Abstract
Antidepressant induced increases in neurogenesis and neurotrophin mobilization in rodents and primates are proposed to be necessary for behavioral efficacy. The current study examines the relationship between the effects of fluoxetine treatment on behavior, cell proliferation and the neurotrophin BDNF in females. Female MRL/MpJ mice were treated acutely (5 and 10 mg/kg) or chronically (2.5, 5 and 10 mg/kg b.i.d.) with fluoxetine and tested in the tail suspension test (TST) and or novelty induced hypophagia test (NIH) respectively. Mice treated chronically with fluoxetine received 4 (100 mg/kg) injections of 5-bromo-2′-deoxyuridine (BrdU) on the last 4 days of treatment to measure DNA synthesis. The other half of the hippocampus and the frontal cortex were removed and examined for BDNF levels. Fluoxetine treatment decreased immobility in the TST and latency to eat in the NIH test, but only the highest dose of fluoxetine significantly altered behavior in both tests. Chronic treatment with 5 and 10 mg/kg of fluoxetine significantly increased cell proliferation and BDNF levels in the hippocampus. Only chronic treatment with the highest of fluoxetine increased BDNF levels in the frontal cortex. Behavioral measures in the NIH test correlated with BDNF levels in the frontal cortex but not in the hippocampus or with cell proliferation in the hippocampus. These data suggest that females require high doses of fluoxetine for behavioral efficacy regardless of elevations of neurogenesis and BDNF mobilization in the hippocampus. Elevations in BDNF levels in the frontal cortex are related to the behavioral efficacy of fluoxetine.
Keywords: Neurogenesis, antidepressants, sex differences, depression, behavior
Female mice treated with high doses of fluoxetine (10 mg/kg) produce more new cells in the hippocampus than males [14]. The behavioral ramifications of this increased level of cell proliferation remain to be determined. Antidepressants are thought to alter depression and anxiety associated behavior through their actions on cell proliferation and neurotrophin mobilization [10, 24]. Women have a 2-fold higher incidence of depression than men [18]. The majority of experiments examining the biological basis of depression and pharmacological agents are conducted in male animals [17]; therefore it remains to be determined whether changes in adult neurogenesis and neurotrophin mobilization are involved with behavioral antidepressant efficacy in females.
A variety of behavioral tests have been used to measure the effects of acute and chronic antidepressant treatments in rodents [9]. The novelty induced hypophagia test / novelty suppressed feeding test has good face validity as it requires chronic treatment with antidepressants to induce behavioral alterations which are associated with decreases in anxiety. Originally it was reported that irradiating the hippocampus to block cell proliferation also blocked the ability of antidepressants to decrease the latency to consume food in the novelty suppressed feeding test [25]. However, recent studies indicate that whether or not cell proliferation is necessary for behavioral efficacy in this test seems to be strain [16] and species dependent [5]. Antidepressant efficacy can also be measured with tests which only require acute treatment. Both the forced swim test and tail suspension test show good predictive value as treatment with a number of antidepressants decrease immobility in both tests [9].
The current study examined the behavioral effects of antidepressants in female mice by measuring the effects following acute and chronic treatment with the selective serotonin reuptake inhibitor (SSRI) fluoxetine. These studies used female mice from the MRL/MpJ strain because male MRL/MpJ mice are highly responsive to the neurogenic and behavioral effects of chronic antidepressant treatments [3]. The behavioral responses to acute and chronic fluoxetine were examined using the tail suspension test and the novelty induced hypophagia test, respectively. The effects of chronic fluoxetine treatment on cell proliferation in the hippocampus and BDNF levels in the hippocampus and frontal cortex were also measured in the same individuals and then used to examine biological correlates to chronic treatment effects.
