Abstract
We analyzed 60 patients with idiopathic early allograft loss (defined as death or retransplantation <90 days) to determine the relative contribution of preformed donor specific HLA alloantibodies (DSA) to this endpoint and defined strict criteria for the diagnosis of antibody-mediated rejection (AMR) in liver allografts. Inclusion criteria encompassed availability of a pre-transplant serum sample and both post-reperfusion and follow-up tissue specimens for “blinded” retrospective re-review of histology and C4d staining. AMR was diagnosed based on the presence of all 4 strict criteria: 1) DSA in serum; 2) histopathological evidence of diffuse microvascular injury/microvasculitis, consistent with antibody-mediated injury; 3) diffuse C4d staining in the portal microvasculature with or without staining in the sinusoids or central veins in at least one sample; and 4) exclusion of other causes of a similar type of injury. Patients thought to be experiencing definite AMR on the basis of routine histopathology alone showed the highest levels of DSA sensitization. Forty percent of patients with pre-transplant DSA with a pattern of bead saturation after serial dilutions developed AMR. One additional multiparous female developed, what appeared to be, a strong “recall” response resulting in combined AMR and ACR causing graft failure. A contribution of DSA to allograft failure could not be excluded in three additional patients who received marginal grafts. In conclusion, liver allograft recipients with high mean fluorescence intensity (MFI) preformed DSA despite dilution seem to be at risk for clinically significant allograft injury, and possibly loss, from AMR often in combination with ACR.
Keywords: Antibody mediated rejection, liver transplantation, rejection, early allograft loss
Introduction
Although antibody mediated rejection (AMR) can cause or contribute to early allograft loss in ABO-compatible liver transplantation (LT), most programs do not routinely monitor for this potential cause of allograft dysfunction and loss. The most convincing case series of hyperacute liver allograft AMR highlighted 3 cases of simultaneous liver-kidney transplants where hyperacute rejection of the kidney was abruptly followed by precipitous liver allograft loss, which developed over a period of days, instead of hours (1). The liver histology demonstrated diffuse coagulative necrosis with IgM and C1q positive staining consistent with hyperacute rejection. However, the perceived infrequency of these findings resulted in a lack of standardized diagnostic criteria for liver AMR.
Unlike hyperacute rejection, early liver allograft injury and loss was found more frequently in crossmatch positive patients (2, 3), but not all crossmatch positive patients experienced graft dysfunction or loss. Those that did more commonly had a persistently positive post-transplant crossmatch, falling complement levels, circulating immune complexes, and refractory thrombocytopenia (4–6) - a clinical profile that supports the diagnosis of AMR in patients with appropriate histology and C4d staining. This phenomenon has been reaffirmed as a rare but ongoing issue in our current era of immunosuppression (7–13). Early in the course of liver allograft injury from DSA, the histology mimics preservation injury (5, 14), which often evolves into acute “cellular” rejection (ACR), and later can progress into vanishing bile duct syndrome or chronic rejection (12, 13, 15–20). Patients are often diagnosed with “ACR” when they have AMR or combined AMR and ACR for 2 reasons: 1) a broad spectrum of findings, including those that appear to be attributable to DSA, have traditionally been attributed to “cellular” rejection; 2) AMR of the liver is often quickly followed by “cellular” rejection and an accompanying cellular infiltrate (10). However, more study is needed in this area.
Recent data enable identification and characterization of donor-specific HLA alloantibodies (DSA) that increase the risk of acute and chronic rejection in sensitized recipients (7, 8, 12, 18, 19, 21, 22). Unique morphologic features, many of which were previously attributed to ACR, aid in the early diagnosis of AMR in LT patients with circulating DSA, but more precision, reliability, and reproducibility are needed (8, 10, 12). Most of these features can be seen in animal models of AMR, and pathognomonically show diffuse microvascular injury that can evolve into hemorrhagic necrosis (23–26). As part of this diagnostic effort that includes the clinical profile described above and standard histology, C4d staining in formalin-fixed paraffin-embedded tissue can point toward AMR as a cause of injury (10, 11), but sensitivity is improved in fresh tissue (8, 9). Despite these advances, only when AMR is accurately diagnosed early and successfully treated can graft outcomes improve, since delay in the diagnosis of AMR usually results in substantial allograft injury or failure (27).
Potential complications from preformed DSA resulted in some centers recommending utilization of crossmatch negative donors when possible (28), but poor predictive power combined with recipient illness severity precluded implementation. The goals of this study, therefore, were to: a) define strict criteria for the diagnosis of acute AMR in liver allograft recipients; b) identify the prevalence of preformed DSA and subsequent AMR in patients with previously unexplained liver allograft loss within 90 days of LT; and c) identify preformed DSA and early histopathologic characteristics in at-risk sensitized recipients.
Methods
Case Selection and Analysis
From 1/1989 to 7/2010 3137 ABO compatible liver transplants without another organ were performed, and 337 (10.7%) patients experienced allograft loss within 90 days. Patients were excluded from the study for the following reasons: lack of a pre-LT sample (n=51), death with a functioning graft (n=86), known cause (-s) of graft loss or death in our database or upon re-review of the clinical history (n=123), and lack of back-table, post-reperfusion, or follow-up biopsies (n=17). All the remaining 60 patients experienced unexplained graft loss and were included in the study.
Serum samples and liver tissue are prospectively collected the day before or of transplant and obtained from the Annette C. and Harold C. Simmons Transplant Institute biorepository after institutional review board approval. Blinded pretransplant serum samples were analyzed in all patients. In addition, all patients had at least one back-table or post-perfusion liver biopsy and at least one follow up liver biopsy, explant or autopsy tissue stained and re-reviewed. All serum and tissue samples are linked with prospectively collected clinical and laboratory data that is locked into the Liver Transplant Research Database System (LTRDS).
