 1 (1.56) | |
| Surgeries | Mean (SD) | 1.55 (2.74) | 1.08 (2.75) | 1.82 (2.79) | 0.16 (0.51) | NA | 0.24 (0.61) | |
| Steroid History | None | Number (Proportion) | 11 (20%) | 9 (20.5%) | 2 (18.2%) | 9 (18%) | 4 (25%) | 5 (14.7%) |
| Previous Steroid Use | Number (Proportion) | 34 (61.8%) | 26 (59.1%) | 8 (72.7%) | 25 (50%) | 10 (62.5%) | 15 (44.1%) | |
| Current Steroid Use | Number (Proportion) | 10 (18.2%) | 9 (20.5%) | 1 (9.1%) | 16 (32%) | 2 (12.5%) | 14 (41.2%) | |
| Last Steroid Use (months) | Median (SD) | 15 (41.7) | 12 (38.7) | 84 (44.4) | 3 (34.9) | 24 (16.0) | 1 (40.5%) | |
| Current Narcotic Use | Number (Proportion) | 7 (12.7%) | 5 (11.4%) | 2 (18.2%) | 4 (8%) | 2 (12.5%) | 2 (5.88%) | |
| Antidepressant Use | Number (Proportion) | 6 (11.1%) | 5 (11.4%) | 1 (9.1%) | 6 (12%) | 2 (12.5%) | 4 (11.8%) | |
Disease Activity
Disease activity scores were calculated using the Crohn's Disease Activity Index (CDAI) for patients with CD (22) and the Seo Activity Index (SAI) for patients with UC. (23) Activity indices were chosen over endoscopic scores due to ease, non-invasiveness and prior validation. The CDAI is a well validated index for CD addressing eight different parameters. Using the CDAI, disease activity for CD was classified as: remission - less than 150; mild disease - between 150 and 220; moderate disease – between 220 and 450; and severe disease – greater than 450. The SAI is a well validated index for UC employing five different parameters; it has the advantage of being more sensitive and has prognostic implications. (24) Using the SAI disease activity for UC was classified as: remission - less than 120; mild disease - between 120 and 150; moderate disease – between 150 and 220; and severe disease – greater than 220. (Table 2)
Table 2. Disease Activity, SIBDQ and Beck Depression Indices.
| Measurement | CD | UC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Disease Activity | Grade | All | Remission | Active | All | Remission | Active | ||
| Number | 55 | 44 | 11 | 50 | 16 | 34 | |||
| SIBDQ | Mean (SD) | 50.6 (8.89) | 52.2 (7.93) | 44.2 (9.97) | 52 (1.18) | 58.5 (9.0) | 44.9 (11.5) | ||
| CDAI (CD only) | Mean (SD) | 78.3 (7.27) | 60.7 (4.10) | 210.2 (61.1) | |||||
| Remission | Number (Proportion) | 44 (80%) | 44 (100%) | ||||||
| Mild | Number (Proportion) | 7 (12.7%) | 7 (63.6%) | ||||||
| Moderate | Number (Proportion) | 4 (7.3%) | 4 (36.4%) | ||||||
| Severe | Number (Proportion) | 0 (0%) | 0 (0%) | ||||||
| SEO index (UC only) | Mean (SD) | 195.9 (143) | 107.0 (6.58) | 237.5 (158) | |||||
| Remission | Number (Proportion) | 16 (32%) | 16 (100%) | ||||||
| Mild | Number (Proportion) | 11 (22%) | 11 (32.4%) | ||||||
| Moderate | Number (Proportion) | 8 (16%) | 8 (23.5%) | ||||||
| Severe | Number (Proportion) | 15 (30%) | 15 (44.1%) | ||||||
| BDI-II | Median (SD) | 8 (7.38) | 8 (6.60) | 12 (8.89) | 8.5 (9.13) | 6 (10.3) | 10 (8.66) | ||
| BDI-PC | Median (SD) | 2 (2.67) | 2 (2.51) | 4 (2.93) | 1.5 (3.11) | 1 (3.69) | 2 (2.86) | ||
Health-Related Quality of Life
HR-QOL was measured using the Short Inflammatory Bowel Disease Questionnaire (SIBDQ). (25) Clinically reliable, this questionnaire has been validated against the much larger IBDQ-36. It addresses the same four subscale dimensions (bowel, systemic, emotional, and social) with ten simple self-administered questions. Each individual question is rated on a 7-point Likert scale from 1 (severe problem) to 7 (no problem) with a maximum score of 70 points representing optimum HR-QOL. (Table 2)
Psychological Status
Psychological status was assessed using the Beck Depression Inventory-II (BDI-II) (26) and the derived Beck Depression Inventory for Primary Care (BDI-PC). (27) (Table 2)
The BDI-II is a self-administered questionnaire that consists of a 21-item rating inventory. It has been validated to both diagnose and monitor depression in medically ill patients including those with intestinal symptoms. Questions directed at somatic symptoms of depression may overlap with symptoms of many illnesses such as appetite, weight loss, general fatigue and concerns for overall health. Patients were asked to rate symptoms on a scale ranging from 0 to 3. A maximum score of 63 could be achieved, with greater scores indicating worsening depression. Using the BDI-II, depression could be classified as follows: none – less than 14; mild depression – 14-19; moderate depression – 20-28; and severe depression – 29-63. The Beck Depression Inventory for Primary Care (BDI-PC) is constructed from a set of seven non-somatic items from the BDI-II with the goal of allowing primary care physicians to easily and quickly screen for the presence of depression while minimizing the possibility of yielding spuriously high estimates of depression for patients with medical problems. This seven item self-report instrument includes: sadness, loss of pleasure, pessimism, past failure, self-dislike, self-criticism, and suicidal thoughts or wishes. The BDI-PC was scored on a 0-3 scale for each item with the maximum score being 21. A BDI-PC cutoff of 4 and above was shown to provide maximum clinical efficacy in identifying patients with and without major depressive disorders (27).
