Abstract
The objective of this study was to increase the understanding of the “second-hit” response in thermal injury. The authors hypothesized that prior thermal injury increases the endotoxin-induced inflammatory response of intestinal mucosa. Mice underwent sham or 25% TBSA scald injury. Seven days after injury, mice were injected with lipopolysaccharide. Blood, jejunum, and colon specimens were obtained at intervals. Serum, jejunal, and colon inflammatory cytokine levels were measured by enzyme-linked immunosorbent assay. Jejunal and colon nuclear factor (NF)-κB activation was measured by electrophoretic mobility shift assay. After remote thermal injury, lipopolysaccharide exposure led to an acute increase in serum interleukin (IL)-6, IL-10, and chemokine keratinocyte-derived chemokine (KC) levels. This correlated with lipopolysaccharide-induced increased IL-6 in colon and chemokine KC in the jejunum and colon in burned mice when compared with sham-injured mice. Lipopolysaccharide-induced NF-κB activation occurred more rapidly in jejunum and colon from burned mice compared with sham-injured mice. Prior thermal injury accelerates lipopolysaccharide-induced inflammatory cytokine production systemically in jejunum and colon. The “second hit” of lipopolysaccharide led to earlier intestinal NF-κB activation in burned mice compared with sham-injured mice. These results indicate that there is a heightened inflammatory response by jejunum and colon in response to a “second hit” of lipopolysaccharide after burn injury.
Severe thermal injury represents a significant inducing factor of the systemic inflammatory response syndrome. Systemic inflammation response syndrome and sepsis remain leading causes of death in intensive care units.1–3 Approximately 50% of patients admitted with burn injuries suffer from infectious complications that contribute to nearly 75% of burn-related deaths.4 In these patients, the acute inflammatory response to the burn may be nonlethal, but the “second hit” of subsequent infection increases their morbidity and mortality.5 Thus, understanding the effect of injury on the inflammatory response to infection is imperative to improving outcomes of burned patients.
The role of the intestine in the inflammatory response to injury and infection is not completely understood. Despite constant exposure of intestinal mucosa to high levels of bacterial products, most notably lipopolysaccharide (LPS), this tissue exhibits minimal baseline inflammation. In the setting of injury or other stressors, the intestinal mucosa may become more sensitive to LPS, leading to increased local inflammation.6,7 Such perturbations in this basal state could be clinically important considering evidence that injury-induced gut inflammation contributes significantly to the systemic inflammatory response.8,9
An augmented intestinal response to LPS may result in increased local inflammatory cytokine production, mucosal inflammation, and intestinal barrier dysfunction.10 In vitro experiments have demonstrated that intestinal epithelial cells treated with pro-inflammatory cytokines respond with increased Toll-like receptor (TLR)-4 expression and an augmented LPS response.11 Meanwhile, in vivo studies have demonstrated that burn injury leads to increased interleukin (IL)-6 mRNA in intestinal lamina propria and gut bacterial translocation.12 In addition, ex vivo enterocytes harvested from previously burned animals produced more IL-6 in the presence of LPS when compared with enterocytes from control animals.13 A recent study from our laboratory14 suggests that the “second hit” of LPS may result in increased intestinal IL-6 levels after LPS treatment and increased late activation of the transcription factor activated protein (AP)-1, but the early inflammatory response to LPS in animals who have underdone previous thermal injury is unknown. Collectively, these findings indicate that injury predisposes the gut toward an exaggerated response to LPS. However, the mechanisms by which injury alters the intestinal LPS response remain elusive.
With the aim of understanding the immediate intestinal response to a “second hit” of infection after injury, we investigated the effect of prior burn injury on the immediate inflammatory response after subsequent LPS exposure in mice. We hypothesized that prior burn injury would increase LPS-induced production of inflammatory cytokines in intestinal mucosa, possibly through altered nuclear factor (NF)-κB signaling in the intestinal mucosa.
METHODS
Induction of Thermal Injury
Male C57/BL6 mice weighing 22 to 29 g were purchased from The Jackson Laboratory (Bar Harbor, ME), fed standard laboratory diet and water ad libitum, and acclimated for 1 week in a climate-controlled room with a 12-hour light-dark cycle. Experiments were approved by the Institutional Animal Care and Use Committee at the University of Cincinnati.
Thermal injury was induced as described previously with minor modifications.15 Briefly, mice (n = 3 in each group) were anesthetized with pentobarbital 60 mg/kg intraperitoneal (IP) injection as well as inhaled isoflurane (1.5%). Dorsal fur was removed by clipping, and mice were exposed to 90°C water for 9 seconds resulting in a 25% TBSA full-thickness burn. Sham mice were exposed to room temperature water. All mice were resuscitated immediately with 1ml normal saline IP and allowed to recover in a warmed oxygen tent.