Histopathological Analysis
Twelve patients had back-table liver biopsies, 59 patients had post-reperfusion liver biopsies, 30 patients had indication follow-up liver biopsies, 48 patients had explant samples, and 2 patients had autopsy tissue. All these samples were re-evaluated, initially, without knowledge of the DSA or C4d staining results by pathologists experienced in liver and transplantation pathology (SMS, AJD). All formalin-fixed, paraffin-embedded tissue samples, with the exception of all back table and one post-reperfusion biopsy (insufficient tissue), were stained for C4d. Evaluation of C4d was based on endothelial cell staining of the portal microvascular, sinusoidal, and central vein endothelium, as previously described (10). AMR was determined to be a substantial contributor to allograft loss only when the following 4 criteria were all met: 1) DSA in serum; 2) diffuse microvascular injury seen on H&E, consistent with an antibody-mediated injury (5, 10); 3) diffuse C4d staining in the portal microvasculature with or without staining present in the sinusoids or central veins in at least one sample; and 4) a clinical profile consistent with AMR and that reasonably excluded other insults that might cause similar injury. Diffuse C4d staining was defined as strong staining of portal vein branches and portal capillaries in a majority of portal tracts. In cases with evidence of 2–3 criteria without a precise etiology for allograft loss, DSA could not be excluded as a contributor to allograft failure.
HLA tissue typing
All recipients and donors were typed for HLA-A, -B, -DRB1, -DRB345 and -DQ using commercially available serologic typing trays or by molecular methods (Terasaki HLA tissue typing trays and Micro SSP™ or LabType® SSO, respectively; One Lambda Inc., Canoga Park, CA). Donors’ HLA-Cw typing was available in half of the cases. Prior to 1998, serology was used exclusively. Since 1998, all donor class I and II HLA typing as well as patient class II was performed by molecular methods, whereas patient class I is performed by serology.
HLA IgG antibody determination
All sera were anonymized and sent to the Terasaki Foundation Laboratory for DSA evaluation. Detection of HLA IgG antibodies was performed using LABScreen single antigen class I (lot 6) and II (lot 8) beads (One Lambda Inc., Canoga Park, CA) according to the manufacturer’s protocol. A normalized trimmed value over 1,000 mean fluorescence intensity (MFI) was considered positive; when the value exceeded 10,000 MFI for a single DSA, serial dilutions (neat, 1:3, 1:9, 1:27, and 1:81) were performed. Bead saturation was defined as an antibody that maintained a similar MFI (with at least an MFI >15,000) regardless of dilution (to ≥ 1:27).
IgG subclass DSA and C1q evaluation
All DSA-positive sera were further evaluated for IgG subclasses and C1q-binding antibodies. Evaluation of IgG subclass DSA was performed using the same LABScreen single antigen beads that were used for the initial HLA IgG antibody determination but replacing the secondary antibody with an antibody specific for each of the IgG subclasses as previously described (19). A normalized trimmed value >500 MFI was defined as positive for IgG subclasses. C1q-fixing antibody determinations used the C1qScreen kit (One Lambda Inc., Canoga Park, CA) according to the manufacturer’s protocol. A normalized trimmed mean >1,000 MFI was considered positive for C1q-fixing antibodies.
Statistical Analysis
Patient characteristics were reported with median values with interquartile ranges where appropriate and compared using the likelihood ratio chi-square test for categorical values and Kruskal-Wallis test for continuous variables in Tables where appropriate. Statistical significance was defined as a P<0.05. SAS 9.1 was used for all statistical analyses.
Results
Table 1 details characteristics of the 60 patients with unexplained early allograft loss divided into 3 groups: 1) patients who experienced AMR as the probable primary cause of early allograft failure (n=3); 2) patients in whom DSA could not be excluded as a contributor to early allograft failure (n=3); and 3) patients without definite histopathological features of AMR (n=54). Only female gender was statistically significantly more common in patients with AMR, which is expected; there were no other statistically significant differences between the groups’ demographics.
Table 1.
Patient and donor characteristics (with interquartile ranges where appropriate) for the 60 recipients with early graft loss (<90 days post-liver transplant).
| AMR Changes Present | Possible Contribution from DSA | No Definitive Evidence of AMR | ||
|---|---|---|---|---|
| Number | 3 | 3 | 54 | |
| Age | 56 (47–58) | 52 (40–55) | 50 (41–58) | |
| Model for End-Stage Liver Disease | 27(12–40) | 16 (13–33) | 17 (12–26) | |
| Pre-operative dialysis | 1(33%) | 0% | 3 (5%) | |
| Cold ischemia time (hours) | 11.8 (7.5–12.2) | 8.4 (7.8–8.7) | 9.6 (8.2–11.7) | |
| Warm ischemia time (minutes) | 62 (52–84) | 52 (50–55) | 56 (46–68) | |
| OR time (hours) | 7.8 (5.1–7.9) | 6.3(4.8–8.2) | 6.8(5.8–7.8) | |
| Time to graft loss (days) | 19 (19–40) | 3 (0–54) | 5.5 (3–19) | |
| Male Sex# | 0% | 1(33%) | 38 (70%) | |
| Race | African-American | 0% | 0% | 1(2%) |
| Hispanic | 0% | 1(33%) | 8(15%) | |
| Other | 0% | 0% | 1(2%) | |
| Caucasian | 3 (100%) | 2(67%) | 44(81%) | |
| Liver Disease | Hepatitis C | 0% | 2(67%) | 14(26%) |
| PSC/PBC/AIH | 2 (67%) | 0% | 9(17%) | |
| Alcohol | 1(33%) | 0% | 8(15%) | |
| NASH/CC | 0% | 1(33%) | 6(11%) | |
| Metabolic/congenital | 0% | 0% | 3(5%) | |
| Other | 0% | 0% | 14(26%) | |
| Hepatocellular Carcinoma | 0% | 0% | 5(9%) | |
| Donor age | 42 (37–45) | 56(55–59) | 50(36–59) | |
| Donor race | Caucasian | 3 (100%) | 2(67%) | 37(68%) |
| African-American | 0% | 1(33%) | 10(19%) | |
| Other | 0% | 0% | 7(13%) | |
| Induction | 1(33%) | 1(33%) | 16(30%) | |
| CNI* | Tacrolimus | 66% | 33% | 29% |
| Cyclosporine | 0% | 0% | 52% | |
| None | 33% | 66% | 19% | |
| Steroids* | 100% | 66% | 60% | |
| Sirolimus* | 0% | 0% | 12% | |
| Mycophenolate* | 0% | 66% | 35% | |
P=0.02; no other statistically significant differences were found between the groups.