Materials and Methods
Animals were experimentally naïve adult female MRL/MpJ mice (Jackson Laboratories, Bar Harbor, ME, USA) age 8–10 weeks at the beginning of all studies. Male MRL/MpJ mice have an enhanced capacity for regeneration without scarring [13] and behavioral and cellular sensitivity to the effects of both acute and chronic antidepressant treatment [2] making this mouse strain ideal for modeling antidepressant efficacy. Mice were pair housed for all experiments except the TST study in which they were group housed (5 to a cage). In all studies mice were housed in polycarbonate cages and maintained on a 12-h light/dark cycle (lights on at 07:00 hours) in a temperature (22°C) and humidity-controlled colony. Animals were given ad libitum access to food and water. Adequate measures were taken to minimize pain and discomfort to the animals and all procedures were in conducted in accordance with the guidelines published in the NIH Guide for Care and Use of Laboratory Animals. All protocols were approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
Drugs were given by intraperitoneal (i.p.) injection. Fluoxetine hydrochloride was purchased from Anawa (Zurich, Switzerland) and prepared fresh daily. The doses were calculated according to the base weight of the drug and administered in a volume of 10 ml/kg. 5-Bromo-2-deoxyuridine (BrdU; Roche Applied Sciences Indianapolis, IN) was dissolved in warm saline and administered by i.p. injection at a volume of 10 ml/kg. Mice were administered injections of 0.9% saline or fluoxetine (2. 5, 5 or 10 mg/kg) twice daily. Fluoxetine was given twice daily because due to its long half –life this dosing strategy results in relatively stable plasma levels [14].
For the cell proliferation, BDNF, and NIH study, animals (n = 60; 10 per dose) received antidepressant treatment for 26 days. To conduct the NIH study, mice were first trained to consume Reese’s peanut butter chips from a plastic petri dish. Training sessions (15 min-first exposure, 5 min - all subsequent exposures) were conducted in the home cage with a divider separating the pair. Mice received 4 training sessions prior to antidepressant treatment and 2 training sessions at the end of antidepressant treatment. After 21 days of antidepressant treatment, the latency to eat in the home cage was recorded. The following day, mice were tested in a novel cage environment. The novel environment differed from the home cage via room location, lighting, smell and lack of bedding. Mice were placed into the novel cage for 5 min and latency to eat was recorded. Immediately after exposure to the novel cage, mice received the first of 4 once daily injections of BrdU (100 mg/kg). Mice were sacrificed 24 hours after the last injection.
For the tail suspension test study a different cohort of mice (n= 30; 10 per dose) were given a single injection of fluoxetine (5 and 10 mg/kg). Mice were injected with their first dose of antidepressant or saline and were tested 30 min later in an automated TST device (Med Associates, St Albans, VT). Mice were suspended by their tails with tape from an aluminum bar connected to a strain gauge for 6 min. The duration of immobility was calculated as the time the force of the animal’s movements were below a preset threshold (breathing only). Optimum thresholds were originally determined by comparing manually scored videotapes with automated scores [8].
Labeling of BrdU was measured in cells displaying the nuclear marker 7-aminoactinomycin D (7-AAD) by flow cytometry as previously described and validated [3, 4]. Mice were decapitated, their brains quickly removed, and the hippocampus and frontal cortex were dissected out. For the hippocampus only the right lobe was analyzed, as cell counts have not been shown to differ between hemispheres [3]. The hippocampus was minced, and digested using an enzymatic cocktail (0.5 mL, 1 mg/mL papain, Roche Applied Sciences Indianapolis, IN; 0.1 M L-cysteine, Sigma St. Louis, MO) and incubated in a dry heat block at 37°C for 15 minutes. Enzymatic digestion was blocked using Hibernate-A (Brain Bits Springfield, IL) containing 10% heat-inactivated fetal bovine serum (FBS; Gibco Grand Island, NY). Tissue was then mechanically triturated to form a single cell suspension and spun in a centrifuge at 2000 rpm for 5 min.