This study was approved by the Yale School of Medicine Human Investigation Committee and all patients gave their consent to participate in the study.
Statistical Analysis
All data were analyzed in the R statistical package (http://www.r-project.org/). Descriptive analyses were performed with all demographic and clinical variables. Mann-Whitney U test was used to compare the SIBDQ score for patients with active or remission disease activity and depression and non-depression status. Simple regression was performed with the dependent variable SIBDQ to determine which independent factors influenced HR-QOL. Simple regression analysis was also performed on four subscale dimensions (bowel, systemic, emotional, and social) of SIBDQ on BDI scores. To assess the effect of all exploratory variables, multiple linear regression analyses were performed on the global SIBDQ scores. The adaptive variable selection procedure based on Akaike's information criterion (AIC) was used to select informative variables.
Results
Demographic and Clinical Characteristics
A total of 129 patients who agreed to participate signed a standard consent form after a full explanation of study purposes and methodology. 17 patients did not return fully completed questionnaires, 5 patients did not obtain the necessary laboratory work, and 2 patients withdrew from the study leaving a final enrollment of 105 patients. The majority (n=19) of those with incomplete measurements were patients with CD who, presumably dropped out as a consequence of the required time commitment necessary to maintain a CDAI seven-day symptom diary.
The clinical and demographic characteristics of patients included in the study are shown in Table 1. Using the appropriate index to determine disease activity (CDAI for CD and SAI for UC), larger proportion of patients with active disease is observed in UC (n=34) as compared to CD (n=11). (Table 2) As shown in Table 3, 20% of CD patients and 18% of UC patients have never been treated with steroids, whereas 18% of CD patients and 32% of UC patients were taking steroids at the time. There is no statistically significant difference observed in steroid, antidepressant or other medication use between patients in remission and those with active disease. Nor is there any difference in steroid, antidepressant or other medication use between CD and UC patients.
Table 3.
Significant single factors determining quality of life (SIBDQ score) in CD and UC. Not significant (N.S.) is shown for P value > 0.1. Only variables appearing to be significant in one of three categories (all patients, patients in remission and active diseases) are shown.
| Clinical, Demographic and Psychological Factors | All Patients (CD: n = 55; UC: n = 50) | Remission (CD: n = 44; UC: n = 16) | Active Disease (CD: n = 11; UC: n = 34) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β Coefficient | R-squares | Pvalue | β Coefficient | R-squares | Pvalue | Beta Coefficient | R-squares | Pvalue | ||
| Crohn's Disease | Steroids | -6.211 | 0.0566 | 0.0445 | -5.676 | 0.0634 | 0.0545 | -20.00 | 0.296 | 0.0486 |
| Biologic | 5.708 | 0.066 | 0.0325 | 5.135 | 0.0633 | 0.0548 | N.S. | |||
| Narcotic | -10.16 | 0.132 | 0.00374 | -10.13 | 0.148 | 0.0057 | N.S. | |||
| Hospitalization | -0.464 | 0.0640 | 0.0348 | -0.420 | 0.0672 | 0.0494 | N.S. | |||
| Anti-depressant | -9.633 | 0.0997 | 0.0108 | -7.877 | 0.0802 | 0.035 | -20.00 | 0.296 | 0.0486 | |
| Anti-anxiety | -7.738 | 0.0606 | 0.0389 | N.S. | -4.361 | 0.401 | 0.0217 | |||
| BDI-II | -0.808 | 0.440 | 2.10×10-8 | -0.733 | 0.357 | 1.11E-05 | -0.767 | 0.273 | 0.0205 | |
| CDAI Score | -0.0543 | 0.182 | 0.000683 | -0.0484 | 0.0401 | 0.102 | -0.0547 | 0.0141 | 0.313 | |
| Ulcerative Colitis | Age | 0.250 | 0.0954 | 0.0166 | N.S. | 0.351 | 0.192 | 0.00553 | ||
| Disease Duration | 0.427 | 0.113 | 0.00976 | N.S. | 0.500 | 0.177 | 0.00773 | |||
| Steroids | -8.195 | 0.0893 | 0.0199 | N.S. | -7.014 | 0.0646 | 0.080 | |||