Day 1 immunosuppression
CNI, calcineurin inhibitor; PSC, primary sclerosing cholangitis; PBC, primary biliary cirrhosis; AIH, autoimmune hepatitis; NASH, non-alcoholic steatohepatitis; CC, cryptogenic cirrhosis
Preformed DSA was found in 53% of the total patient population, but there were differences in the DSA characteristics between the 3 groups (Table 2). The candidate cases were identified on the basis of H&E alone, but the results correlated with median MFI of each patient’s single highest class I DSA that was 20,266, 20,109 and 2,783 (P=0.03); and class II DSA that was 11,794, 5,414, and 2,385 (P=0.07) respectively for those with AMR, those where DSA could not be excluded as a contributor to allograft loss, and those without AMR. DSA-positive patients had between 1–8 distinct DSAs detected. In addition, an effort to capture the potential detrimental effects of multiple DSAs by adding the sum of each individual undiluted DSA (MFISUM) showed an even stronger correlation with the histopathological findings. The median MFISUM was considerably higher in those with AMR (95,988) vs. those where DSA could not be excluded as a contributing factor to allograft loss (40,752) vs. those without AMR (3,643; P=0.002). Since MFISUM does not capture the true “amount” of DSA (because of bead saturation), we performed dilutional analyses. Eighty percent of patients with at least one DSA with bead saturation at 1:27 or 1:81 dilutions had AMR as a probable primary reason for or non-excludable contributor to allograft loss, and 2 of the 3 cases of AMR showed focal or diffuse microvascular endothelial cell C4d staining on post-reperfusion biopsy.
Table 2.
Donor-Specific Antibodies in patients with early graft loss (<90 days) (A) the class, (B) the median MFI, (C) number of patients with newly identified causes of graft loss divided by DSA presence or absence.
| (A) | ||||
|---|---|---|---|---|
| AMR | Possible Contribution DSA | No AMR | P-value | |
| Class I | 0% | 2 (67%) | 7 (13%) | 0.002 |
| Class II | 0% | 0% | 15 (28%) | |
| Class I & II | 3 (100%) | 1 (33%) | 4 (7%) | |
| None | 0% | 0% | 28 (52%) | |
| (B) | ||||
|---|---|---|---|---|
| Median* (IQR) | AMR | Possible Contribution from DSA | No AMR | p-value |
| Class I | 20266 (4815–26234) | 20109(16804–22717) | 2783(1436–11527) | 0.03 |
| Class II | 11794 (9429–24865) | 4416 | 2385(1287–4944) | 0.07 |
| MFISUM | 95988(26336–131135) | 40752(39691–63284) | 3643(1436–12805) | 0.002 |
| (C) | ||||
|---|---|---|---|---|
| Causes of graft loss | DSA saturation^ | DSA + (no saturation) | DSA− | Total |
| AMR +/− ACR | 2 (40%) | 1 (4%) | 0 | 3 (5%) |
| ACR/Ischemia | 2 (7%) | 5 (18%) | 7 (11%) | |
| Fat/Ischemia | 11 (41%) | 6 (21%) | 17 (28%) | |
| Vascular | 4 (15%) | 8 (29%) | 12 (20%) | |
| Unknown | 3 (60%)** | 9 (33%)** | 9 (32%) | 21 (35%) |
DSA saturation = at least one DSA that maintained a similar MFI (>15,000) regardless of dilution (to ≥1:27)
Median of positive patients
2 patients with DSA saturation and 1 patient without bead saturation had DSA as a non-excludable contributor allograft failure.
The details of the DSAs, laboratory parameters, and histology for each of the 3 cases of AMR are shown in Figures 1–3. The first 2 cases (Figure 1–2) share multiple high MFI class I and II DSAs, some with bead saturation at ≥1:27 dilutions, several of the IgG3 subclass, and many C1q positive. These 2 patients also shared a slightly delayed high aminotransferase peak, platelet consumption, and persistent bilirubin elevation. The third case was a multiparous female with moderate MFI DSAs of the IgG1 subclass that were C1q negative who experienced an apparent “recall” AMR response characterized by a delay in the aminotransferase elevations and platelet consumption.
Figure 1.
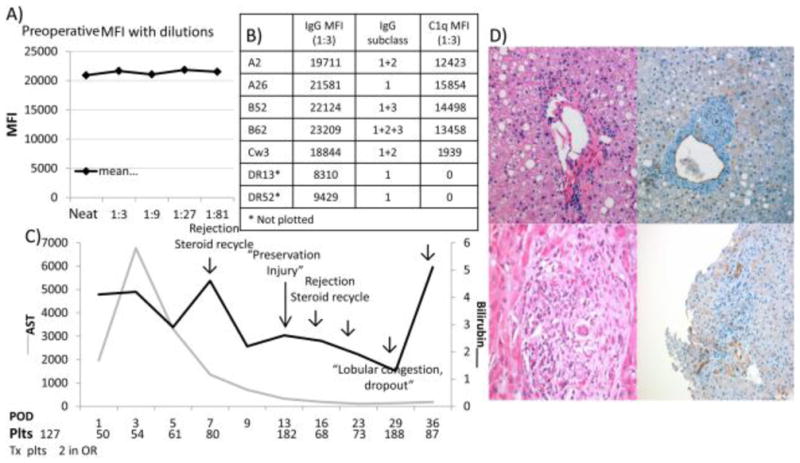
Patient 1 was a 56-year-old female with a gynecological history significant for 1 still birth and with a MELD score of 12 who underwent primary LT for AIH with a 37-year-old male donor after 12.2 hours of cold ischemia in 1990. She had (A) saturation of single-antigen beads of numerous DSAs at a 1:81 dilution against class I before transplantation, (B) 7 distinct DSAs (MFIs reported at a 1:3 dilution) in all, and (C) a clinical course characterized by early postoperative platelet consumption and recurrent rejection. (D) The postreperfusion biopsy showed endothelial reactivity and diffuse C4d positivity (top panels), and this was followed by evidence of combined AMR and ACR on day 16 characterized by diffuse microvascular endothelial cell injury (bottom panels).
Figure 3.