The supernatant was removed and the resultant cells were then fixed and stained using the FITC BrdU Flow Kit (BD Biosciences San Jose, CA). Prior to analysis, cells were filtered through a cell strainer cap (30 μm) to remove debris. The data was collected on a BD FACS Canto system at the University of Pennsylvania Flow Cytometry Core Facility. Background signal was controlled for by staining tissue from animals that had not been injected with BrdU. All data was collected an analyzed using BD FACSDiva software (BD Biosciences, San Jose, CA).
The left unilateral hippocampus and the whole frontal cortex were used to measure BDNF levels 24 hr after cessation of antidepressant treatment. Tissue was flash frozen in isopentane and placed in at freezer at −80° C until analysis. BDNF protein levels were quantified using a commercially available sandwich ELISA kit (Promega, Madison, WI). The tissue was homogenized in 0.75 mL of lysis buffer (100 mM PIPES pH 7.0, 500 mM NaCl, 2 mM EDTA, 0.1% sodium azide, 2% bovine serum albumin, 0.2% Triton X-100, 5 μg/mL aprotinin, 0.1μg/mL pepstatin A, 0.5 μg/mL antipain). The homogenate was centrifuged at 13,000 rpm for 30 min at 4°C. The supernatant was removed and the amount of BDNF protein in each sample was measured from the supernatant in duplicate by ELISA following the manufactures instructions. BDNF levels were normalized to the wet tissue weighs.
Statistical analysis was performed using analysis of variance (ANOVA) and post hoc analysis was performed using Newman-Keuls when appropriate. All analyses were performed as one way ANOVAs using Statistica software (StatSoft Inc, Tulsa, OK).
Results
Acute treatment with fluoxetine significantly reduced the duration of immobility in the tail suspension test [F 2,20 = 3.28, p < 0.05]. Only 10 mg/kg significantly reduced immobility compared to saline treated controls (p = 0.05) (Fig 1a).
Figure 1.
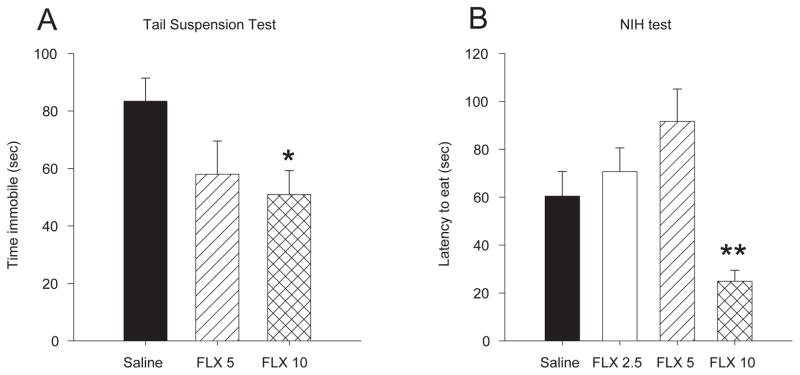
Dose dependent effects of fluoxetine treatment on depression associated behaviors in female mice. A) Acute treatment with 10 mg/kg but not 5 mg/kg of fluoxetine reduced immobility in the tail suspension test. B) Chronic treatment with 10 mg/kg/day but not 5 mg/kg/day of fluoxetine decreased latency to eat in the novel cage during the NIH test. Bars represent mean values ± SEM. Asterisk (*) denotes significant differences, multiple asterisks indicate level of significance (* p < 0.05, ** p < 0.01).
Chronic treatment with fluoxetine reduced the latency to eat in the NIH test [F 3, 32 = 7.97, p < 0.001]. Mice treated with 10 mg/kg/day had shorter latencies to eat in a novel environment than all other doses and saline treated controls (p values < 0.001) (Fig 1b). Fluoxetine treatment did not alter latency to eat in the home cage [F 3, 34 = 1.00, p > 0.05] or the amount eaten in the home cage [F 3, 36 = 0.35, p > 0.05] or novel cage [F 3, 36 = 1.60, p > 0.05].