| CRP | -0.188 | 0.0637 | 0.0427 | N.S. | -0.169 | 0.0665 | 0.0766 | |||
| BDI-II | -0.782 | 0.356 | 2.93×10-6 | -0.639 | 0.509 | 0.00116 | -0.8421 | 0.382 | 5.95×10-5 | |
| SEO Index | -0.0264 | 0.0849 | 0.0227 | N.S. | N.S. | |||||
Relationship between Health-Related Quality of Life and Depression Indices
As shown in Table 2, the mean SIBDQ score in CD patients is 50.6 and in UC patients is 52.0. In patients with CD the mean SIBDQ scores are significantly lower in patients with active disease than those in remission (44.2 vs 52.2, P = 0.0125), indicating a decreased HR-QOL for patients with active CD. Similarly in patients with UC the mean SIBDQ scores are significantly lower in patients with active disease than those in remission (44.9 vs 58.5, P = 0.0262), indicating a decreased HR-QOL for patients with active UC. As shown in Table 2, the median BDI-II score for CD patients (8) is not significantly different from the median BDI-II score for those with UC (8.5). There is no significant difference in the median BDI-II score between those with active disease and those in remission for either CD or UC patients. From the BDI-II, the median BDI-PC score is calculated to be 2 for CD patients and 1.5 for UC patients. There is no significant difference in the median BDI-PC score between those with active disease and those in remission for either CD or UC patients. The BDI-II and the BDI-PC are highly correlated with each other in both CD and UC (correlation coefficient = 0.884 and 0.918, respectively).
Based on a BDI-PC cutoff of 4, the prevalence of depression (BDI-PC >= 4) is 25% (n=26) for all IBD patients. In specific, 29% (n=16) of those with CD and 20% (n=10) of those with UC met the depression criterion. The prevalence of depression is similar in those with active disease versus those in remission [29% (n=13) vs 22% (n=13)].
To understand the effect of depression and disease activity on quality of life, patients were dichotomized into depression (BDI-PC >= 4) and non-depression (BDI-PC < 4) groups; as well as patients in active disease and in remission. Overall, the patients with depression have lower quality of life SIBDQ scores as compared to non-depressed patients. This holds true no matter whether they had CD or UC, whether or not their disease is in remission or active status or both. It indicates a poorer HR-QOL for patients who are depressed regardless of their disease state or disease activity (Figure 1A and Figure 1B). All the comparisons between depressed and non-depressed patients are statistically significant except in active CD patients (Table, Supplemental Digital Content 1, http://links.lww.com/IBD/A133, rows 3, 4, 5). For example, the SIBDQ scores in depression and non-depression patients regardless of the disease activity are significantly different (P = 3.95×10-5 for CD, P = 0.00141 for UC, Mann-Whitney U test). In active CD patients, the average SIBDQ sore is not significantly lower in depressed patients as compared to non-depressed (P = 0.126), however, the mean score is over 11 points higher (mean = 50.4 vs 39.0). This might be due to the small samples size in this group (6 with depression and 5 without depression). For the effect of disease activity, SIBDQ is generally lower for patients with active disease as compared to patients in remission (P = 0.0125 for CD, P = 0.00262 for UC, Table, Supplemental Digital Content 1, http://links.lww.com/IBD/A133, row 6). The reduction is statistically weaker in separated groups of depressed or non-depressed patients (Table, Supplemental Digital Content 1, http://links.lww.com/IBD/A133, rows 1, 2). No significant difference of SIBDQ score is observed between CD patients in both depression and non-depression patients (P = 0.252 and 0.247). In UC, the quality of life for patients without depression is still significantly lower in active UC (P = 0.00139) as compared to patients with depression (P = 0.667).
Figure 1.