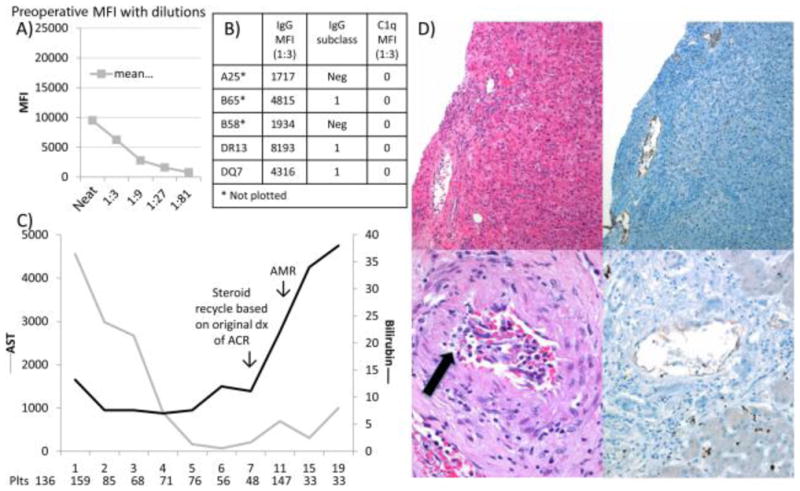
Patient 3 was a 58-year-old multiparous female with a MELD score of 27 who underwent primary LT for PBC with a 58-year-old male donor after 11.8 hours of cold ischemia in 2004. She had (A,B) 5 distinct preformed lower MFI class I and II DSAs (MFIs reported at a 1:3 dilution) and (C) a clinical course characterized by an apparent recall response. (D) Although the postreperfusion biopsy was unremarkable (not shown), the day 7 indication liver biopsy showed combined AMR and ACR (top panels). The autopsy sample on day 20 showed severe combined AMR and ACR with active lymphocytic intimal inflammation, intimal foam cells (arrow), and continued C4d positivity despite the postmortem nature of the tissue sample (bottom panels).
Figure 2.
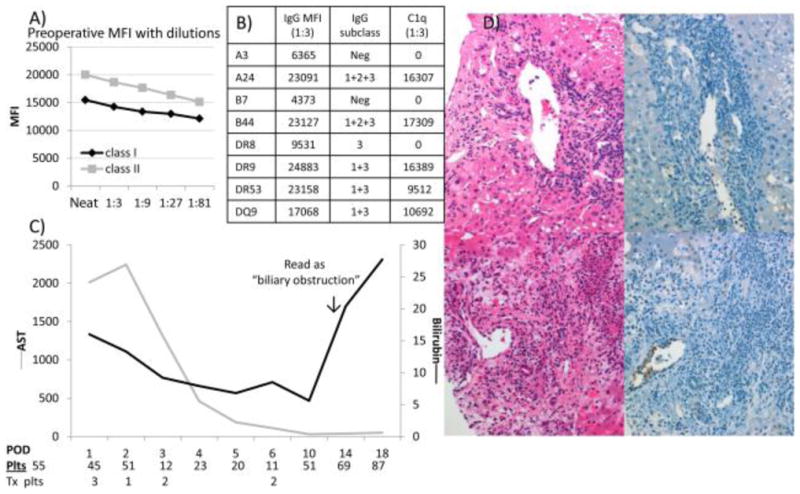
Patient 2 was a 47-year-old primiparous female with a MELD score of 40 who underwent primary LT for alcohol-induced cirrhosis with a 45-year-old female donor after 7.6 hours of cold ischemia in 2005. (A) She had high mean class I and II single-antigen beads at a 1:81 dilution of numerous class I and II DSAs, (B) 8 distinct DSAs were found, and (C) her clinical course was characterized by early postoperative platelet consumption. (D) The postreperfusion biopsy showed endothelial reactivity and C4d positivity (top panels), and this evolved into combined AMR and ACR in her only liver biopsy performed on day 12, which was characterized by very severe endothelial cell reactivity and damage (bottom panels).
All 3 cases with definite evidence of AMR had histology characterized primarily by portal microvascular endothelial cell hypertrophy or near hobnailing, eosinophilic and neutrophilic portal capillaritis/inlet venulitis, portal edema, ductular reaction, with diffuse portal microvascular C4d staining. All 3 cases where DSA could not be excluded as a contributor to allograft loss had lower C1q values despite 2 having DSA that reached bead saturation at ≥1:27 dilution. The full case report details of all 6 cases can be found in supplemental data, and Table 3 outlines patient, donor, and DSA characteristics of all DSA positive cases. The remaining cases, except where indicated, had post-perfusion and follow up tissue samples that were C4d negative.
Table 3.
Patient, donor and antibody characteristics for the 32 patients with pre-liver transplant DSA present. Although all patients originally had unexplained graft loss, a retrospective etiology was able to be determined in many.
| ACR | fat | Pres. Injury | MFISUM | C1qSUM | IgG3SUM | days-graft loss | age | Age | CIT | MELD | Cause | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 10 | 1 | 115,791 | 58,195 | 2,805 | 40 | 56 | 37 | 12.2 | 12 | AMR*^ |
| 2 | ACR2 | mod-40 | 3 | 131,596 | 70,209 | 39,037 | 19 | 47 | 45 | 7.6 | 40 | AMR/ACR**^ |
| 3 | ACR1 | mild-20 | 1 | 20,975 | 0 | 0 | 19 | 58 | 42 | 11.8 | 27 | AMR/ACR^ |
| 4 | 0 | mod-40 | 0 | 70,396 | 11,331 | 0 | 0 | 52 | 59 | 8.4 | 16 | Fat/?DSA |
| 5 | ACR3 | 0 | 1 | 40,752 | 33,688 | 790 | 3 | 40 | 56 | 7.8 | 13 | ACR/?DSA |
| 6 | 0 | 5 | 1 | 39,691 | 2,824 | 0 | 54 | 55 | 55 | 8.7 | 33 | ?DSA |
| 7 | 0 | 5 | 3 | 31,130 | 10,938 | 11,985 | 3 | 43 | 21 | 13.2 | 20 | unknown |
| 8 | 0 | mild-5 | 1 | 30,863 | 21,049 | 8,185 | 4 | 50 | 51 | 6.3 | 16 | unknown |
| 9 | 0 | mild-5 | 1 | 12,805 | 0 | 0 | 19 | 58 | 50 | 13.8 | 22 | unknown |
| 10 | 0 | 5 | 3 | 8,076 | 0 | 0 | 12 | 42 | 28 | 7.5 | 13 | unknown |
| 11 | 0 | mod-15 | 3 | 7,851 | 0 | 0 | 6 | 37 | 28 | NA | 28 | unknown |
| 12 | 0 | 5 | 3 | 6,711 | 0 | 1,024 | 1 | 51 | 38 | 14.1 | 14 | unknown |
| 13 | ACR1 | 0 | 2 | 4,944 | 0 | 6,248 | 3 | 17 | 34 | 6.4 | 32 | unknown |
| 14 | 0 | 0 | 0 | 2,679 | 0 | 0 | 10 | 38 | 16 | 6.4 | 36 | unknown |
| 15 | 0 | 5 | 3 | 2,650 | 0 | 0 | 3 | 45 | 42 | 10.8 | 17 | unknown |
| 16 | ACR1 | 0 | 1 | 2,121 | 0 | 0 | 2 | 52 | 45 | 4.3 | 8 | unknown |
| 17 | 0 | mod | 1 | 21,880 | 0 | 0 | 1 | 38 | 23 | 9.9 | 21 | ischemia |
| 18 | 0 | mod | 3 | 16,565 | 0 | 0 | 38 | 62 | 74 | 8.9 | 22 | ischemia/fat |
| 19 | 0 | 0 | 1 | 15,915 | 0 | 0 | 33 | 33 | 38 | 11 | 30 | vascular |
| 20 | 0 | mild | 3 | 14,839 | 0 | 1,153 | 3 | 59 | 40 | 9.6 | 15 | ischemia |
| 21 | 0 | mild-5 | 2 | 6,956 | 0 | 0 | 5 | 63 | 63 | 7.6 | 13 | vascular |
| 22 | 0 | 30 | 3 | 3,749 | 0 | 830 | 4 | 62 | 53 | 8.3 | 19 | ischemia/fat |
| 23 | ACR1 | 70 | 3 | 3,538 | 0 | 0 | 1 | 16 | 52 | 11.7 | 9 | ischemia/fat |
| 24 | 0 | 60 | 3 | 3,338 | 0 | 0 | 38 | 60 | 58 | NA | 35 | ischemia/fat |
| 25 | ACR3 | 0 | 3 | 2,863 | 0 | 0 | 6 | 54 | 47 | 13.3 | 8 | ischemia/ACR |
| 26 | 0 | 0 | 3 | 1,443 | 0 | 0 | 2 | 58 | 40 | 8.9 | 17 | vascular |
| 27 | 0 | 50 | 2 | 1,436 | 0 | 0 | 3 | 67 | 32 | 10.3 | 20 | ischemia/fat |