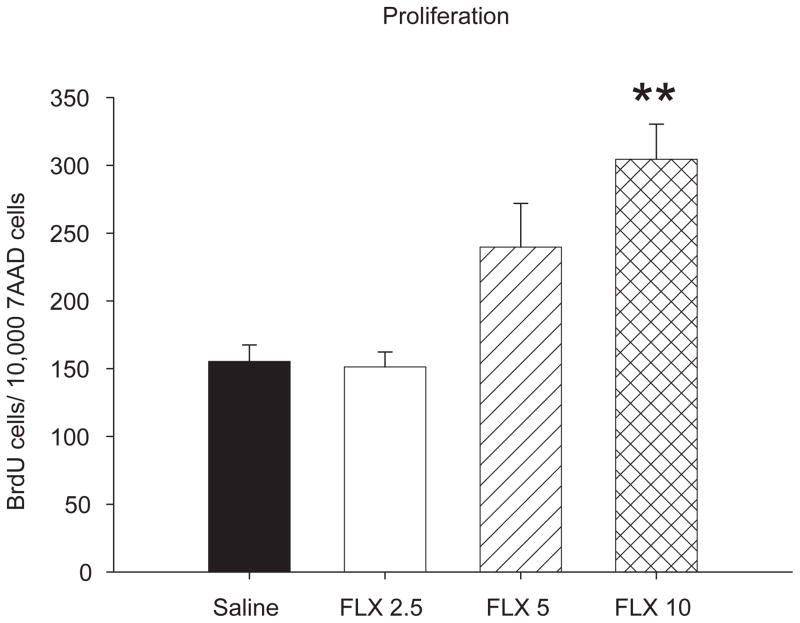
Cell proliferation in the hippocampus was increased following chronic treatment with fluoxetine [F 3,34 = 9.73, p < 0.001] (Fig. 2). Post hoc analysis indicated that treatment with 5 mg/kg/day of fluoxetine caused a 1.5-fold increase in cell proliferation compared to animals given saline or treated with 2.5 mg/kg fluoxetine (p values < 0.05). Chronic treatment with 10 mg/kg/day of fluoxetine resulted in a 1.8-fold increase in cell proliferation above saline controls or animals treated with 2.5 mg/kg fluoxetine (p values < 0.001). Changes in cell proliferation did not correlate with latency to eat in the novel cage of the NIH test [r = −0.195, p > 0.05] or the amount of chips eaten [r = −0.001, p > 0.05].
Figure 2.
Chronic treatment with fluoxetine produced dose dependent patterns of cell proliferation in the hippocampus of female mice. Treatment with 5 and 10 mg/kg/day increased cell proliferation when compared to saline treated animals and mice treated with 2. 5 mg/kg/day. Bars represent mean values ± SEM. Asterisk (*) denotes significant differences, multiple asterisks indicate level of significance (* p < 0.05, ** p < 0.01).
