Abstract
Melatonin is an important secondary messenger in plant innate immunity against the bacterial pathogen Pseudomonas syringe pv. tomato (Pst) DC3000 in the salicylic acid (SA)- and nitric oxide (NO)-dependent pathway. However, the metabolic homeostasis in melatonin-mediated innate immunity is unknown. In this study, comparative metabolomic analysis found that the endogenous levels of both soluble sugars (fructose, glucose, melibose, sucrose, maltose, galatose, tagatofuranose and turanose) and glycerol were commonly increased after both melatonin treatment and Pst DC3000 infection in Arabidopsis. Further studies showed that exogenous pre-treatment with fructose, glucose, sucrose, or glycerol increased innate immunity against Pst DC3000 infection in wild type (Col-0) Arabidopsis plants, but largely alleviated their effects on the innate immunity in SA-deficient NahG plants and NO-deficient mutants. This indicated that SA and NO are also essential for sugars and glycerol-mediated disease resistance. Moreover, exogenous fructose, glucose, sucrose and glycerol pre-treatments remarkably increased endogenous NO level, but had no significant effect on the endogenous melatonin level. Taken together, this study highlights the involvement of sugars and glycerol in melatonin-mediated innate immunity against bacterial pathogen in SA and NO-dependent pathway in Arabidopsis.
Since melatonin (N-acetyl-5-methoxytryptamine) was first discovered and examined in plants by Dubbels et al. and Hattori et al. in 19951,2, it has drawn progressively more attention of plant scientists3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34. Initially, the studies focused on the distribution of melatonin in plants, by comparing the differences of endogenous melatonin levels in different plant organs and in numerous plant species, and investigating the effects of various stress treatments, senescence, light, dark, the location of growth, growth state, harvest time of seeds on the endogenous melatonin levels3,4,5,6,7,8,9,10,11,12,13,14. In recent ten years, plant biologists have paid more attentions to investigating the biological roles of melatonin in plants due to exogenous application of melatonin15,16,17,18,19,20,21,22,23,24,25,26,27 or using melatonin-deficient and melatonin-enrich transgenic plants by genetic modulation of melatonin synthetic or metabolic-related genes28,29,30,31,32,33,34.
To date, the in vivo roles of melatonin in plant seed germination, primary root and lateral root development, circadian rhythms and photoprotection, flowering time and vegetative growth, fruit ripening and natural senescence have been described15,16,17,18,19,20. Also, melatonin protects against stresses including drought, salt, osmotic stress, oxidative stress, heat and cold stresses, copper stress, plant-pathogen interaction, etc21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42. Some biological roles of melatonin in plants are similar to those in animals, such as regulation of circadian rhythms12,36,37,38,39,40, scavenging of reactive nitrogen species (RNS) and reactive oxygen species (ROS)13,42,43,44,45,46, and regulation of innate immunity22,32,33,41,47,48.
In animals, melatonin is involved in innate immunity by acting on basophils, eosinophils, eosinophils, monocytes-macrophages, neutrophils, dendritic cells, mast cells and natural killer cells47,48. In plants, at least four reports have revealed the protective role of melatonin in plant innate immunity22,32,33,41. It was found that exogenous melatonin pre-treatment improved resistance of apple to Marssonina apple blotch (Diplocarpon mali) by modulating activities of antioxidant enzymes, hydrogen peroxide (H2O2) level and transcripts of pathogensis-related proteins (PRs) during plant-pathogen interaction22. Lee et al. found that melatonin-treatment increased disease resistance against pathogen attack such as Pseudomonas syringe pv. tomato (Pst) DC3000 by up-regulating a series of defense genes that were activated by salicylic acid (SA) and ethylene (ET) in Arabidopsis and tobacco32. Using the knockout mutant of serotonin N-acetyltransferase (SNAT) which is the penultimate enzyme in melatonin biosynthesis pathway, Lee et al. found that snat knockout mutants exhibited susceptibility to pathogen Pst DC3000 (avrRpt2) infection that coincided with decreased endogenous melatonin and SA levels as well as reduced induction of defense genes, this indicated that melatonin-elicited pathogen resistance is SA-dependent in Arabidopsis33. Recently, Shi et al. found that melatonin positively regulated plant innate immunity against bacterial pathogen in SA- and nitric oxide (NO)-dependent pathway41. Zhao et al. investigated the effect of exogenous melatonin on carbohydrate metabolism especially sucrose metabolism, and found that melatonin-mediated sucrose metabolism is contributed to pathogen resistance49. However, the comprehensive metabolic homeostasis including amino acids, organic acids, sugars and sugar alcohols in response to melatonin and bacterial pathogen remains unclear.