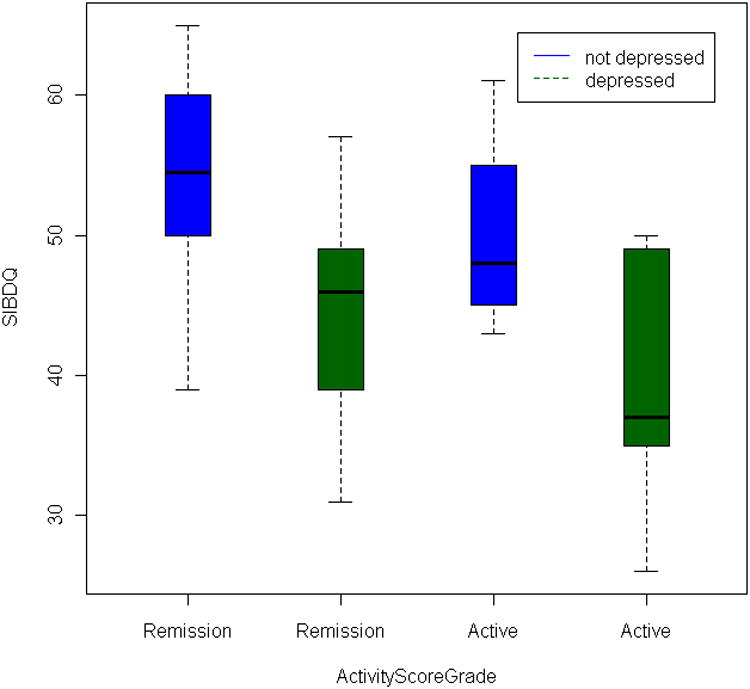
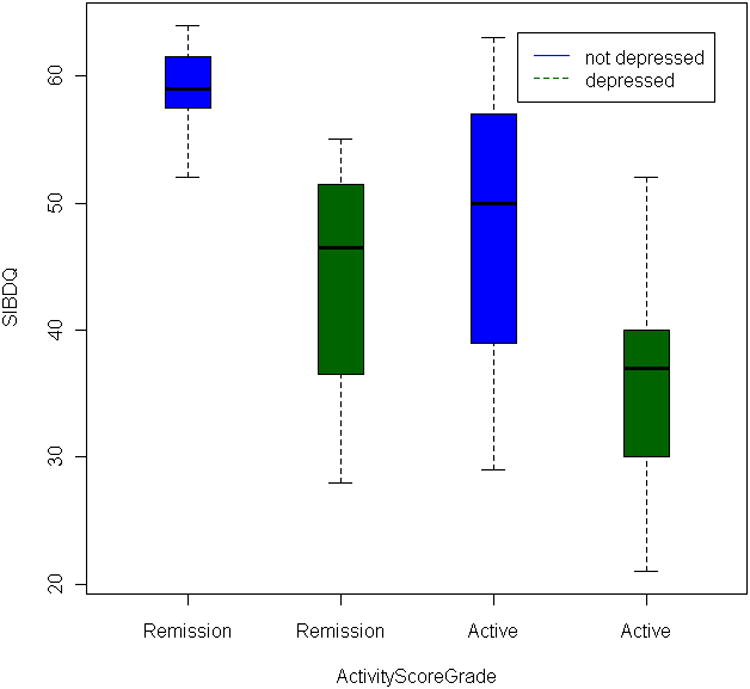
Boxplot of the effect of disease activity and depression score on SIBDQ. The y-axis displays the SIBDQ quality of life score, and x-axis displays the four categories classified by depression status and disease activity. The blue and green box shows patients without depression and with depression. The median SIBDQ score is displayed as the horizontal bar within each colored box.
A. Crohn's disease patients
B. Ulcerative Colitis patients
Factors Determining the Quality of Life
To explore the effects of other demographic, clinical and psychological variables in determining the HL-QOL, simple regression analysis was first performed on all CD and UC patients, patients in remission or active diseases, respectively. The SIBDQ score is the dependent variable. Demographic and clinical measurements including BDI-II and CDAI/SAI scores are explanatory variables. In all patients, among variables other than depression BDI score and disease activity CDAI/SAI score, narcotic use decreases SIBDQ score in CD (P = 0.00374, Table 3), and in UC, longer disease duration increases SIBDQ score (P = 0.00976, Table 4). Narcotic use also decreases SIBDQ score in CD remission patients, and longer disease duration increase SIBDQ in UC active patients. In all CD patients, current steroid use, larger number of hospitalization, anti-depressant use, anti-anxiety drug use show a trend of decreasing SIBDQ scores, while biologic use shows a trend of increasing SIBDQ score. In all UC patients, steroid use decreases SIBDQ, while increased age displays trend of increasing SIBDQ. Regarding to psychological variables, BDI-II decreases SIBDQ (P = 2.10×10-8 in CD, P = 2.93×10-6 in UC). The negative relationship is also observed in CD remission patients, as well as both UC remission and active patients. In CD active patients (n = 11), a negative relationship is also observed (P = 0.0205). The disease activity decreases the SIBDQ in CD patients (P = 0.000683), and a decreasing trend is observed in UC patients (P = 0.0227). Additionally, a significant difference of BDI scores for patients using anti-depressants or not (P = 0.324 in CD and P = 0.536 in UC) is not observed.
Table 4.