Seven days after initial burn or sham injury, induction of the second hit was achieved with IP injection of LPS, 10 mg/kg based on average weight (E. coli 0111:B4, Calbiochem La Jolla, CA, or Sigma, St. Louis, MO). IP injection of normal saline was used as a negative control. At intervals, mice were killed, and blood was obtained by cardiac puncture, separated by centrifugation, and serum stored at −80°C until analysis. Small and large bowel were removed and flushed with saline. Jejunal mucosa was harvested by scraping as described previously,16 whereas colon was procured whole. Specimens were snap-frozen in liquid nitrogen and stored at −80°C until analysis.
Tissue Extraction
Nuclear and cytoplasmic fractions were prepared as described previously.16 All steps were carried out on ice. Tissue samples were homogenized in 0.5 ml of buffer A (10 mmol/L N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid [pH 7.9], 1.5 mmol/L MgCl2, 10 mmol/L KCl, 1 mmol/L dithiothreitol, and 1 mmol/L phenylmethanesulfonylfluoride [PMSF]), incubated for 10 minutes, and then centrifuged at 850g for 10 minutes at 4°C. The pellets were resuspended in 1.5 times cell volume of buffer A with 0.1% Triton X-100, incubated for 10 minutes, and centrifuged as mentioned earlier. The supernatant was removed and saved as the cytoplasmic fraction. The pellet was resuspended in 300 µl of buffer A, centrifuged as mentioned earlier, and resuspended in 1 cell volume of a buffer of 20 mmol/LN-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (pH 7.9), 25% glycerol (volume per volume), 420 mmol/L NaCl, 1.5 mmol/L MgCl2, and 0.2 mmol/L EDTA. After incubation for 30 minutes, the nuclear fraction was recovered by centrifugation at 20,000g for 15 minutes at 4°C. Fractions were assayed for protein concentration (BCA Protein Assay Kit, Pierce, Rockford, IL) and stored at −80°C until analysis.
Cytokine analysis was performed as described previously.17 Jejunal scrapings were sonicated for two 10-second periods in 1 ml phosphate-buffered saline containing Complete Protease Inhibitor Cocktail Tablets (Roche, Indianapolis, IN) and 2 mM PMSF (Sigma). Colon whole bowel specimens were first homogenized and then sonicated for one 10-second period in this same solution. Samples were centrifuged at 12,000g at 4°C for 45 minutes. Supernatant density was determined using BCA Protein Assay Kit (Pierce). IL-6, IL-10, and chemokine KC was measured using commercially available ELISA kits (R&D Systems, Minneapolis, MN, and Quansys Biosystems, Logan, UT) per the manufacturer’s instructions. Serum cytokines are expressed as pictogram per milliliter and intestinal cytokines as nanogram per gram protein.
Electrophoretic Mobility Shift Assay
Nuclear extracts of jejunal or colonic tissue were analyzed by electrophoretic mobility shift assay as described previously.16 Double-stranded consensus oligonucleotide of NF-κB (Promega, Madison, WI) was end-labeled with (32P) γ-adenosine triphosphate (Perkin Elmer, Boston, MA) using polynucleotide kinase T4 (Promega). End-labeled probe was purified from unincorporated (32P) γ-adenosine triphosphate using a purification column (Bio-Rad Laboratories, Hercules, CA). Binding reactions (total volume 15 µl) with equal amounts of nuclear extracts (15–20 µg) and oligonucleotide and buffer containing 20% glycerol (vol/vol), 50 mM Tris-HCl, pH 7.9, 2.5 mM EDTA, 2.5 mM dithiothreitol, 5 mM MgCl2, 250 mM NaCl, and 0.25 µg/µl poly[d(I-C)] (USB Corporation, Cleveland, OH) were incubated at room temperature for 30 minutes. For supershift reactions, 1 µl of antibodies to p50, p52, crel, or p65 was added to samples for 30 minutes before addition of oligonucleotide. Samples were subjected to electrophoretic separation on a nondenaturing 5% polyacrylamide gel at 100 V. Blots were dried at 53°C for 3 hours and analyzed by exposure to PhosphorImager screen (GE Healthcare, Piscataway, NJ) or autoradiography film.
Statistical Analysis
Analysis of variance followed by Tukey’s post hoc test was used to compare mean values between groups. A P value <.05 was considered significant. Cytokine concentrations are expressed as mean ± SEM.