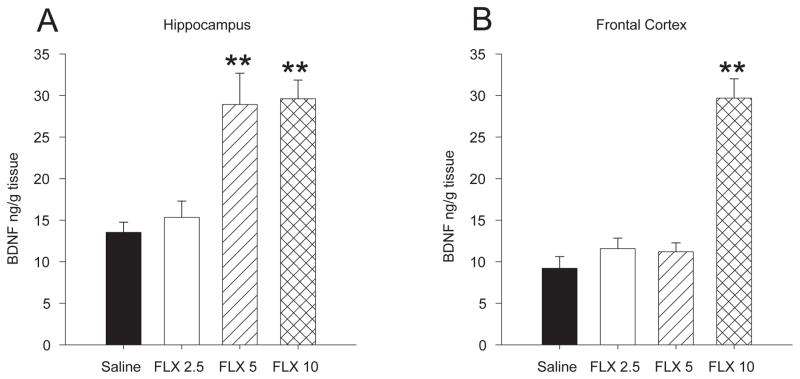
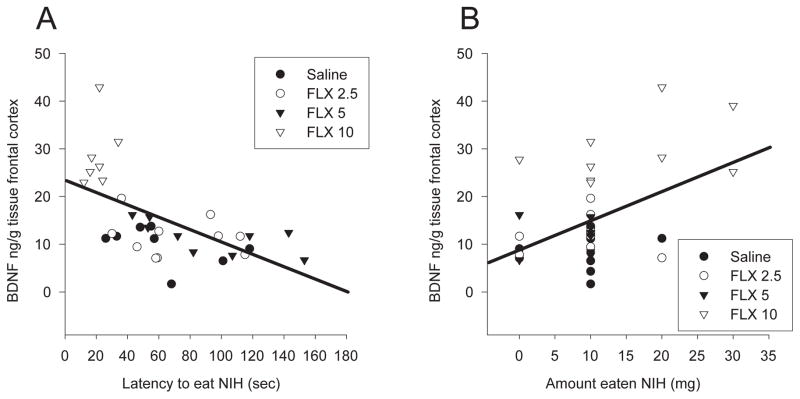
Chronic fluoxetine treatment also increased BDNF levels in the hippocampus [F 3,36 = 12.03, p < 0.001] and the frontal cortex [F 3, 34 = 36.29, p < 0.001]. In the hippocampus, treatment with both 5 and 10 mg/kg/day resulted in a 2-fold increase in BDNF levels compared to animals treated with saline or 2.5 mg/kg fluoxetine (p values < 0.001) (Fig. 3a). In the frontal cortex 10 mg/kg produced a significant ~ 3-fold increase in BDNF levels compared to all other groups (p values < 0.001) (Fig. 3b). BDNF levels in the hippocampus did not correlate with latency to eat in the novel cage of the NIH test [r = −0.189, p > 0.05] or the amount eaten [r = 0.127, p > 0.05]. However, BDNF levels in the frontal cortex correlated with both latency to eat in the novel cage [r = −0.591, p < 0.05] (Fig 4a) and the amount eaten [r = 0.467, p < 0.05] (Fig. 4b) for the NIH test.
Figure 3.
Chronic treatment with fluoxetine increased BDNF levels in the hippocampus and frontal cortex of female mice in a dose dependent manner. A) Treatment with 5 and 10 mg/kg/day increased BDNF levels in the hippocampus of female mice. B) Only the 10 mg/kg/day dose increased BDNF levels in the frontal cortex of female mice. Bars represent mean values ± SEM. Asterisk (*) denotes significant differences, multiple asterisks indicate level of significance (* p < 0.05, ** p < 0.01).
Figure 4.
BDNF levels in the frontal cortex correlate with behavior in the NIH test. A) Female mice with higher levels of BDNF in the frontal cortex had a shorter latency to eat in the novel cage of the NIH test. B) Females with higher levels of BDNF in the frontal cortex ate more peanut butter chips in the novel cage of the NIH test.
Discussion
Treatment with 10 mg/kg of fluoxetine reduced immobility in the tail suspension test, and continued treatment with 10 mg/kg/day for 21 days decreased the latency to eat a palatable food in the novelty induced hypophagia test, indicating that females were only behaviorally responsive to high doses of fluoxetine. Chronic treatment of female mice with fluoxetine elevated cell proliferation in a dose dependent manner. Both 5 and 10 mg/kg/day of fluoxetine increased cell proliferation whereas a low dose (2.5 mg/kg/day) did not. The highest dose of fluoxetine (10 mg/kg/day) produced larger increases in cell proliferation than any other dose used. It has been established that around 80–90% of new cells generated in the hippocampus develop a neuronal phenotype 28 days after labeling [6, 7, 11, 20] suggesting that the majority of proliferating cells measured in this region would become neurons. Changes in cell proliferation were accompanied by increases in BDNF levels. Both 5 and 10 mg/kg/day of fluoxetine, the doses that increased cell proliferation also increased BDNF levels in the hippocampus. Only the highest dose (10 mg/kg/day) altered BDNF levels in the frontal cortex. These alterations in BDNF mobilization in the frontal cortex were the only measures to correlate with behavior in the NIH test. Therefore, these data dissociate the effects of antidepressants on cell proliferation and BDNF levels in the hippocampus from behavioral efficacy, as the 5 mg/kg dose of fluoxetine which was sufficient to increase cell proliferation and BDNF levels in the hippocampus was not sufficient to alter behavior.