Factors determining Quality of Life (SIBDQ). Variables after backward variable selection based on AIC criterion are displayed with their β coefficient and P values. In Crohn's Disease, the adjusted R-square for multiple regression is 0.611. In Ulcerative Colitis, the adjusted R-square is 0.530.
| Crohn's Disease | Ulcerative Colitis | ||||
|---|---|---|---|---|---|
| Variables | β Coefficient | P value | Variables | β Coefficient | P value |
| Disease Location | -1.569 | 0.126 | Gender | -5.693 | 0.0211 |
| Hospitalization | -0.27 | 0.108 | Disease Duration | 0.264 | 0.0370 |
| Anti-depressant | -4.535 | 0.111 | Immunologic | 4.138 | 0.171 |
| Biologic | 5.344 | 0.00780 | BDI-II | -0.75 | 2.02×10-6 |
| Narcotic | -4.978 | 0.0741 | SEO Index | -0.02446 | 0.00492 |
| CRP | -0.091 | 0.115 | |||
| BDI-II | -0.591 | 8.22×10-6 | |||
| CDAI Score | -0.0192 | 0.110 | |||
To explore the effects of combined demographic, clinical and psychological variables in SIBDQ, multiple regression was then carried out on all available variables. For psychological measurement, BDI-II was selected as the explanatory variable, as high correlation is observed between the BDI-II and BDI-PC scores (Pearson's coefficient 0.88 in CD and 0.92 in UC). Because there are over twenty variables, a backward elimination variable selection procedure was performed so that the resulting multiple regression model reduced the number of predictor variables and achieved the highest information criterion. Table 4 shows the remaining variables after stepwise variable selection. For all CD patients, eight variables remain after variable selection, they are: disease location, hospitalization, anti-depressant use, biologic use, narcotic use, C-reactive protein (CRP) use, BDI-II and CDAI score. The proportion of the variance explained by the linear components (adjusted R square) is 61.1%, while BDI-II alone explains 44.0% (Table 4). As to statistical significance, the use of biologic agents increases SIBDQ by 5.344 (P = 0.00780), and each unit increase of BDI-II decreases SIBDQ by 0.591 (P = 8.22×10-6). For UC patients, five variables remain after variable selection, they are gender, disease duration, immunologic, BDI-II and SAI. The proportion of the variance explained by the predictor variables (adjusted R square) is 53.0% (Table 4), while BDI-II alone explains 38.2%. Statistical significant factors include BDI-II and SAI. Each unit increase of BDI-II decreased SIBDQ by 0.75 (P = 2.02×10-6), and each unit increase of SAI score decreases SIBDQ by 0.0245 (P = 0.00492).
Discussion
The heterogeneous nature of IBD makes generalizations about psychopathology and quality of life difficult. To date, few studies have taken into account the dual role of depression and disease activity as predictors of HR-QOL in patients with IBD. Our study is in alignment with previous studies that have shown psychiatric disorders being an independent risk for poor HR-QOL in IBD patients. (28) However, previous studies did not show a difference in the degree of psychiatric disturbance and its effect on HR-QOL, between patients with CD and UC, after controlling for disease activity. (28) Instead, our study demonstrates that depression may be a stronger risk factor for poor HR-QOL than disease activity alone. We demonstrate that BDI-II is the most important predictor of the SIBDQ score. In our IBD samples, patients with depression have a significantly worse HR-QOL and this difference remain true regardless of whether or not patients have CD or UC, have active disease, or are in remission. Although disease activity is an important contributor to HR-QOL, depression is the most important predictor of the HR-QOL. A recent clinical review supports the general belief that the presence of IBD is associated with a higher prevalence of depression. (12) In our study, there is a high prevalence – one-fourth - of depression in our cohort that did not differ on the basis of disease type or other basic demographic factors. Our findings are consistent with other studies that demonstrate an increased risk of depression in patients with IBD similar to other chronic diseases. (29-33) Comparative studies have typically reported higher levels of anxiety or depression in IBD patients with active disease than those in remission. (34-37) Similar to a recent study utilizing the Hospital Anxiety and Depression Scale (HADS) (20), our study did not detect a significant difference in depressive symptoms or depression prevalence between patients in clinical remission or with active disease. Unlike HADS, BDI-II used in our study is particularly useful because it quantifies the severity of depression using both somatic and affective components. (38)
Strengths of the current study are the well-validated patient-based indices used to quantify depressive symptoms and HR-QOL that are easy to adapt to a busy clinical practice. Depression is difficult to accurately assess in patients with chronic gastrointestinal disorders as many current instruments may be influenced by the disease process itself, i.e., fatigue, sleep disturbance, weight loss and/or changes in appetite. The BDI-II has been validated in medically ill patients including those with chronic gastrointestinal disorders and the BDI-PC is a validated screen for depression using non-somatic criteria. In our study the BDI-II and the BDI-PC correlated very well together, making use of the BDI-PC a practical tool to use in busy outpatient IBD clinics.