RESULTS
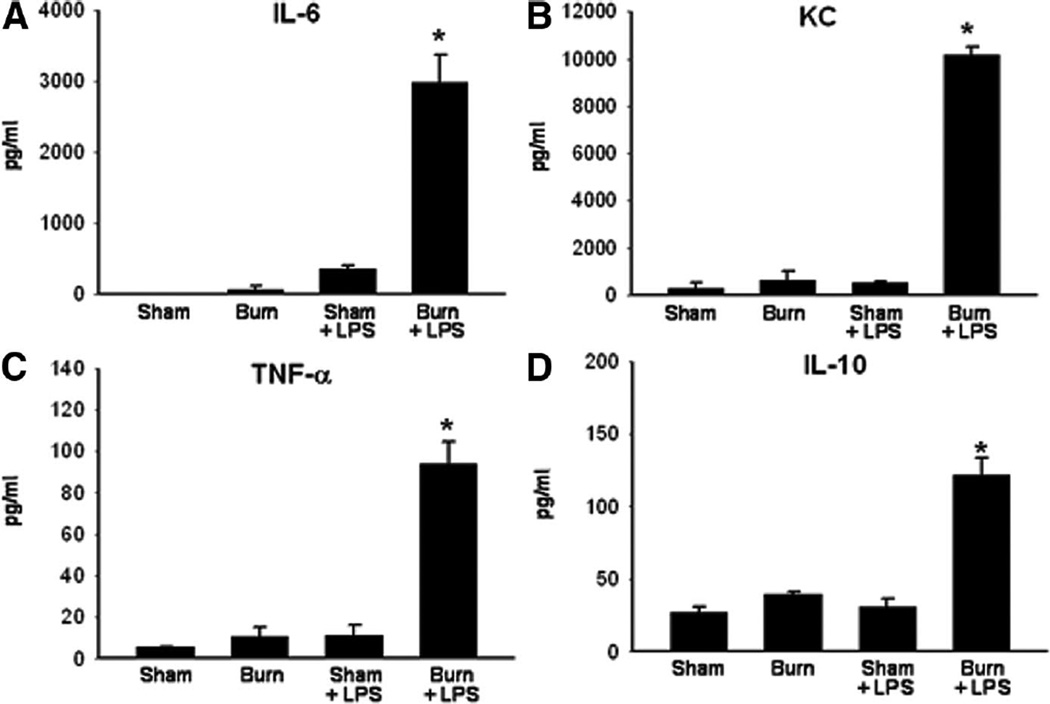
Thermal injury causes an intense initial systemic inflammatory response resulting in increased proinflammatory cytokine levels that subsequently resolve. Based on the previous experiments in our laboratory, mice subjected to burn injury have increased serum levels of multiple proinflammatory cytokines, including IL-6 and tumor necrosis factor (TNF)-α, during the first 24 hours after injury (data not shown). By postburn day (PBD) 7, this initial inflammatory response resolved, and there were no significant differences between sham and burn mice in serum levels of IL-6, TNF-α, or other markers tested including KC and IL-10 (Figure 1). To determine whether the initial burn injury altered a subsequent response to LPS, we allowed 7 days for the acute inflammatory response to burn injury to subside before evaluating the systemic and intestinal response to endotoxin challenge. To induce the “second hit,” mice were injected with 10 mg/kg LPS. When we examined serum from burned and sham-injured mice after LPS injection, we noted significantly increased levels of IL-6, KC, TNF-α, and IL-10 within 20 minutes of LPS injection (Figure 1).
Figure 1.
Serum ELISA for (A) interleukin (IL)-6, (B) KC, (C) tumor necrosis factor (TNF)-α, and (D) IL-10 in mice treated with sham or burn injury, allowed to recover for 7 days, then injected with normal saline or LPS. *P < .05 in burned/LPS-injected mice compared with all other groups (n = 3 for each group).