In this study, comparative metabolomic analysis using gas chromatography time-of-flight mass spectrometry (GC-TOF-MS) was performed to assay primary metabolites after melatonin treatment and Pst DC3000 infection in Arabidopsis. Further studies investigated the association among sugars and sugar alcohols, melatonin-mediated disease response, and SA and NO signaling in Arabidopsis. Based on collective results herein, we highlight the involvement of sugars and glycerol in melatonin-mediated innate immunity against bacterial pathogen in SA and NO-dependent pathway in Arabidopsis.
Results
The effects of exogenous melatonin treatment and Pst DC3000 infection on the endogenous levels of primary metabolites
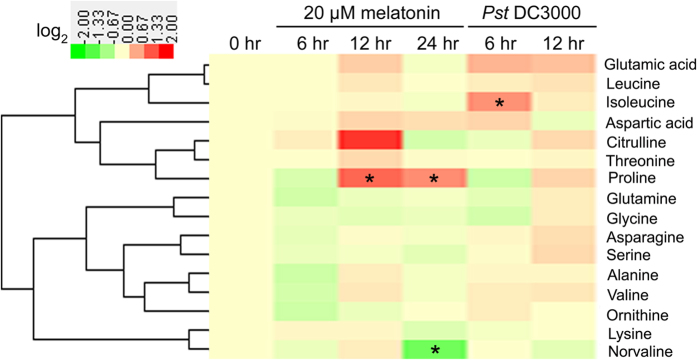
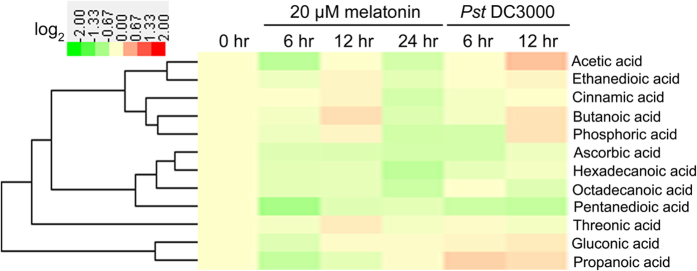
To gain additional insight into the metabolic homeostasis which is commonly affected by exogenous melatonin and bacterial pathogen Pst DC3000 infection, comparative metabolomic analysis using GC-TOF-MS was performed to quantify the primary metabolites after stress treatments. Totally, 51 metabolites including 16 amino acids, 12 organic acids, 18 sugars and 5 sugar alcohols were reproducibly examined in Arabidopsis leaves after different treatments (Table 1 and Supplemental Table S1). Among 16 assayed amino acids, exogenous melatonin significantly increased proline level after treatment for 12 hrs and 24 hrs, but reduced norvaline level after treatment for 24 hrs, and Pst DC3000 significantly increased isoleucine level after infection for 6 hrs (Fig. 1 and Supplemental Table 1). However, both exogenous melatonin treatment and Pst DC3000 infection had no significant effect on the endogenous levels of assayed 12 organic acids (Fig. 2 and Supplemental Table 1).
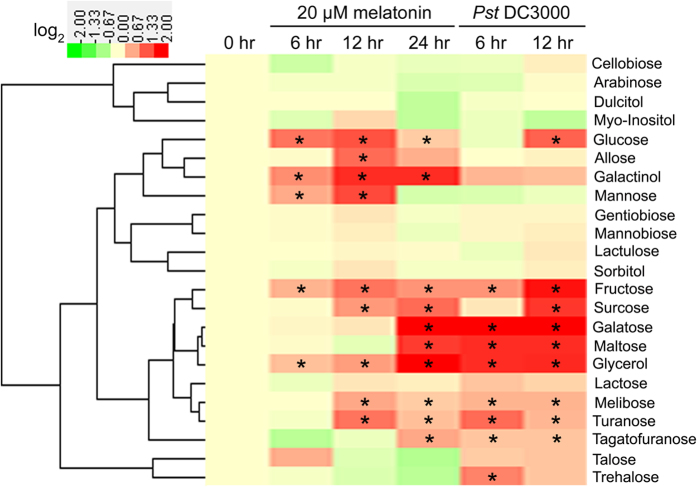
Table 1. Concentrations of 18 sugars and 5 sugar alcohols in response to melatonin and Pst DC3000 treatments in Arabidopsis. The concentrations of metabolites were expressed as μg g−1 FW. The data represent the means of three biological repeats ± SDs. Gray background indicates significant increased metabolite in comparison to 0 hr.