| 28 | 0 | 65 | 3 | 1,267 | 0 | 0 | 17 | 50 | 53 | 11.5 | 10 | ischemia/fat |
| 29 | 0 | mod-5 | 1 | 1,287 | 0 | 0 | 11 | 60 | 54 | 5.7 | 34 | vascular |
| 30 | ACR1 | 0 | 3 | 1,279 | 1,774 | 929 | 3 | 41 | 21 | 14.3 | 17 | ischemia/ACR |
| 31 | 0 | 30 | 2 | 1,166 | 0 | 0 | 2 | 52 | 42 | 9.6 | 14 | ischemia/fat |
| 32 | 0 | severe | 3 | 1,040 | 0 | 0 | 3 | 67 | 10 | 8.7 | 12 | ischemia/fat |
Diffuse C4d staining was found on post-reperfusion liver biopsy
Focal C4d staining was found on post-reperfusion liver biopsy
Diffuse C4d staining was found on indication liver biopsy
ACR, acute cellular rejection; microvesicular fat was described as mild or mod (moderate); macrovesicular steatosis was assigned as a number which was the percent; Pres. Injury, preservation injury on post-reperfusion liver biopsy; DSA, donor specific antibody; MFI, mean fluorescence intensity; MFIsum, sum of each distinct DSA; C1q, single antigen beads test to detect C1q-fixing antibodies; CIT, cold ischemia time in hours; MELD, model for end-stage liver disease; AMR, antibody mediated rejection
Although the primary evaluation of clinicopathologic parameters did not reveal a reason for allograft loss in all cases, re-evaluation of the clinical profile, reperfusion, post-LT, and explant pathology resulted in identification of the cause of allograft failure in 65% of patients (Table 2C): severe ischemia compounded by either underlying severe donor macrovesicular steatosis(28%) or acute cellular rejection without evidence of AMR (11%) and unrecognized hepatic artery and portal vein thrombosis or suboptimal liver perfusion (20%) were common.
Discussion
Liver allografts are more resistant to preformed DSA causing early or acute AMR than other solid organ transplants, but accumulating evidence favors those who recognized AMR as a potential cause of liver injury (1–3, 8, 14, 28). Recognized risk factors identified in the era before single antigen bead technology allowed accurate HLA DSA determinations were: high titer preformed antibodies, persistence of the anti-donor antibodies after transplantation, and otherwise unexplained thrombocytopenia and hypocomplementemia (4–6, 14, 29).
In an effort to further refine risk factors and establish an early AMR diagnosis, we analyzed the 60 cases of idiopathic early (<90 days post-LT) allograft loss in patients with precisely defined preformed HLA alloantibodies using stringent criteria that included: 1) DSA in serum 2) histopathological evidence of diffuse microvascular endothelial cell injury/microvasculitis; 3) diffuse strong C4d positivity in at least one of the tissue samples; and 4) reasonable exclusion of other causes of injury that might result in similar findings. Importantly, this study shows that strict adherence to routine histopathological findings of diffuse microvascular endothelial cell reactivity, which approaches “hobnailing” and microvasculitis reliably point toward recipients with the highest levels of sensitization. When these findings are combined with diffuse C4d positivity, evidence of serum sensitization, and exclusion of other insults that might cause a similar injury pattern, one can confidently establish an acute AMR diagnosis. These observations should serve as an impetus for further study of the correlation between routine histopathology, C4d deposition, and DSA in liver allograft recipients.
Different C4d staining patterns have been described, and although a consensus agreement regarding a diagnostic pattern is not available, diffuse portal microvascular positivity in formalin-fixed, paraffin-embedded samples, is emerging as most strongly correlated with DSA-induced injury (8–12). In our opinion, however, when attempting to establish a diagnosis of AMR in this new era of sensitive DSA detection, stringent criteria should be used to avoid over diagnosis. C4d negative renal allograft AMR has been identified, and likely also occurs in the liver, but until more is learned about liver AMR, we favor taking this conservative approach (30).
Interestingly, 53% of all patients with early allograft failure had preformed DSA with MFI >1000. This is a substantially higher percentage than in our recently reported population of 1270 consecutive liver transplant recipients (36%) (13). Moreover, dilutional studies of strong pre-transplant DSAs found 5 patients with at least one DSA with a pattern of bead saturation at ≥ 1:27 dilution. Two of these five (40%) showed convincing evidence of AMR in tissue specimens, as defined above. In another two patients (40%), antibody mediated injury could not be excluded as a contributor to early allograft loss. These observations are consistent with previous, less specific, dilutional analyses using conventional lymphocytotoxic crossmatches (2, 4).