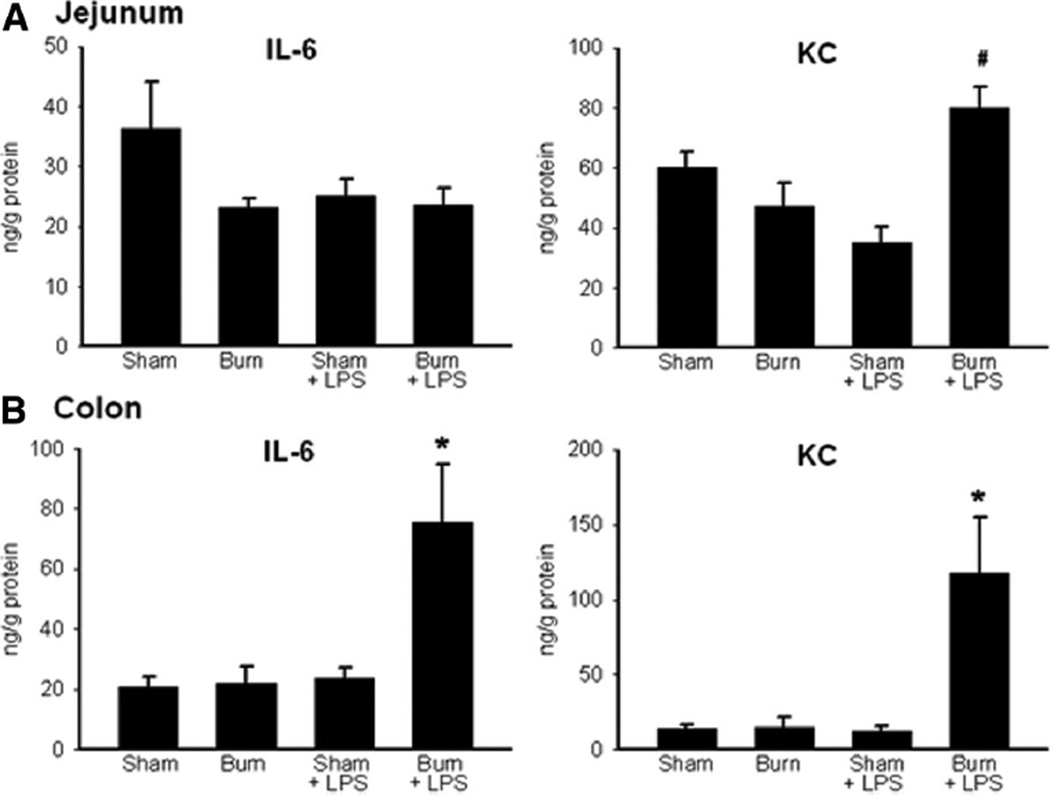
When we examined the intestinal samples on PBD 7, there were no differences in jejunum or colon levels of IL-6 or KC in sham compared with burn-injured mice (Figure 2), similar to our results of serum cytokine analysis. Mice receiving LPS after burn injury showed significantly increased jejunal KC levels compared with sham mice receiving LPS (Figure 2A). Jejunum IL-6 levels were unchanged between these groups at this time point (Figure 2A). In the colon, both IL-6 and KC (Figure 2B) were significantly increased after LPS injection in mice with prior burn injury when compared with sham injury. When we analyzed samples from jejunum and colon for TNF-α and IL-10, we found no differences between groups (data not shown).
Figure 2.
ELISA for interleukin (IL)-6 and KC in the jejunum (A) and colon (B) from mice treated with sham or burn injury, recovered for 7 days, then treated with NS or LPS injection. #P < .05 in jejunum burned/LPS-injected mice compared with sham/LPS and burn only groups. *P < .05 in the colon from burn/LPS-injected mice compared with all other groups. N = 3 for each group.
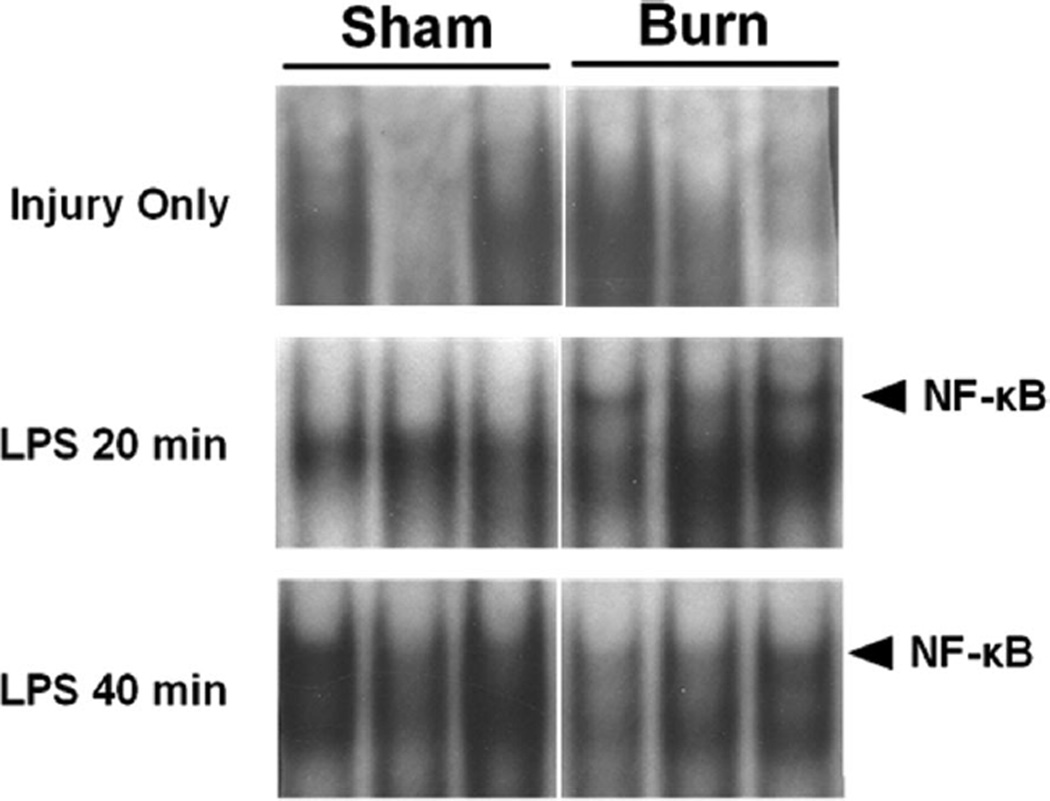
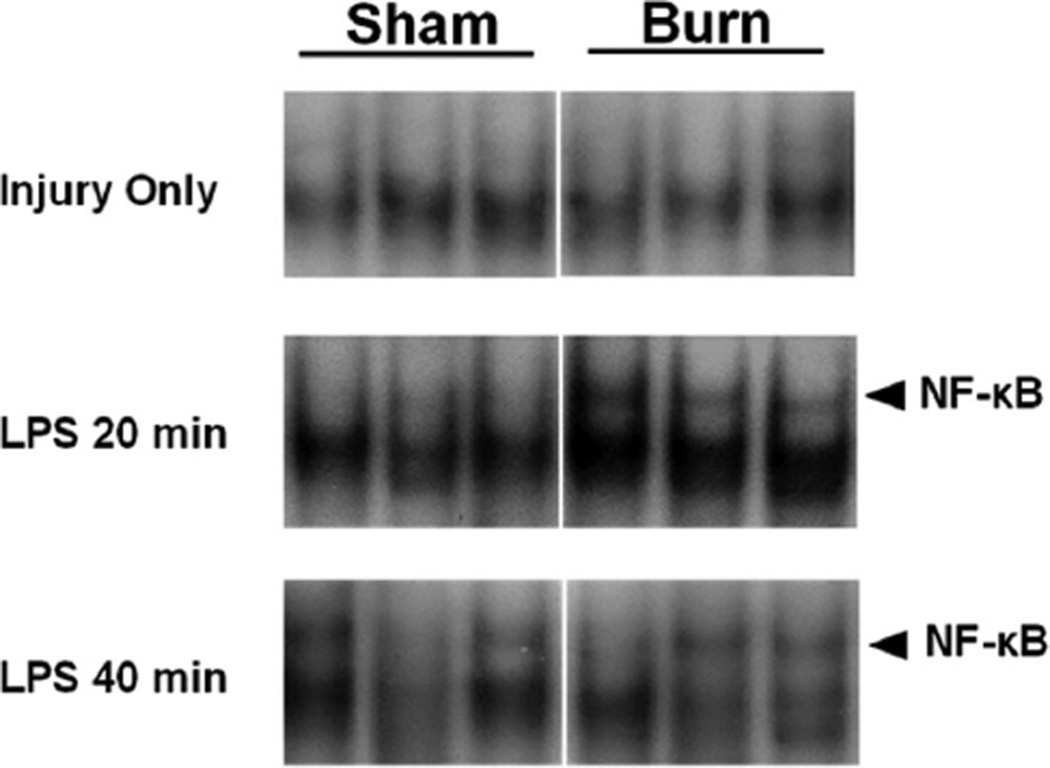