Yet all HR-QOL instruments including disease specific items have shortcomings. First, questionnaires with a large number of items are time consuming and difficult to integrate into clinical practice, whereas shorter scales such as the SIBDQ may be easier to complete but have reduced sensitivity to detect clinically significant change. Second, since HR-QOL was assessed by a disease-specific index, the SIBDQ, an overlap could exist between disease activity, depression and HR-QOL that might artificially magnify the correlation between these factors thereby limiting our ability to detect other dependent factors. To this end, a generic HR-QOL life measure such as SF-8 might be a future alternative measure (39) to explore the extent of confounding between HR-QOL, SIBDQ and other factors. Lastly, the SIBDQ consists of four subphenotypes – bowel, systemic, social and emotional – and it is possible that heterogeneity among these four subphenotypes might differently impact the BDI score, i.e., the emotional subscore might be more influential for measuring depression. Therefore, we did a regression analysis, using the subphenotype score as the dependent variable and BDI-II score as the predictor. As seen in Table, Supplemental Digital Content 2, http://links.lww.com/IBD/A134 all of the four subphenotypes as well as the total SIBDQ for both CD and UC vs BDI-II are statistically significant, indicating that the BDI affects all components of the SIBDQ and not just the emotional component. This reiterates the importance of the influence of the BDI-II on the SIBDQ and therefore HR-QOL. In addition to limitations of HR-QOL measurements, we noted that of 24 patients who dropped out for various reasons, 19 CD patients dropped out presumably because they failed to mail back a 7 day symptom diary necessary to compute the CDAI score. The uncertainty of these dropped out patients' wellbeing and depression score may pose bias in the analysis. In regards to the measurement standards of disease activity, activity indices (CDAI and SAI) were chosen due to ease, non-invasiveness and prior validation. In recent years, however, endoscopic evidence of disease becomes a standard medical practice in determining the disease activity. Therefore, endoscopic mucosal visualization for remission and disease activity assessment is necessary for future studies in this direction.
We focus on disease activity and depression in determining the HR-QOL in this study. Other than disease activity and depression, demographic and clinical factors might be associated with HR-QOL, albeit in a lesser degree. However, under certain categories, small sample size may limit the power to identify association signals. For instance, narcotic use is significant in all CD patients and patients in remission (R-square 0.132 and 0.148, P = 0.00374 and 0.0057), but not in active disease patients (Table 3). In fact, a detailed power calculation shows that for an R-square value of 0.16, the power with 15 samples (similar to the number of active disease CD patients) is 18.6%; while the power in 50 and 40 samples (similar to all CD patients and remission patients) are 68.8% and 56.9% (Table, Supplemental Digital Content 3, http://links.lww.com/IBD/A135). Therefore, larger sample size may be needed for studies with adequate power to detect other demographic and clinical factors associated with HR-QOL.
All patients enrolled in this study were seen in an IBD clinic at a tertiary care center. Patients with a complicated course of disease may be over represented in our patient population thereby decreasing the generalizability of our results. That being said, the majority of patients were in clinical remission and only a small proportion had severe disease as determined by their respective activity index score. Furthermore, the proportion of patients found to have depressive symptoms and the mean SIBDQ scores are comparable to previous studies enrolled from tertiary and non-tertiary centers. (34, 35)
As measures of HR-QOL become a common clinical outcome, it is important to further investigate the role of factors beyond disease activity, especially those that may be modifiable and thus appropriate targets for intervention. Like all studies with a cross-sectional design, it is impractical to infer causal effects between disease severity, psychiatric disorders and HR-QOL. Despite this, our results provide further evidence that depression is a common co-morbidity in patients with IBD, and its presence contributes to a decreased HR-QOL. There is a need for well-designed and properly controlled longitudinal prospective studies to adequately clarify the relationship between psychological co-morbidities and IBD.
It is becoming clearer that physicians who evaluate and treat patients with IBD should focus on the presence of symptoms beyond those corresponding solely to disease activity. Screening for depression may be part of routine clinical evaluation by gastroenterologists. We believe that assessment of psychological disorders in the gastrointestinal clinic along with appropriate management will improve the quality of care provided to our patients and ultimately may result in improved HR-QOL for them.