| No. | Sugars or sugar alcohols | 0 hr | Melatonin-6 hr | Melatonin-12 hr | Melatonin-24 hr | Pst DC3000-6 hr | PstDC3000-12 hr |
|---|---|---|---|---|---|---|---|
| 1 | Allose | 3.53 ± 0.54 | 3.60 ± 0.62 | 7.84 ± 0.59 | 5.39 ± 0.86 | 3.51 ± 0.11 | 3.79 ± 0.13 |
| 2 | Arabinose | 8.62 ± 2.16 | 8.22 ± 1.30 | 8.17 ± 0.69 | 7.04 ± 0.11 | 7.24 ± 0.44 | 8.79 ± 0.68 |
| 3 | Cellobiose | 9.34 ± 1.26 | 7.13 ± 0.68 | 8.91 ± 0.51 | 8.32 ± 0.43 | 8.45 ± 0.58 | 10.12 ± 0.89 |
| 4 | Fructose | 74.30 ± 7.20 | 112.38 ± 11.48 | 158.10 ± 27.24 | 131.08 ± 3.16 | 134.27 ± 7.11 | 268.58 ± 35.93 |
| 5 | Galatose | 4.24 ± 0.28 | 4.38 ± 0.88 | 4.88 ± 1.47 | 31.13 ± 2.67 | 26.29 ± 1.30 | 17.96 ± 3.29 |
| 6 | Gentiobiose | 12.09 ± 0.88 | 12.35 ± 0.34 | 13.70 ± 0.96 | 11.41 ± 0.19 | 12.56 ± 0.53 | 12.91 ± 0.52 |
| 7 | Glucose | 8.61 ± 1.47 | 18.31 ± 2.30 | 22.02 ± 5.79 | 11.40 ± 0.53 | 7.84 ± 1.44 | 20.18 ± 1.55 |
| 8 | Lactose | 6.19 ± 0.61 | 5.66 ± 1.69 | 7.15 ± 0.92 | 6.89 ± 0.66 | 8.63 ± 1.92 | 7.83 ± 0.47 |
| 9 | Lactulose | 22.20 ± 2.15 | 22.33 ± 2.70 | 23.07 ± 2.34 | 21.78 ± 2.08 | 20.14 ± 2.10 | 25.08 ± 4.20 |
| 10 | Maltose | 6.55 ± 2.31 | 6.67 ± 2.47 | 5.70 ± 1.99 | 18.91 ± 2.19 | 20.28 ± 1.16 | 20.89 ± 3.16 |
| 11 | Mannobiose | 16.79 ± 0.91 | 17.22 ± 0.94 | 18.20 ± 1.22 | 15.45 ± 0.47 | 17.57 ± 2.07 | 18.54 ± 0.64 |
| 12 | Mannose | 58.09 ± 4.50 | 92.19 ± 13.16 | 155.53 ± 24.37 | 46.63 ±9.43 | 48.21 ± 9.27 | 52.71 ± 2.65 |
| 13 | Melibose | 25.66 ± 3.15 | 25.70 ± 2.59 | 41.60 ± 3.74 | 34.10 ± 1.58 | 38.43 ± 1.80 | 38.84 ± 1.64 |
| 14 | Sucrose | 32.15 ± 2.76 | 32.91 ± 3.08 | 55.95 ± 4.17 | 70.88 ± 7.18 | 37.25 ± 2.81 | 95.68 ± 9.59 |
| 15 | Tagatofuranose | 60.10 ± 9.91 | 41.54 ± 17.84 | 54.06 ± 5.12 | 95.59 ± 12.68 | 82.05 ± 11.72 | 80.99 ± 1.88 |
| 16 | Talose | 9.70 ± 3.76 | 15.14 ± 7.53 | 8.18 ± 1.51 | 6.47 ± 2.58 | 12.53 ± 9.07 | 13.34 ± 7.23 |
| 17 | Trehalose | 3.45 ± 0.74 | 3.31 ± 1.14 | 2.85 ± 0.13 | 2.39 ± 0.05 | 6.77 ± 0.21 | 4.71 ± 0.18 |
| 18 | Turanose | 4.07 ± 0.46 | 3.88 ± 1.27 | 8.46 ± 2.34 | 5.75 ± 0.23 | 9.10 ± 1.46 | 6.04 ± 0.29 |
| 19 | Dulcitol | 6.07 ± 1.42 | 6.15 ± 1.21 | 6.12 ± 1.34 | 4.38 ± 0.61 | 5.87 ± 1.16 | 6.01 ± 1.51 |
| 20 | Galactinol | 4.92 ± 1.50 | 9.08 ± 3.72 | 15.40 ±1.11 | 15.08 ± 0.17 | 7.24 ± 1.45 | 6.98 ± 2.22 |
| 21 | Glycerol | 17.29 ± 1.29 | 24.23 ± 2.92 | 29.32 ± 1.99 | 68.52 ± 2.48 | 54.79 ± 1.35 | 55.58 ± 3.36 |
| 22 | Myo-Inositol | 39.65 ± 5.75 | 33.45 ± 0.38 | 48.76 ± 17.61 | 28.43 ± 0.69 | 35.71 ± 10.96 | 28.52 ± 4.46 |
| 23 | Sorbitol | 22.83 ± 2.44 | 21.53 ± 2.69 | 25.94 ± 4.31 | 22.06 ± 3.71 | 21.39 ± 3.21 | 25.09 ± 3.06 |
Figure 1. Heatmap showing the effects of exogenous melatonin treatment and Pst DC3000 infection on the endogenous levels of 16 amino acids.