The rarity (2/59) of diffuse C4d positivity on post-reperfusion liver biopsies in our formalin-fixed, paraffin-embedded archival tissue samples might be related to the high level of sensitization in our recipients that developed definite evidence of AMR or the low sensitivity of our C4d stain, or both, but both of these recipients subsequently developed AMR resulting in allograft loss. This study, however, was biased by including only those who experienced allograft failure. Nevertheless, one might consider performing a post-reperfusion liver biopsy and staining it for C4d in high MFI preformed DSA-positive recipients as an early warning sign for AMR. Regardless, considering the inter-center variability of sensitivity for C4d staining, this finding will need confirmation (8, 9, 11, 12, 31).
Of note, one of the 3 patients who developed AMR had moderate MFI of several non-complement-fixing DSAs and a benign reperfusion biopsy. She was, however, a multiparous female and developed what appeared to be a very strong “recall” response with dramatic AMR with ACR: diffuse microvascular endothelial cell hypertrophy and damage, diffuse C4d positivity on follow-up biopsies, and lymphocytic arteritis with rare intimal foam cells within 27 days of transplantation. The combination of arteritis and diffuse C4d positivity is accepted as evidence of AMR in other solid organ allografts (32–36). Unfortunately, a post-transplant follow-up serum was not available for analysis on any of our AMR cases.
This more detailed analysis confirms previous studies showing that severe AMR is a relatively rare event after transplantation and quickly evolves into combined AMR and ACR (5, 8, 28). It is unknown if current immunosuppression and surgical advances would have altered the outcomes of some of our earlier patients (7, 8, 10, 12). Based on inclusion of only convincing cases in this series, AMR was thought to be the primary cause of early allograft loss in only 1% of all early allograft failures, and a possible contributing factor to early allograft loss in an additional 1% of cases; the figures rise to 5% in historic cases with a previously undetermined cause of failure; and 10% in those with preformed DSA.
Although somewhat speculative, a combination of the diffuse pattern of C4d staining and serology suggests that high MFI class I DSAs that override potential innate protective mechanisms might have the most potential for immediate damage resulting in rapid allograft failure. This contention is based on class I HLA’s diffuse expression throughout the liver microvasculature, unlike class II HLA’s limited expression in the portal capillaries in a subpopulation of donor livers, unless the liver is affected by an inflammatory disorder (23, 37, 38). Because of limited expression, class II DSA may cause slower graft injury, and is associated with an increased risk of acute “cellular” rejection and chronic rejection (12, 13, 18, 21). However, since all definite AMR cases had class I and II DSA present before the transplant, it is possible that class I DSA-mediated injury lead to up-regulation of class II HLA in the liver enabling the pathologic potential of class II DSA. Conversely, no definitive proof of AMR was detected in 80% of DSA-positive recipients. However, most of these had low MFI DSAs. Stringent criteria were used to identify convincing cases, but does not exclude the possibility of more subtle forms of antibody mediated injuries or longer term consequences if the allografts had survived.
Fortunately, and in confirmation of previous studies, lower MFI preformed DSA, and most low titer crossmatch results, by extension, did not result in identifiable graft damage or loss when standard low risk donors are utilized (7, 8, 31, 39, 40). The role DSA plays in allograft loss when marginal organs are transplanted, however, remains unclear. This consideration was evident from our 3 cases, where it was determined that DSA/AMR was not the primary cause of liver allograft loss, but DSA could not be excluded as a contributor to liver allograft failure. Therefore, caution may be needed when matching high-risk donors to patients with preformed high MFI DSA. Although we find this hypothesis interesting, further study is needed to define the precise donor and antibody characteristics that should not be mixed. Therefore, testing of all patients before transplantation and follow up testing to determine persistence post-transplant are needed to define the precise donor and antibody characteristics that cannot be matched.
Finally, this study suffers from several weaknesses. First, it was a retrospective, single center study. Second, we relied entirely on formalin-fixed, paraffin-embedded tissue samples (some decades old), which are known to have inferior C4d staining sensitivity. Third, follow-up serum samples to determine post-operative DSA presence would have been ideal to substantiate the AMR diagnoses, since in the majority of cases DSA disappears yet those where it has been shown to persist have an increased risk of inferior outcomes. And fourth, we cannot exclude the possibility that current standards of care would have improved the outcomes seen in these historical patients. We attempted to mitigate these shortcomings by first reviewing the cases for a characteristic pattern of microvascular injury without knowledge of the DSA status or C4d staining, and then rigorously excluding other causes of allograft injury that might show a similar pattern of histopathology.
In conclusion, high MFI preformed DSA can result in substantial early graft injury from AMR, which usually combines with ACR to cause allograft failure. Although this was a rare event [1% (3/337) of all early graft loss and 5% (3/60) of previously unexplained early graft loss cases], graft loss from AMR occurred in 40% of patients with at least one preformed DSA with bead saturation at ≥ 1:27 dilution, especially in those with a post–reperfusion formalin-fixed, paraffin-embedded biopsy diffusely positive for C4d. Testing all liver transplant candidates for DSA before transplant and conducting protocol post-reperfusion biopsies and follow up single antigen bead testing would be ideal to determine which highly sensitized patients might be at risk and should be carried out at centers dedicated to study the phenomenon. Broader recommendations to carry out similar testing in low risk patients at all centers must be proven cost-effective prior to wide spread implementation.