Supplementary Material
References
- 1.Kucharzik T, Maaser C, Lugering A, et al. Recent understanding of IBD pathogenesis: Implications for future therapies. Inflammatory Bowel Diseases. 2006;12:1068–1083. doi: 10.1097/01.mib.0000235827.21778.d5. [DOI] [PubMed] [Google Scholar]
- 2.Bernklev T, Jahnsen J, Lygren I, et al. Health-related quality of life in patients with inflammatory bowel disease measured with the short form-36: Psychometric assessments and a comparison with general population norms. Inflammatory Bowel Diseases. 2005;11:909–918. doi: 10.1097/01.mib.0000179467.01748.99. [DOI] [PubMed] [Google Scholar]
- 3.Patten SB, Beck CA, Kassam A, et al. Long-term medical conditions and major depression: Strength of association for specific conditions in the general population. Canadian Journal of Psychiatry-Revue Canadienne De Psychiatrie. 2005;50:195–202. doi: 10.1177/070674370505000402. [DOI] [PubMed] [Google Scholar]
- 4.Scott KM, Bruffaerts R, Tsang A, et al. Depression-anxiety relationships with chronic physical conditions: Results from the World Mental Health surveys. Journal of Affective Disorders. 2007;103:113–120. doi: 10.1016/j.jad.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 5.Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions' of DSM-IV disorders in the national comorbidity survey replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan MD, LaCroix AZ, Baum C, et al. Functional status in coronary artery disease: A one-year prospective study of the role of anxiety and depression. American Journal of Medicine. 1997;103:348–356. doi: 10.1016/s0002-9343(97)00167-8. [DOI] [PubMed] [Google Scholar]
- 7.Kessler RC, Ormel J, Demler O, et al. Comorbid mental disorders account for the role impairment of commonly occurring chronic physical disorders: Results from the national comorbidity survey. Journal of Occupational and Environmental Medicine. 2003;45:1257–1266. doi: 10.1097/01.jom.0000100000.70011.bb. [DOI] [PubMed] [Google Scholar]
- 8.Porcelli P, Zaka S, Centonze S, et al. Psychological distress and levels of disease activity in Inflammtory Bowel Disease. Italian Journal of Gastroenterology. 1994;26:111–115. [PubMed] [Google Scholar]
- 9.Porcelli P, Leoci C, Guerra V. A prospective study of the relationship between disease activity and psychologic distress in patients with inflammatory bowel disease. Scandinavian Journal of Gastroenterology. 1996;31:792–796. doi: 10.3109/00365529609010354. [DOI] [PubMed] [Google Scholar]
- 10.Vanhemert AM, Hengeveld MW, Bolk JH, et al. Psychiatric disroders in relation to medical illness among patients of a general medical outpatient clinic. Psychological Medicine. 1993;23:167–173. doi: 10.1017/s0033291700038952. [DOI] [PubMed] [Google Scholar]
- 11.Leue C, van Os J, Neeleman J, et al. Bidirectional associations between depression/anxiety and bowel disease in a population based cohort. Journal of Epidemiology and Community Health. 2005;59:434–435. doi: 10.1136/jech.2004.028175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graff LA, Walker JR, Bernstein CN. Depression and Anxiety in Inflammatory Bowel Disease: A Review of Comorbidity and Management. Inflammatory Bowel Diseases. 2009;15:1105–1118. doi: 10.1002/ibd.20873. [DOI] [PubMed] [Google Scholar]
- 13.Mawdsley JE, Rampton DS. Psychological stress in IBD: New insights into pathogenic and therapeutic implications. Gut. 2005;54:1481–1491. doi: 10.1136/gut.2005.064261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schipper H, Clinch J, Powell V. Definitions and conceptual issues. Spilker, B. 1990:11–24. [Google Scholar]
- 15.Rubin GP, Hungin APS, Chinn DJ, et al. Quality of life in patients with established inflammatory bowel disease: a UK general practice survey. Alimentary Pharmacology & Therapeutics. 2004;19:529–535. doi: 10.1111/j.1365-2036.2004.1873.x. [DOI] [PubMed] [Google Scholar]
- 16.Blondel-Kucharski F, Chircop C, Marquis P, et al. Health-related quality of life in Crohn's disease: A prospective longitudinal study in 231 patients. American Journal of Gastroenterology. 2001;96:2915–2920. doi: 10.1111/j.1572-0241.2001.4681_b.x. [DOI] [PubMed] [Google Scholar]
- 17.Casellas F, Arenas JI, Baudet JS, et al. Impairment of health-related quality of life in patients with inflammatory bowel disease: A Spanish multicenter study. Inflammatory Bowel Diseases. 2005;11:488–496. doi: 10.1097/01.mib.0000159661.55028.56. [DOI] [PubMed] [Google Scholar]
- 18.Han SW, McColl E, Barton JR, et al. Predictors of quality of life in ulcerative colitis. Inflammatory Bowel Diseases. 2005;11:24–34. doi: 10.1097/00054725-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Sainsbury A, Heatley RV. Review article: psychosocial factors in the quality of life of patients with inflammatory bowel disease. Alimentary Pharmacology & Therapeutics. 2005;21:499–508. doi: 10.1111/j.1365-2036.2005.02380.x. [DOI] [PubMed] [Google Scholar]