For exogenous melatonin treatments, 28-day-old of soil-grown WT (Col-0) Arabidopsis plants were watered with nutrient solution containing 20 μM melatonin from below in pots with plants. For pathogen infection, 28-day-old soil-grown WT (Col-0) Arabidopsis plant leaves were infected with bacterial suspension (OD600 = 0.002) containg 10 mM MgCl2 and 0.05% silwet L-77. For cluster analysis, all these metabolite levels were quantified as log2 value of fold change in relative to the WT plants at 0 hr of treatment which was set as 1.0. The detailed concentrations were shown in Table S1. The asterisk (*) indicates significant difference in comparison to 0 hr of treatment at p < 0.05.
Figure 2. Heatmap showing the effects of exogenous melatonin treatment and Pst DC3000 infection on the endogenous levels of 12 organic acids.
For exogenous melatonin treatments, 28-day-old of soil-grown WT (Col-0) Arabidopsis plants were watered with nutrient solution containing 20 μM melatonin from below in pots with plants. For pathogen infection, 28-day-old soil-grown WT (Col-0) Arabidopsis plant leaves were infected with bacterial suspension (OD600 = 0.002) containg 10 mM MgCl2 and 0.05% silwet L-77. For cluster analysis, all these metabolite levels were quantified as log2 value of fold change in relative to the WT plants at 0 hr of treatment which was set as 1.0. The detailed concentrations were shown in Table S1.
Interestingly, both exogenous melatonin treatment and Pst DC3000 infection significantly increased the endogenous levels of multiple sugars and sugar alcohols (Fig. 3 and Table 1). Among these sugars and sugar alcohols, the endogenous levels of fructose, glucose, melibose, sucrose, maltose, galatose, tagatofuranose, turanose and glycerol commonly increased after melatonin treatment and Pst DC3000 infection (Fig. 3 and Table 1). The results indicate the possible involvement of sugars and sugar alcohols in melatonin-mediated plant disease response against bacterial pathogen Pst DC3000.
Figure 3. Heatmap showing the effects of exogenous melatonin treatment and Pst DC3000 infection on the endogenous levels of 18 sugars and 5 sugar alcohols.
For exogenous melatonin treatments, 28-day-old of soil-grown WT (Col-0) Arabidopsis plants were watered with nutrient solution containing 20 μM melatonin from below in pots with plants. For pathogen infection, 28-day-old soil-grown WT (Col-0) Arabidopsis plant leaves were infected with bacterial suspension (OD600 = 0.002) containg 10 mM MgCl2 and 0.05% silwet L-77. For cluster analysis, all these metabolite levels were quantified as log2 value of fold change in relative to the WT plants at 0 hr of treatment which was set as 1.0. The detailed concentrations were shown in Table 1. The asterisk (*) indicates significant difference in comparison to 0 hr of treatment at p < 0.05.
The effects of exogenous sugar and glycerol treatments on plant disease resistance
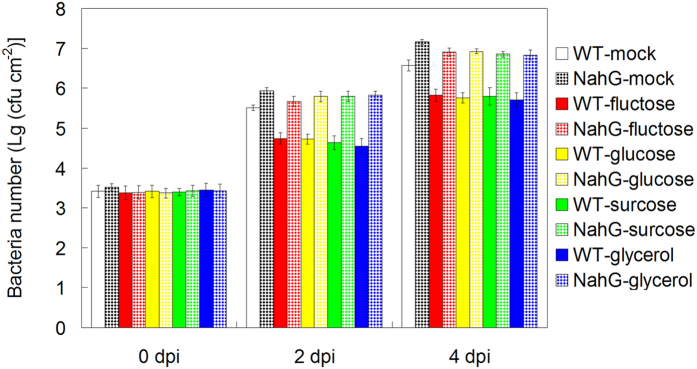
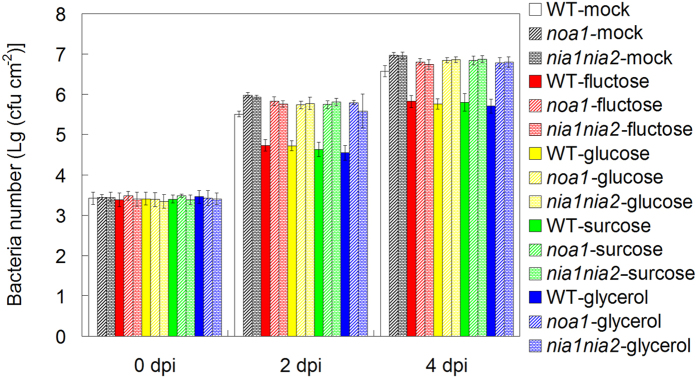
To further investigate the possible relation among sugars and sugar alcohols, melatonin and plant disease resistance against bacterial pathogen Pst DC3000, we chose three popular sugars (fructose, glucose and sucrose) and glycerol for further analysis. Using quantification of bacteria number in Pst DC3000-infected leaves, we found that 5 mM fructose, or 5 mM glucose, or 5 mM sucrose, or 5 mM glycerol pre-treated plants showed significantly lower bacterial propagation at both 2 days post infection (dpi) and 4 dpi of Pst DC3000 (Fig. 4).