Abbreviations
- AMR
antibody mediated rejection
- DSA
donor specific HLA antibodies
- LT
liver transplantation
- LTRDS
liver transplant research database system
- MFI
mean fluorescence intensity
- POD
post-operative day
References
- 1.Starzl TE, Demetris AJ, Todo S, Kang Y, Tzakis A, Duquesnoy R, et al. Evidence for hyperacute rejection of human liver grafts: The case of the canary kidneys. Clin Transplant. 1989;3:37–45. [PMC free article] [PubMed] [Google Scholar]
- 2.Takaya S, Duquesnoy R, Iwaki Y, Demetris J, Yagihashi A, Bronsther O, et al. Positive crossmatch in primary human liver allografts under cyclosporine or FK 506 therapy. Transplant Proc. 1991;23(1 Pt 1):396–9. [PMC free article] [PubMed] [Google Scholar]
- 3.Ogura K, Koyama H, Takemoto S, Terasaki PI, Busuttil RW. Significance of a positive crossmatch on outcome in human liver transplantation. Transplant Proc. 1992 Aug;24(4):1465. [PubMed] [Google Scholar]
- 4.Manez R, Kelly RH, Kobayashi M, Takaya S, Bronsther O, Kramer D, et al. Immunoglobulin G lymphocytotoxic antibodies in clinical liver transplantation: studies toward further defining their significance. Hepatology. 1995 May;21(5):1345–52. doi: 10.1002/hep.1840210519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demetris AJ, Nakamura K, Yagihashi A, Iwaki Y, Takaya S, Hartman GG, et al. A clinicopathological study of human liver allograft recipients harboring preformed IgG lymphocytotoxic antibodies. Hepatology. 1992 Sep;16(3):671–81. doi: 10.1002/hep.1840160310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakamura K, Yagihashi A, Iwaki Y, Takaya S, Hartman GG, Murase N, et al. The lymphocytotoxic crossmatch in liver transplantation: a clinicopathologic analysis. Transplant Proc. 1991 Dec;23(6):3021–2. [PMC free article] [PubMed] [Google Scholar]
- 7.Castillo-Rama M, Castro MJ, Bernardo I, Meneu-Diaz JC, Elola-Olaso AM, Calleja-Antolin SM, et al. Preformed antibodies detected by cytotoxic assay or multibead array decrease liver allograft survival: role of human leukocyte antigen compatibility. Liver Transpl. 2008 Apr;14(4):554–62. doi: 10.1002/lt.21408. [DOI] [PubMed] [Google Scholar]
- 8.Kozlowski T, Rubinas T, Nickeleit V, Woosley J, Schmitz J, Collins D, et al. Liver allograft antibody-mediated rejection with demonstration of sinusoidal C4d staining and circulating donor-specific antibodies. Liver Transpl. 2011 Apr;17(4):357–68. doi: 10.1002/lt.22233. [DOI] [PubMed] [Google Scholar]
- 9.Kozlowski T, Andreoni K, Schmitz J, Hayashi PH, Nickeleit V. Sinusoidal C4d deposits in liver allografts indicate an antibody-mediated response: diagnostic considerations in the evaluation of liver allografts. Liver Transpl. 2012 Jun;18(6):641–58. doi: 10.1002/lt.23403. [DOI] [PubMed] [Google Scholar]
- 10.Lunz J, Ruppert KM, Cajaiba MM, Isse K, Bentlejewski CA, Minervini M, et al. Re-examination of the lymphocytotoxic crossmatch in liver transplantation: can C4d stains help in monitoring? Am J Transplant. 2012 Jan;12(1):171–82. doi: 10.1111/j.1600-6143.2011.03786.x. [DOI] [PubMed] [Google Scholar]
- 11.Sakashita H, Haga H, Ashihara E, Wen MC, Tsuji H, Miyagawa-Hayashino A, et al. Significance of C4d staining in ABO-identical/compatible liver transplantation. Mod Pathol. 2007 Jun;20(6):676–84. doi: 10.1038/modpathol.3800784. [DOI] [PubMed] [Google Scholar]
- 12.Musat AI, Agni RM, Wai PY, Pirsch JD, Lorentzen DF, Powell A, et al. The significance of donor-specific HLA antibodies in rejection and ductopenia development in ABO compatible liver transplantation. Am J Transplant. 2011 Mar;11(3):500–10. doi: 10.1111/j.1600-6143.2010.03414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Leary JG, Kaneku H, Jennings LW, Banuelos N, Susskind BM, Terasaki PI, et al. Preformed class II donor-specific antibodies are associated with an increased risk of early rejection after liver transplantation. Liver Transpl. 2013 Jun 18; doi: 10.1002/lt.23687. [DOI] [PubMed] [Google Scholar]
- 14.Manez R, Kobayashi M, Takaya S, Bronsther O, Kramer D, Bonet H, et al. Humoral rejection associated with antidonor lymphocytotoxic antibodies following liver transplantation. Transplant Proc. 1993 Feb;25(1 Pt 2):888–90. [PMC free article] [PubMed] [Google Scholar]
- 15.Batts KP, Moore SB, Perkins JD, Wiesner RH, Grambsch PM, Krom RA. Influence of positive lymphocyte crossmatch and HLA mismatching on vanishing bile duct syndrome in human liver allografts. Transplantation. 1988;45(2):376–9. doi: 10.1097/00007890-198802000-00026. [DOI] [PubMed] [Google Scholar]
- 16.Ludwig J, Wiesner RH, Batts KP, Perkins JD, Krom RA. The acute vanishing bile duct syndrome (acute irreversible rejection) after orthotopic liver transplantation. Hepatology. 1987 May-Jun;7(3):476–83. doi: 10.1002/hep.1840070311. [DOI] [PubMed] [Google Scholar]
- 17.Donaldson PT, Alexander GJ, O’Grady J, Neuberger J, Portmann B, Thick M, et al. Evidence for an immune response to HLA class I antigens in the vanishing-bileduct syndrome after liver transplantation. Lancet. 1987 Apr 25;1(8539):945–51. doi: 10.1016/s0140-6736(87)90293-5. [DOI] [PubMed] [Google Scholar]
- 18.O’Leary JG, Kaneku H, Susskind BM, Jennings LW, Neri MA, Davis GL, et al. High mean fluorescence intensity donor-specific anti-HLA antibodies associated with chronic rejection Postliver transplant. Am J Transplant. 2011 Sep;11(9):1868–76. doi: 10.1111/j.1600-6143.2011.03593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaneku H, O’Leary JG, Taniguchi M, Susskind BM, Terasaki PI, Klintmalm GB. Donor-specific human leukocyte antigen antibodies of the immunoglobulin G3 subclass are associated with chronic rejection and graft loss after liver transplantation. Liver Transpl. 2012 Aug;18(8):984–92. doi: 10.1002/lt.23451. [DOI] [PubMed] [Google Scholar]