Previously it has been reported that male MRL/MpJ mice respond with the greatest increase in cell proliferation to the 5 mg/kg/day dose and that this dose was also sufficient to decrease latency to eat in the NIH test. [3]. While the current study did not directly compare males and females, it suggests that there may be sex differences in both the effects of fluoxetine on behavior and hippocampal neurogenesis, which should be examined in future studies. Sex differences in the behavioral effects of fluoxetine may be due to sex differences in the metabolism of fluoxetine. Female MRL/MpJ mice had larger ratios of the metabolite norfluoxetine to fluoxetine and developed higher circulating levels of norfluoxetine in the brain and plasma following chronic treatment with fluoxetine [14]. With a longer half-life than fluoxetine, norfluoxetine can maintain increased extracellular serotonin levels via serotonin transporter inhibition and may be a mechanism for these behavioral effects [23]. Serotonin transporter knock out rats have decreased levels of BDNF in the frontal cortex and hippocampus. [21]. BDNF in the forebrain is necessary for the efficacy of antidepressants in acute behavioral tests in both sexes, such as the forced swim test, and conditional genetic deletion of BDNF in the forebrain leads to the development of anhedonia specifically in female mice [22].
There has been a lack of agreement in the literature on the effects of antidepressants on neurogenesis in females. Some studies have found that doses that are sufficient to increase cell proliferation in males do not increase cell proliferation in females [12, 15] whereas others have found increases in cell proliferation in females occur when higher doses of antidepressants are used [1, 19]. The current study is the first in females to examine the relationship between the dose dependent effects of fluoxetine treatment on cell proliferation, neurotrophins and behavior. These data suggest that some of the inconsistencies in the literature are likely due to different dosing strategies, although strain and species differences may also contribute.
Research Highlights.
High doses but not low doses of fluoxetine increase neurogenesis in the hippocampus of female mice.
High doses but not low doses of fluoxetine increase BDNF protein levels in the hippocampus and frontal cortex of female mice.
The highest dose of fluoxetine tested (10 mg/kg/day) altered depression associated behavior in female mice, but lower doses did not alter behavior.
The effects of fluoxetine on depression associated behavior in females correlated with BDNF protein levels in the frontal cortex, but not BDNF protein levels or neurogenesis in the hippocampus.
Acknowledgments
This research was supported by USPHS grant MH86599. The authors thank Darrick T. Balu for his assistance with the flow cytometry and behavioral techniques. The authors also thank Charles H. Pletcher Jr. at the University of Pennsylvania Flow cytometry Center for assistance with the flow cytometry techniques used in this paper
References
- 1.Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science. 2007;317:819–823. doi: 10.1126/science.1144400. [DOI] [PubMed] [Google Scholar]
- 2.Balu DT. Enhanced sensitivity of the MRL/MpJ mouse to the neuroplastic and behavioral effects of acute and chronic antidepressant treatments. Exp Clin Psychopharmacol. 18:71–77. doi: 10.1037/a0017295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balu DT, Hodes GE, Anderson BT, Lucki I. Enhanced sensitivity of the MRL/MpJ mouse to the neuroplastic and behavioral effects of chronic antidepressant treatments. Neuropsychopharmacology. 2009;34:1764–1773. doi: 10.1038/npp.2008.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balu DT, Hodes GE, Hill TE, Ho N, Rahman Z, Bender CN, Ring RH, Dwyer JM, Rosenzweig-Lipson S, Hughes ZA, Schechter LE, Lucki I. Flow cytometric analysis of BrdU incorporation as a high-throughput method for measuring adult neurogenesis in the mouse. J Pharmacol Toxicol Methods. 2009;59:100–107. doi: 10.1016/j.vascn.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, Palha JA, Almeida OF, Sousa N. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry. 2009;14:764–773. 739. doi: 10.1038/mp.2008.119. [DOI] [PubMed] [Google Scholar]