Figure 4. The effects of exogenous treatments of fructose, glucose, sucrose and glycerol on innate immunity against Pst DC3000 in Arabidopsis.
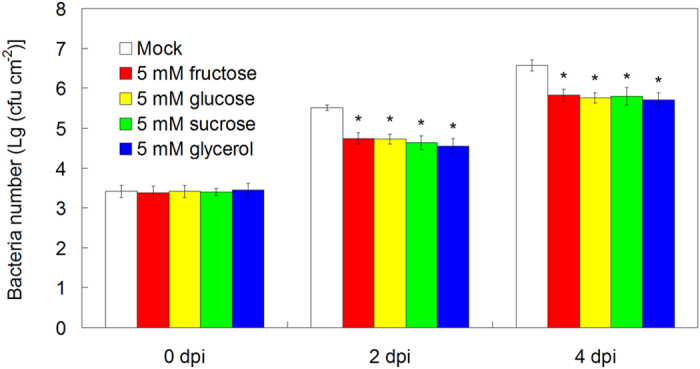
For the assay, 26-day-old soil-grown WT (Col-0) Arabidopsis plants were watered with nutrient solution containing mock, 5 mM fructose, 5 mM glucose, 5 mM sucrose and 5 mM glycerol from below in pots with plants for 2 days, and then 28-day-old soil-grown WT (Col-0) Arabidopsis plant leaves were infected with bacterial suspension (OD600 = 0.002) containg 10 mM MgCl2 and 0.05% silwet L-77. At 0, 2 and 4 days post infection (dpi), the bacterial populations in the leave discs were determined. The results shown are the means ± SDs (n = 6), and p < 0.05 was considered as significant difference that was marked with asterisk (*).
Sugar and glycerol regulate plant disease resistance in SA- and NO-dependent pathway
Both SA and NO positively regulated innate immunity against Pst DC3000 infection, and they are essential for melatonin-mediated plant disease response in Arabidopsis32,33,41. To dissect how sugars (fructose, glucose and sucrose) and glycerol mediated disease resistance, we examined the effects of these sugars and sugar alcohols on wild type (WT, Col-0) plants, SA-deficient plants (NahG overexpressing plants) and NO deficient mutants (noa1 and nia1nia2). Without sugars (fructose, glucose and sucrose) and glycerol pre-treatment, NahG, noa1 and nia1nia2 displayed significantly more bacterial propagation in comparison WT (Col-0) plants at both 2 dpi and 4 dpi of Pst DC3000 (Figs 5 and 6). Notably, the effect of 5 mM fructose, or 5 mM glucose, or 5 mM sucrose, or 5 mM glycerol pre-treatment on the disease resistance was alleviated in NahG, noa1 and nia1nia2 mutants, as evidenced by the bacterial propagation in Pst DC3000 infected leaves of these mutants (Figs 5 and 6).
Figure 5. The involvement of SA in fructose, glucose, sucrose and glycerol-mediated innate immunity against Pst DC3000 in Arabidopsis.
For the assay, 26-day-old soil-grown WT (Col-0) and NahG Arabidopsis plants were watered with nutrient solution containing mock, 5 mM fructose, 5 mM glucose, 5 mM sucrose and 5 mM glycerol from below in pots with plants for 2 days, and then 28-day-old soil-grown WT (Col-0) and NahG Arabidopsis plant leaves were infected with bacterial suspension (OD600 = 0.002) containg 10 mM MgCl2 and 0.05% silwet L-77. At 0, 2 and 4 days post infection (dpi), the bacterial populations in the leave discs were determined. The results shown are the means ± SDs (n = 6).
Figure 6. The involvement of NO in fructose, glucose, sucrose and glycerol-mediated innate immunity against Pst DC3000 in Arabidopsis.
For the assay, 26-day-old soil-grown WT (Col-0) and NO deficient mutants (noa1 and nia1nia2) were watered with nutrient solution containing mock, 5 mM fructose, 5 mM glucose, 5 mM sucrose and 5 mM glycerol from below in pots with plants for 2 days, and then 28-day-old soil-grown WT (Col-0) and NO deficient mutants (noa1 and nia1nia2) were infected with bacterial suspension (OD600 = 0.002) containg 10 mM MgCl2 and 0.05% silwet L-77. At 0, 2 and 4 days post infection (dpi), the bacterial populations in the leave discs were determined. The results shown are the means ± SDs (n = 6).