- 20.O’Leary JG, Klintmalm GB. Impact of donor-specific antibodies on results of liver transplantation. Curr Opin Organ Transplant. 2013 Jun;18(3):279–84. doi: 10.1097/MOT.0b013e3283614a10. [DOI] [PubMed] [Google Scholar]
- 21.O’Leary JG, Gebel HM, Ruiz R, Bray RA, Marr JD, Zhou XJ, et al. Class II alloantibody and mortality in simultaneous liver-kidney transplantation. Am J Transplant. 2013 Apr;13(4):954–60. doi: 10.1111/ajt.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tait BD, Susal C, Gebel HM, Nickerson PW, Zachary AA, Claas FH, et al. Consensus guidelines on the testing and clinical management issues associated with HLA and non-HLA antibodies in transplantation. Transplantation. 2013 Jan 15;95(1):19–47. doi: 10.1097/TP.0b013e31827a19cc. [DOI] [PubMed] [Google Scholar]
- 23.Demetris AJ, Murase N, Nakamura K, Iwaki Y, Yagihashi A, Valdivia L, et al. Immunopathology of antibodies as effectors of orthotopic liver allograft rejection. Semin Liver Dis. 1992 Feb;12(1):51–9. doi: 10.1055/s-2007-1007376. [DOI] [PubMed] [Google Scholar]
- 24.Knechtle SJ, Kolbeck PC, Tsuchimoto S, Coundouriotis A, Sanfilippo F, Bollinger RR. Hepatic transplantation into sensitized recipients. Demonstration of hyperacute rejection. Transplantation. 1987 Jan;43(1):8–12. doi: 10.1097/00007890-198701000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Knechtle SJ, Wolfe JA, Burchette J, Sanfilippo F, Bollinger RR. Infiltrating cell phenotypes and patterns associated with hepatic allograft rejection or acceptance. Transplantation. 1987 Feb;43(2):169–72. doi: 10.1097/00007890-198702000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura K, Murase N, Becich MJ, Furuya T, Todo S, Fung JJ, et al. Liver allograft rejection in sensitized recipients. Observations in a clinically relevant small animal model. Am J Pathol. 1993 May;142(5):1383–91. [PMC free article] [PubMed] [Google Scholar]
- 27.Paterno F, Shiller M, Tillery G, O’Leary JG, Susskind B, Trotter J, et al. Bortezomib for acute antibody-mediated rejection in liver transplantation. Am J Transplant. 2012 Sep;12(9):2526–31. doi: 10.1111/j.1600-6143.2012.04126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takaya S, Bronsther O, Iwaki Y, Nakamura K, Abu-Elmagd K, Yagihashi A, et al. The adverse impact on liver transplantation of using positive cytotoxic crossmatch donors. Transplantation. 1992;53(2):400–6. doi: 10.1097/00007890-199202010-00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobayashi M, Yagihashi A, Manez R, Takaya S, Noguchi K, Konno A, et al. Posttransplant donor-specific T-lymphocytotoxic antibody in liver transplant patients with a positive crossmatch. Transplant Proc. 1992 Dec;24(6):2510–1. [PMC free article] [PubMed] [Google Scholar]
- 30.Mengel M, Sis B, Haas M, Colvin RB, Halloran PF, Racusen LC, et al. Banff 2011 Meeting report: new concepts in antibody-mediated rejection. Am J Transplant. 2012 Mar;12(3):563–70. doi: 10.1111/j.1600-6143.2011.03926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taner T, Gandhi MJ, Sanderson SO, Poterucha CR, De Goey SR, Stegall MD, et al. Prevalence, course and impact of HLA donor-specific antibodies in liver transplantation in the first year. Am J Transplant. 2012 Jun;12(6):1504–10. doi: 10.1111/j.1600-6143.2012.03995.x. [DOI] [PubMed] [Google Scholar]
- 32.Burns AM, Ma L, Li Y, Yin D, Shen J, Xu J, et al. Memory alloreactive B cells and alloantibodies prevent anti-CD154-mediated allograft acceptance. J Immunol. 2009 Feb 1;182(3):1314–24. doi: 10.4049/jimmunol.182.3.1314. [DOI] [PubMed] [Google Scholar]
- 33.Bingaman AW, Farber DL. Memory T cells in transplantation: generation, function, and potential role in rejection. Am J Transplant. 2004 Jun;4(6):846–52. doi: 10.1111/j.1600-6143.2004.00453.x. [DOI] [PubMed] [Google Scholar]
- 34.Floyd TL, Koehn BH, Kitchens WH, Robertson JM, Cheeseman JA, Stempora L, et al. Limiting the amount and duration of antigen exposure during priming increases memory T cell requirement for costimulation during recall. J Immunol. 2011 Feb 15;186(4):2033–41. doi: 10.4049/jimmunol.1003015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Itescu S, Tung TC, Burke EM, Weinberg A, Moazami N, Artrip JH, et al. Preformed IgG antibodies against major histocompatibility complex class II antigens are major risk factors for high-grade cellular rejection in recipients of heart transplantation. Circulation. 1998 Aug 25;98(8):786–93. doi: 10.1161/01.cir.98.8.786. [DOI] [PubMed] [Google Scholar]
- 36.Itescu S, Tung TC, Burke EM, Weinberg AD, Mancini D, Michler RE, et al. An immunological algorithm to predict risk of high-grade rejection in cardiac transplant recipients. Lancet. 1998 Jul 25;352(9124):263–70. doi: 10.1016/S0140-6736(98)09475-6. [DOI] [PubMed] [Google Scholar]
- 37.Ballardini G, Mirakian R, Bianchi FB, Pisi E, Doniach D, Bottazzo GF. Aberrant expression of HLA-DR antigens on bileduct epithelium in primary biliary cirrhosis: relevance to pathogenesis. Lancet. 1984 Nov 3;2(8410):1009–13. doi: 10.1016/s0140-6736(84)91108-5. [DOI] [PubMed] [Google Scholar]
- 38.Demetris AJ, Lasky S, Van Thiel DH, Starzl TE, Whiteside T. Induction of DR/IA antigens in human liver allografts. An immunocytochemical and clinicopathologic analysis of twenty failed grafts. Transplantation. 1985 Nov;40(5):504–9. doi: 10.1097/00007890-198511000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takaya S, Duquesnoy R, Iwaki Y, Demetris J, Yagihashi A, Bronsther O, et al. Positive crossmatch in primary human liver allografts under cyclosporine or FK 506 therapy. Transplant Proc. 1991 Feb;23(1 Pt 1):396–9. [PMC free article] [PubMed] [Google Scholar]
- 40.Scornik JC, Soldevilla-Pico C, Van der Werf WJ, Hemming AW, Reed AI, Langham MR, Jr, et al. Susceptibility of liver allografts to high or low concentrations of preformed antibodies as measured by flow cytometry. Am J Transplant. 2001 Jul;1(2):152–6. doi: 10.1034/j.1600-6143.2001.10209.x. [DOI] [PubMed] [Google Scholar]