- 20.Vidal A, Gomez-Gil E, Sans M, et al. Health-related quality of life in inflammatory bowel disease patients: The role of psychopathology and personality. Inflammatory Bowel Diseases. 2008;14:977–983. doi: 10.1002/ibd.20388. [DOI] [PubMed] [Google Scholar]
- 21.Lennard-Jones JE. Classification of inflammatory bowel disease. Scandinavian journal of gastroenterology Supplement. 1989;170:2–6. doi: 10.3109/00365528909091339. discussion 16-19. [DOI] [PubMed] [Google Scholar]
- 22.Vanhees PAM, Vanelteren PH, Vanlier HJJ, et al. An index of inflammatory activity in patients with Crohn's disease. Gut. 1980;21:279–286. doi: 10.1136/gut.21.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seo M, Okada M, Yao T, et al. An index of disease activity in patients with ulcerative colitis. American Journal of Gastroenterology. 1992;87:971–976. [PubMed] [Google Scholar]
- 24.Seo M, Okada M, Yao T, et al. Evaluation of disease activity in patients with moderatey active ulcerative colitus: comparisons between a new activity index and Truelove and Witts' classification. American Journal of Gastroenterology. 1995;90:1759–1763. [PubMed] [Google Scholar]
- 25.Irvine EJ, Zhou Q, Thompson AK, et al. The short inflammatory bowel disease questionnaire: A quality of life instrument for community physicians managing inflammatory bowel disease. American Journal of Gastroenterology. 1996;91:1571–1578. [PubMed] [Google Scholar]
- 26.Beck AT, Guth D, Steer RA, et al. Screening for major depression disorders in medical inpatients with the Beck Depression Inventory for Primary Care. Behaviour Research and Therapy. 1997;35:785–791. doi: 10.1016/s0005-7967(97)00025-9. [DOI] [PubMed] [Google Scholar]
- 27.Steer RA, Cavalieri TA, Leonard DM, et al. Use of the Beck Depression Inventory for Primary Care to screen for major depression disorders. General Hospital Psychiatry. 1999;21:106–111. doi: 10.1016/s0163-8343(98)00070-x. [DOI] [PubMed] [Google Scholar]
- 28.Guthrie E, Jackson J, Shaffer J, et al. Psychological disorder and severity of inflammatory bowel disease predict health-related quality of life in ulcerative colitis and Crohn's disease. American Journal of Gastroenterology. 2002;97:1994–1999. doi: 10.1111/j.1572-0241.2002.05842.x. [DOI] [PubMed] [Google Scholar]
- 29.Lashner B. In: Disease Management Project: Inflammatory Bowel Disease. Clinic TC, editor. 2005. [Google Scholar]
- 30.Sheffield BF, Carney MWP. Crohn's Disease, psychosomatic illness. British Journal of Psychiatry. 1976;128:446–450. doi: 10.1192/bjp.128.5.446. [DOI] [PubMed] [Google Scholar]
- 31.Blazer DG, Kessler RC, McGonagle KA, et al. The prevalence and distribution of major depression in a national community sample: the national comorbidity survey. American Journal of Psychiatry. 1994;151:979–986. doi: 10.1176/ajp.151.7.979. [DOI] [PubMed] [Google Scholar]
- 32.Cavanaugh S, Clark DC, Gibbons RD. Diagnosing depression in the hospitalized medically ill. Psychosomatics. 1983;24:809–815. doi: 10.1016/S0033-3182(83)73151-8. [DOI] [PubMed] [Google Scholar]
- 33.Rodin G, Craven J, Littlefield C. Depression in the medically ill : an integrated approach. New York: Brunner/Mazel; 1991. [Google Scholar]
- 34.Calvet X, Gallardo O, Coronas R, et al. Remission on thiopurinic immunomodulators normalizes quality of life and psychological status in patients with Crohn's disease. Inflammatory Bowel Diseases. 2006;12:692–696. doi: 10.1097/00054725-200608000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Levenstein S, Prantera C, Varvo V, et al. Psychological stress and disease activity in ulcerative colitis: a multidimensional cross-sectional study. American Journal of Gastroenterology. 1994;89:1219–1225. [PubMed] [Google Scholar]
- 36.Simren M, Axelsson J, Gillberg R, et al. Quality of life in inflammatory bowel disease in remission: The impact of IBS-like symptoms and associated psychological factors. American Journal of Gastroenterology. 2002;97:389–396. doi: 10.1111/j.1572-0241.2002.05475.x. [DOI] [PubMed] [Google Scholar]
- 37.Maunder RG, Greenberg GR, Hunter JJ, et al. Psychobiological subtypes of ulcerative colitis: pANCA status moderates the relationship between disease activity and psychological distress. American Journal of Gastroenterology. 2006;101:2546–2551. doi: 10.1111/j.1572-0241.2006.00798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 39.Fukuhara S, Suzukamo Y. Manual of the SF-8 Japanese edition. Kyoto: Institute for Health Outcomes & Process Evaluation Research; 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