The effects of exogenous sugar and glycerol treatments on endogenous NO production
Moreover, we also examined the effects of exogenous treatments of fructose, glucose, sucrose and glycerol on endogenous levels of melatonin and NO in Arabidopsis. After the treatments using these sugars and sugar alcohols for 3, 6, 12 and 24 hrs, no significant difference of endogenous melatonin level was determined between mock and sugars or glycerol-treated Arabidopsis leaves (Fig. 7a). Using both the hemoglobin assay and the NO-sensitive fluorescence assay to detect endogenous NO, we found that 5 mM fructose, or 5 mM glucose, or 5 mM sucrose, or 5 mM glycerol treatment significantly increased endogenous NO level after 3 to 24 hrs of treatment (Fig. 7b,c).
Figure 7. The effects of exogenous treatments of fructose, glucose, sucrose and glycerol on endogenous levels of melatonin (a) and NO (b) (c) in Arabidopsis.
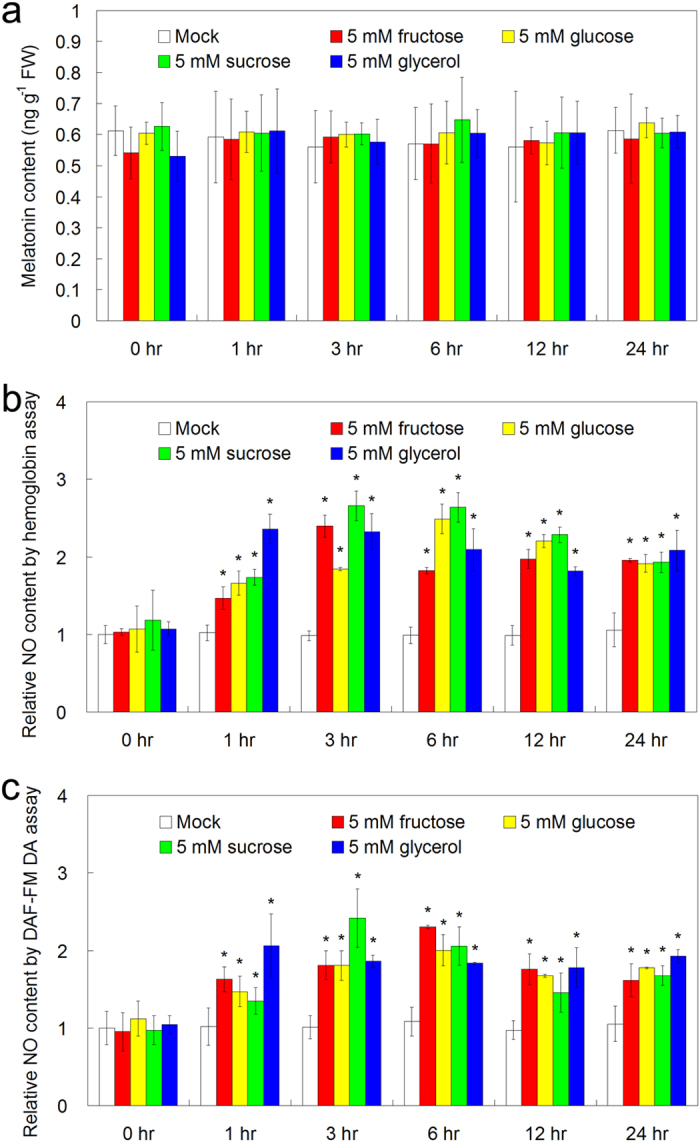
For the assay, 26-day-old soil-grown WT (Col-0) Arabidopsis plants were watered with nutrient solution containing mock, 5 mM fructose, 5 mM glucose, 5 mM sucrose and 5 mM glycerol from below in pots with plants for 0, 3, 6, 12 and 24 hrs, respectively, and plant leaves were used for the determination. Endogenous NO level in the leaves were assayed using the hemoglobin assay (b) and the DAF-FM DA assay (c). The results shown are the means ± SDs (n = 3). The sterisk (*) indicates significant difference in comparison to 0 hr of treatment at p < 0.05.
Discussion
Plants are exposed to changeable and complex environmental conditions, however, as sessible organisms, plants can not change their location to escape the harsh circumstances39,50,51,52,53,54,55,56,57. Bacterial pathogen infection is one of the most major and severe harsh stresses that influence plant growth and result in yield loss in agriculture20,32,33,51,52,58. Once a bacterial pathogen is applied, the pathogen enters the host plant tissues (usually leaves) through natural opening stomata or wounds, leading to high population levels in intercellular spaces of host plants; these results in water-soaked patches and necrotic in infected leaves58,59,60. In the long evolution during plant-pathogen interaction, plants have developed a variety of mechanisms to perceive, recognize and counteract pathogen entry, including pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI) and effector-triggered immunity (ETI)58,59,60.