The LPS signaling pathway converges on transcription factors including NF-κB. This transcription factor plays a central role in regulating inflammation, including, at least in part, the proinflammatory mediators IL-6 and KC. To determine whether the altered expression of IL-6 and KC could be due to changes in NF-κB activation, we evaluated NF-κB in jejunum and colon. On PBD 7, there were no changes in NF-κB DNA binding activity in jejunum from sham compared with burn mice (Figure 3). Sequential treatment with thermal injury followed by LPS injection resulted in an increased intestinal NF-κB activation within 20 minutes of injection (Figure 3). Both burn and sham injured groups demonstrated NF-κB activation by 40 minutes after LPS injection (Figure 3). Similar results were observed in the colon (Figure 4). Supershift analysis showed that this activation band consisted mainly of p65 and p50/p65 complexes (Figure 5). In the normal saline (vehicle control)-injected mice, there was no difference in NF-κB activation between groups.
Figure 3.
Electrophoretic mobility shift assay for nuclear factor (NF)-κ B binding activity in the jejunum after burn or sham injury followed by LPS injection. Samples are from individual mice (n = 3 total for each condition) and are cut from the same blot for each time point.
Figure 4.
Electrophoretic mobility shift assay for nuclear factor (NF)-κB binding activity in the colon after burn or sham injury followed by LPS injection. Samples are from individual mice (n = 3 total for each condition) and are cut from the same blot for each time point.
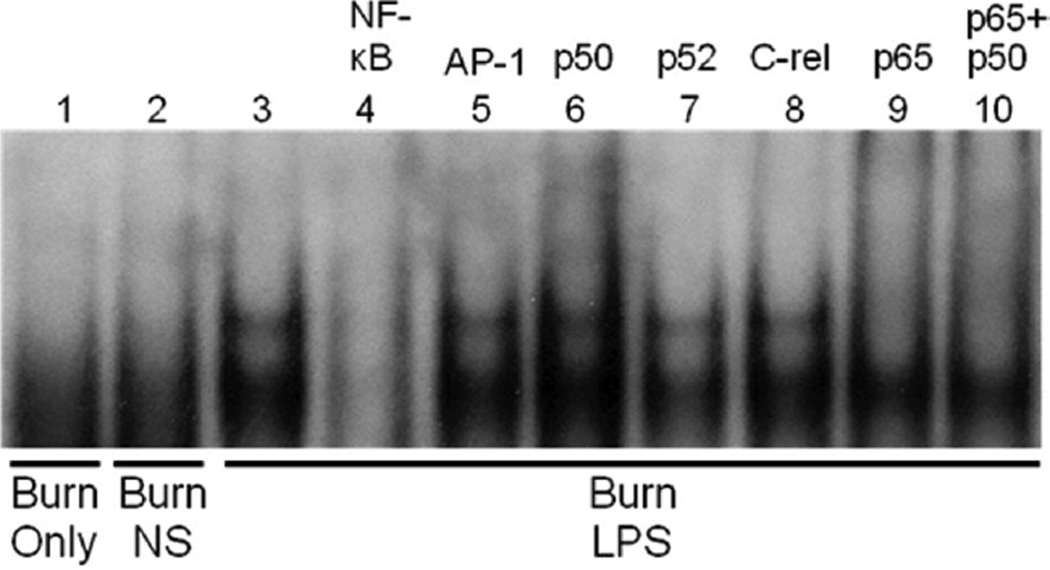
Figure 5.