- 6.Biebl M, Cooper CM, Winkler J, Kuhn HG. Analysis of neurogenesis and programmed cell death reveals a self-renewing capacity in the adult rat brain. Neurosci Lett. 2000;291:17–20. doi: 10.1016/s0304-3940(00)01368-9. [DOI] [PubMed] [Google Scholar]
- 7.Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- 8.Crowley JJ, Jones MD, O’Leary OF, Lucki I. Automated tests for measuring the effects of antidepressants in mice. Pharmacol Biochem Behav. 2004;78:269–274. doi: 10.1016/j.pbb.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 9.Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- 10.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Encinas JM, Vaahtokari A, Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci U S A. 2006;103:8233–8238. doi: 10.1073/pnas.0601992103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engesser-Cesar C, Anderson AJ, Cotman CW. Wheel running and fluoxetine antidepressant treatment have differential effects in the hippocampus and the spinal cord. Neuroscience. 2007;144:1033–1044. doi: 10.1016/j.neuroscience.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 13.Heber-Katz E, Leferovich J, Bedelbaeva K, Gourevitch D, Clark L. The scarless heart and the MRL mouse. Philos Trans R Soc Lond B Biol Sci. 2004;359:785–793. doi: 10.1098/rstb.2004.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodes GE, Hill-Smith TE, Suckow RF, Cooper TB, Lucki I. Sex-specific effects of chronic fluoxetine treatment on neuroplasticity and pharmacokinetics in mice. J Pharmacol Exp Ther. 332:266–273. doi: 10.1124/jpet.109.158717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodes GE, Yang L, Van Kooy J, Santollo J, Shors TJ. Prozac during puberty: distinctive effects on neurogenesis as a function of age and sex. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holick KA, Lee DC, Hen R, Dulawa SC. Behavioral effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology. 2008;33:406–417. doi: 10.1038/sj.npp.1301399. [DOI] [PubMed] [Google Scholar]
- 17.Hughes RN. Sex does matter: comments on the prevalence of male-only investigations of drug effects on rodent behaviour. Behav Pharmacol. 2007;18:583–589. doi: 10.1097/FBP.0b013e3282eff0e8. [DOI] [PubMed] [Google Scholar]
- 18.Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- 19.Lagace DC, Fischer SJ, Eisch AJ. Gender and endogenous levels of estradiol do not influence adult hippocampal neurogenesis in mice. Hippocampus. 2007;17:175–180. doi: 10.1002/hipo.20265. [DOI] [PubMed] [Google Scholar]
- 20.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molteni R, Cattaneo A, Calabrese F, Macchi F, Olivier JD, Racagni G, Ellenbroek BA, Gennarelli M, Riva MA. Reduced function of the serotonin transporter is associated with decreased expression of BDNF in rodents as well as in humans. Neurobiol Dis. 37:747–755. doi: 10.1016/j.nbd.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S, Parada LF, Nestler EJ. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol Psychiatry. 2007;61:187–197. doi: 10.1016/j.biopsych.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 23.Qu Y, Aluisio L, Lord B, Boggs J, Hoey K, Mazur C, Lovenberg T. Pharmacokinetics and pharmacodynamics of norfluoxetine in rats: Increasing extracellular serotonin level in the frontal cortex. Pharmacol Biochem Behav. 2009;92:469–473. doi: 10.1016/j.pbb.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 24.Russo-Neustadt AA, Chen MJ. Brain-derived neurotrophic factor and antidepressant activity. Curr Pharm Des. 2005;11:1495–1510. doi: 10.2174/1381612053764788. [DOI] [PubMed] [Google Scholar]
- 25.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]