To date, there are three major methods to improve plant disease resistance. First is to screen and identify disease-resistant varieties from numerous wild genotypes and apply them in agriculture, and second is to investigate some major genes that confer improved disease resistance and modulate the expression of these genes by genetic breeding51,52,61. Moreover, screening protective molecules such as melatonin, SA, NO, hydrogen sulfide (H2S) in plant innate immunity and exogenous applications of them can also enhance plant disease resistance20,32,33,60,61,62,63,64,65. In recent years, the safety of transgenic crops has attracted many critics and many people can not accept such products66,67,68. Thus, exogenous applications of the protective molecules such as melatonin may be acceptable and effective.
SA signaling is the most important pathway of plant defense response against pathogen infection. These include the induction of endogenous SA in response to pathogen infection, the perception of SA by SA receptors (Nonexpressor of Pathogenesis Related Protein 3 (NPR3) and NPR4), the release of NPR1 monomers and translocation in nucleus as well as the interaction of nuclear NPR1 and TGACG sequence-specific binding protein (TGA) transcription factors, and the activation of PRs58,59,60,69,70. NO is also an important gaseous molecule and secondary messenger in plant disease response against pathogen infection71,72,73,74,75,76,77,78,79,80. On one hand, NO promotes the nuclear translocation of NPR1 and S-nitrosylation (SNO) of NPR1 and TGA1 by NO enhances the DNA binding activity of TGA1 to the promoters of PRs, resulting in the activation of PRs51,61,71,72,73,74,75,76,77,78. By comparison, SNO of respiratory burst oxidase protein D (RBOHD) by NO blunts SA biosynthesis and decreases RBOHD activity, resulting in reduced reactive oxygen intermediates and cell death during pathogen-triggered hypersensitive response in Arabidopsis79,80. Based on previous studies that investigated the effects of melatonin on disease resistance in WT plants, SA-deficient NahG plants and NO-deficient mutants (noa1 and nia1nia2)32,33,41, the cooperation between melatonin and SA as well as the relation of NO and SA have been revealed, indicating that melatonin conferred enhanced plant innate immunity against bacterial pathogen in both SA- and NO-dependent pathway in Arabidopsis.
As a possible secondary messenger in plant stress responses, melatonin may perceive stress signaling by extensive reprogramming of the transcriptome and the proteome19,23,26,42. Weeda et al. and Shi et al. identified 1308 and 3933 increased or reduced gene transcripts due to exogenous melatonin treatment in Arabidopsis and in bermudagrass, respectively23,26. Wang et al. and Shi et al. identified 309 and 76 differentially expressed proteins as a result of exogenous melatonin administration in apple and in bermudagrass, respectively19,42. Moreover, some important transcription factors that are regulated by melatonin including zinc finger of Arabidopsis thaliana 6 (ZAT6)24, auxin resistant 3 (AXR3)/indole-3-acetic acid inducible 17 (IAA17)25, and class A1 heat shock factors (HSFA1s)27 are involved in melatonin-mediated freezing stress, natural leaf senescence, and heat stress responses, respectively. However, melatonin-mediated metabolomic analysis, especially melatonin-mediated metabolic homeostasis in response to bacterial pathogen, is unknown.
In this study, although 51 primary metabolites including 16 amino acids, 12 organic acids, 18 sugars and 5 sugar alcohols were reproducibly examined in Arabidopsis leaves after exogenous melatonin treatment and Pst DC3000 infection, only 7 sugars (fructose, glucose, melibose, sucrose, maltose, galatose and tagatofuranose) and glycerol commonly increased after these treatments (Figs 1, 2, 3, Table 1 and Supplemental Table S1). Fructose, glucose and sucrose are three popular sugars with wide distributions in plants26,54, so they and glycerol were chosen for further analysis. Further studies showed that exogenous fructose, glucose, sucrose and glycerol pre-treatments enhanced innate immunity against Pst DC3000 infection in WT plants (Fig. 4); these sugars and glycerol pre-treatments increased endogenous NO level but had no significant effect on the endogenous melatonin level (Fig. 7). These results indicated the involvement of sugars of glycerol in melatonin-mediated basal immunity, as well as in SA and NO signaling pathways.