Supershift electrophoretic mobility shift assay (EMSA) characterization of LPS inducible nuclear factor (NF)-κB binding in the jejunum of mice 20 minutes after LPS injection. The activation (upper) band is not present after burn injury alone or after normal saline (NS) vehicle injection (lanes 1–3). Cold competition with NF-κB but not with activated protein (AP)-1 oligonucleotide inhibited binding, demonstrating probe specificity (lanes 4 and 5). Supershift EMSA using specific antibodies directed toward p50, p52, crel, and p65 subunits show that the upper activation band is composed of p65 and p50/p65 components (lanes 6–10). The experiment was performed in triplicate to ensure reproducibility.
DISCUSSION
In this study, we examined the effect of prior thermal injury on the subsequent intestinal immune response to a “second hit” from endotoxin. Our data indicate that prior thermal injury alters the immunologic tone of the jejunum and colon, rendering mice more sensitive to a “second hit” from LPS 7 days later. These data extend our previous findings of increased IL-6 in intestine late after administration of the “second hit” and suggest that LPS administration after prior thermal injury is associated with alterations in proximal signaling events.
Our experiments demonstrated that mice with a history of remote thermal injury respond to LPS with acutely increased systemic levels of proinflammatory cytokines including IL-6, TNF-α, and KC. These cytokines act in a variety of ways on different tissues to initiate the acute phase response and recruit inflammatory cells to sites of inflammation. Coinciding with this increase in proinflammatory cytokines, we noted that serum IL-10, which has primarily anti-inflammatory properties, including inhibition of neutrophil TNF-α and IL-8 release, was elevated in burned mice compared with sham-injured mice after LPS injection. Collectively, these cytokine changes suggest that injury primes the innate immune response to subsequent pathogen exposure, leading to an exuberant proinflammatory response in concert with increased negative feedback loop signaling.
The effect of the “second hit” on serum cytokine levels guided our investigation of intestinal cytokine changes after injury alone and after injury combined with endotoxin exposure. We found that colonic IL-6 levels were especially responsive to the “second hit” of LPS. IL-6 is a pleiotropic cytokine which may, depending on various factors, exert either proinflammatory or anti-inflammatory effects on tissues (reviewed in Refs 18 and 19). IL-6 is produced by intestinal mucosa during critical illness. In previous in vivo models, intestinal IL-6 was increased in the setting of endotoxemia and sepsis20 and has been shown to play a key role in increased intestinal permeability and bacterial translocation after ischemia/reperfusion injury.10
Our results suggest that the chemokine KC, a mouse homologue to human IL-8, increased rapidly after LPS injection in jejunum and colon of mice with prior thermal injury. IL-8 is rapidly synthesized at sites of inflammation and plays an important role in neutrophil chemotaxis and activation. In addition to LPS, other stimuli including cytokines such as TNF-α can induce release of IL-8. Monocytes and macrophages have been thought to be the principle sources of this cytokine, but enterocytes have been shown to produce this chemokine as well.19 Our data suggest that increased KC production is a component of the “two-hit” response to burn injury and subsequent infection. Thus, we sought to explore the mechanisms of increased production of cytokines such as chemokine KC in intestinal mucosa (jejunum) and whole bowel intestinal samples (colon).
Both IL-6 and KC are regulated, at least in part, by the transcription factor NF-κB, which plays a central role in regulating inflammation.21 We found that NF-kB activation occurred earlier (within 20 minutes of LPS exposure) in jejunal mucosa scrapings and colon whole bowel samples in mice with prior burn injury. The source of this NF-κB activation may be enterocytes or other inflammatory cells in the intestine. In either case, our data suggest that NF-κB activation is an important mechanism in the acute inflammatory response of the injury-primed intestine to subsequent inflammatory stimuli. We hypothesized that prior burn injury may predispose intestinal epithelium to an augmented LPS response by upregulation of the LPS receptor TLR-4. However, Western blot analysis of jejunum and colon revealed no difference in the expression of TLR-4 or its essential coreceptor, myeloid differentiation protein-2, 1 week after burn injury.14 Despite evidence of accelerated NF-κB activation and increased proinflammatory cytokine levels in the intestine after LPS injection in mice with prior burn injury, our preliminary data suggest that this phenomenon is not the result of increased intestinal TLR-4 receptor expression in these mice. Instead, it is possible that alterations in intermediary components of the signaling pathway may be involved in the augmented response to LPS.
When patients survive an initial injury, they are at an increased risk for morbidity and mortality from subsequent insults such as sepsis and endotoxemia. Our data indicate that one aspect of this “second hit” is an augmented activation of NF-κB in intestinal mucosa which correlates with acute increases in intestinal and systemic inflammatory cytokines. Synergistic elevation of these markers in mice treated with LPS after a remote burn injury strongly suggest that these conditions also produce an exaggerated systemic inflammatory response and may lead to an increased multiple organ failure. This marked increase of these cytokines and NF-κB activation in jejunal mucosa and colon after LPS exposure in burned mice indicate that the second-hit phenomena may be due to increased intestinal NF-κB-mediated inflammation.
The inflammatory response to thermal injury is complex and seems to lead to long-term alterations in the subsequent response to a “second hit.” Previous experiments suggest that the nature of the “second hit” after previous thermal injury may drastically alter the subsequent host response. A “second hit” of endotoxemia or Pseudomonas aeruginosa infection seems to lead to increased systemic and organ-specific inflammation (current study and Refs. 22 and 23). In contrast, prior thermal injury appears to blunt the inflammatory response to a subsequent “second hit” consisting of cecal ligation and puncture.24 Further experiments are needed to delineate the influence of the type of “second hit” on the inflammatory response after prior thermal injury.
These data expand upon previous work from our group using a two-hit model of remote thermal injury followed by endotoxin injection with analysis at later time points. Four hours after a second hit of LPS, we noted increased tissue levels of IL-6 and augmented binding of AP-1 in the colon of previously burned mice.14 The results reported here showing acutely increased intestinal NF-κB activation in conjunction with our prior observations of later increased AP-1 binding in this two-hit model are consistent with the reported kinetics of LPS-induced time-dependent activation of these transcription factors.25
Our current findings are important because they imply that a prior injury can induce persistent alterations in the inflammatory response to a subsequent insult. A potentiated inflammatory response of the intestine may be detrimental given the key role this organ seems to play in the pathogenesis of the systemic inflammatory response and multiple organ failure. An increased understanding of the altered inflammatory response of the gut after burn injury may allow therapeutic intervention in patients at risk for a “second hit.”
Acknowledgments
Supported by grants no. 8902 (to T.A.P.) and no. 8903 (to C.K.O.) from the Shriners Hospitals for Children.
Footnotes
Presented as an abstract at the 95th Annual American College of Surgeons Clinical Congress, Surgical Forum (Oral Presentation), October 13, 2009, Chicago, IL.
REFERENCES
- 1.Saffle JR, Sullivan JJ, Tuohig GM, Larson CM. Multiple organ failure in patients with thermal injury. Crit Care Med. 1993;21:1673–1683. doi: 10.1097/00003246-199311000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Harris BH, Gelfand JA. The immune response to trauma. Semin Pediatr Surg. 1995;4:77–82. [PubMed] [Google Scholar]
- 3.Baue AE, Durham R, Faist E. Systemic inflammatory response syndrome (SIRS), multiple organ dysfunction syndrome (MODS), multiple organ failure (MOF): are we winning the battle? Shock. 1998;10:79–89. doi: 10.1097/00024382-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Santucci SG, Gobara S, Santos CR, Fontana C, Levin AS. Infections in a burn intensive care unit: experience of seven years. J Hosp Infect. 2003;53:6–13. doi: 10.1053/jhin.2002.1340. [DOI] [PubMed] [Google Scholar]
- 5.Moore FA, Moore EE. Evolving concepts in the pathogenesis of postinjury multiple organ failure. Surg Clin North Am. 1995;75:257–277. doi: 10.1016/s0039-6109(16)46587-4. [DOI] [PubMed] [Google Scholar]
- 6.Williams DL, Ha T, Li C, et al. Modulation of tissue Toll-like receptor 2 and 4 during the early phases of polymicrobial sepsis correlates with mortality. Crit Care Med. 2003;31:1808–1818. doi: 10.1097/01.CCM.0000069343.27691.F3. [DOI] [PubMed] [Google Scholar]
- 7.Paterson HM, Murphy TJ, Purcell EJ, et al. Injury primes the innate immune system for enhanced Toll-like receptor reactivity. J Immunol. 2003;171:1473–1483. doi: 10.4049/jimmunol.171.3.1473. [DOI] [PubMed] [Google Scholar]
- 8.Magnotti LJ, Upperman JS, Xu DZ, Lu Q, Deitch EA. Gut-derived mesenteric lymph but not portal blood increases endothelial cell permeability and promotes lung injury after hemorrhagic shock. Ann Surg. 1998;228:518–527. doi: 10.1097/00000658-199810000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magnotti LJ, Xu DZ, Lu Q, Deitch EA. Gut-derived mesenteric lymph: a link between burn and lung injury. Arch Surg. 1999;134:1333–1340. discussion 1340–1. [PubMed] [Google Scholar]
- 10.Yang R, Han X, Uchiyama T, et al. IL-6 is essential for development of gut barrier dysfunction after hemorrhagic shock and resuscitation in mice. Am J Physiol Gastrointest Liver Physiol. 2003;285:G621–G629. doi: 10.1152/ajpgi.00177.2003. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki M, Hisamatsu T, Podolsky DK. Gamma interferon augments the intracellular pathway for lipopolysaccharide (LPS) recognition in human intestinal epithelial cells through coordinated up-regulation of LPS uptake and expression of the intracellular Toll-like receptor 4-MD-2 complex. Infect Immun. 2003;71:3503–3511. doi: 10.1128/IAI.71.6.3503-3511.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bai X, Xiao G, Tian X. [The relationship between postburn gene expression of modulators in gut associated lymph tissue and the change in IgA plasma cells.] Zhonghua Shao Shang Za Zhi. 2000;16:108–110. [PubMed] [Google Scholar]
- 13.Ogle CK, Mao JX, Wu JZ, Ogle JD, Alexander JW. The 1994 Lindberg Award. The production of tumor necrosis factor, interleukin-1, interleukin-6, and prostaglandin E2 by isolated enterocytes and gut macrophages: effect of lipopolysaccharide and thermal injury. J Burn Care Rehabil. 1994;15:470–477. [PubMed] [Google Scholar]
- 14.Huber NL, Bailey SR, Schuster RM, et al. Remote thermal injury increases LPS-induced intestinal IL-6 production. J Surg Res. 2010;160:190–195. doi: 10.1016/j.jss.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noel JG, Guo X, Wells-Byrum D, et al. Effect of thermal injury on splenic myelopoiesis. Shock. 2005;23:115–122. doi: 10.1097/01.shk.0000154239.00887.18. [DOI] [PubMed] [Google Scholar]
- 16.Pritts TA, Wang Q, Sun X, et al. Induction of the stress response in vivo decreases nuclear factor-kappa B activity in jejunal mucosa of endotoxemic mice. Arch Surg. 2000;135:860–866. doi: 10.1001/archsurg.135.7.860. [DOI] [PubMed] [Google Scholar]
- 17.Tung PH, Wang Q, Ogle CK, Smith CD. Minimal increase in gut-mucosal interleukin-6 during laparoscopy. Surg Endosc. 1998;12:409–411. doi: 10.1007/s004649900692. [DOI] [PubMed] [Google Scholar]
- 18.Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP. The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med. 1998;128:127–137. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- 19.Pritts T, Hungness E, Wang Q, et al. Mucosal and enterocyte IL-6 production during sepsis and endotoxemia—role of transcription factors and regulation by the stress response. Am J Surg. 2002;183:372–383. doi: 10.1016/s0002-9610(02)00812-7. [DOI] [PubMed] [Google Scholar]
- 20.Meyer TA, Wang J, Tiao GM, et al. Sepsis and endotoxemia stimulate intestinal interleukin-6 production. Surgery. 1995;118:336–342. doi: 10.1016/s0039-6060(05)80342-3. [DOI] [PubMed] [Google Scholar]
- 21.Ohmori Y, Fukumoto S, Hamilton TA. Two structurally distinct kappa B sequence motifs cooperatively control LPS-induced KC gene transcription in mouse macrophages. J Immunol. 1995;155:3593–3600. [PubMed] [Google Scholar]
- 22.Adediran SG, Dauplaise DJ, Kasten KR, et al. Early infection during burn-induced inflammatory response results in increased mortality and p38-mediated neutrophil dysfunction. Am J Physiol Regul Integr Comp Physiol. 2010;299:R918–R925. doi: 10.1152/ajpregu.00132.2010. [DOI] [PubMed] [Google Scholar]
- 23.Murphy TJ, Paterson HM, Kriynovich S, et al. Linking the “two-hit” response following injury to enhanced TLR4 reactivity. J Leukoc Biol. 2005;77:16–23. doi: 10.1189/jlb.0704382. [DOI] [PubMed] [Google Scholar]
- 24.Maung AA, Fujimi S, MacConmara MP, et al. Injury enhances resistance to Escherichia coli infection by boosting innate immune system function. J Immunol. 2008;180:2450–2458. doi: 10.4049/jimmunol.180.4.2450. [DOI] [PubMed] [Google Scholar]
- 25.Zhou HR, Islam Z, Pestka JJ. Kinetics of lipopolysaccharide-induced transcription factor activation/inactivation and relation to proinflammatory gene expression in the murine spleen. Toxicol Appl Pharmacol. 2003;187:147–161. doi: 10.1016/s0041-008x(02)00077-7. [DOI] [PubMed] [Google Scholar]