Consistently, Thibaud et al. and Tsutsui et al. revealed the positive regulation of glucose and sucrose in plant disease resistance in SA-dependent way81,82. Both SA and NO confer enhanced disease resistance against bacterial pathogen in Arabidopsis, and the cooperation between them plays important roles in plant innate immunity41,69,70,71,72,73,74,75,76,77,78,79,80. Because melatonin confers enhanced plant innate immunity against bacterial pathogen in both SA and NO-dependent pathway in Arabidopsis41, we therefore investigated the possible cooperation among sugars and glycerol-mediated innate immunity, SA and NO signaling pathways. Interestingly, both SA and NO were essential for fructose, glucose, sucrose and glycerol-conferred enhanced innate immunity, as evidenced by the alleviated effects of them in SA-deficient NahG plants and NO-deficient Arabidopsis mutants (Figs 5, 6). Notably, Zhao et al. [2015] found that exogenous application of melatonin mediated invertase inhibitor (C/VIF)-regulated CWI activity and enhanced sucrose metabolism, thus significantly increased the productions of sucrose, glucose and fructose (by 7 to 9 fold higher)49. Moreover, they found that both melatonin-mediated carbohydrate metabolism including sucrose, xylose and galactose and mediated-activated SA responsive genes are contributed to pathogen resistance49. Both this study and Zhao’s results highlight melatonin-mediated carbohydrate metabolism in melatonin-mediated basal immunity. Additionally, Mandal et al. [2012] found that glycerol increases endogenous NO level and involves in NO-mediated defense signaling in Arabidopsis83. Taken together, this study is the first comprehensive metabolomic analysis including amino acids, organic acids, sugars and sugar alcohols regarding to the protective role of melatonin in innate immunity against a bacterial pathogen in Arabidopsis. In the meanwhile, the relationship among sugars, glycerol, SA and NO signaling pathways were partially revealed in melatonin-mediated basal immunity.
Based on the results herein, a novel model for sugars and glycerol in melatonin-mediated disease resistance against bacterial pathogen in Arabidopsis is proposed in this study (Fig. 8). As previous described, both bacterial pathogen Pst DC3000 infection and exogenous application of melatonin largely increased endogenous melatonin level and accumulations of various sugars (fructose, glucose and surcose) and glycerol; these sugars and sugar alcohol confer enhanced disease resistance against Pst DC3000. As positive regulators of plant innate immunity, both SA and NO are essential for melatonin as well as sugars (fructose, glucose and surcose) and glycerol-mediated disease responses against Pst DC3000 in Arabidopsis.
Figure 8. Model depicting the involvement of sugars and glycerol in melatonin-mediated innate immunity against bacterial pathogen in Arabidopsis.
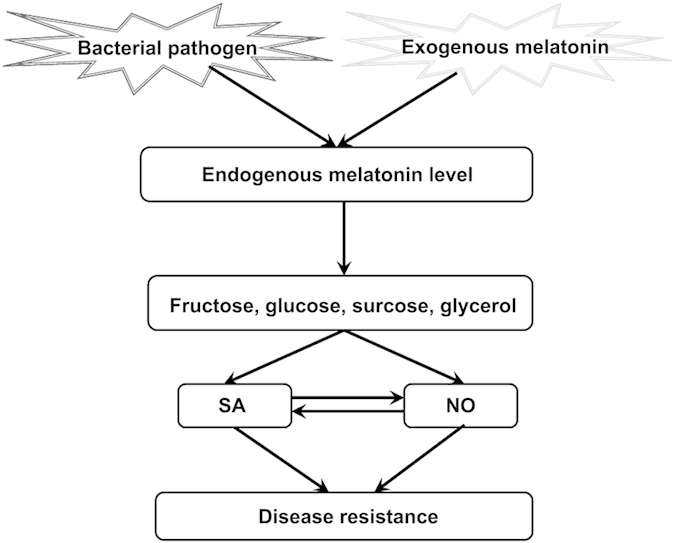
In the current report, we identify the metabolic pathway regarding to the protective role of melatonin in innate immunity against a bacterial pathogen in Arabidopsis. Melatonin treatment increased the accumulations of sugars and glycerol in response to bacterial pathogen infection, and the elevated sugars and glycerol thereafter increase endogenous NO level which confer enhanced innate immunity against bacterial pathogen in SA and NO-dependent pathway in Arabidopsis.
Methods
Plant materials and growth conditions
All Arabidopsis seeds in the background of Columbia-0 (Col-0) ecotype were first stratificated at 4 °C for 3 days in darkness, and thereafter were sown in soil in the growth chamber. The growth chamber was controlled at 23 ± 2 °C, with the irradiance of about 120 μmol quanta m−2 s−1 and 65% relative humidity under 16 hr light and 8 hr dark cycles. Nutrient solution was watered in the soil twice every week, to keep plant growth. The NO-deficient mutants [noa1 (CS6511) and nia1nia2 (CS2356)] were from Arabidopsis Biological Resource Center (ABRC), and SA-deficient NahG (the salicylate hydroxylase-expressing transgenic plant) has been described in Gaffney et al.50.
Infection of bacterial pathogen Pst DC3000
The bacterial pathogen strain of Pst DC3000 was used for the assay plant innate immunity as previously described41,51,52. The strain of Pst DC3000 was streaked out on King’s B (KB) medium containing 50 μg ml−1 rifampicin at 28 °C for 2 days, and fresh bacteria was transferred to new KB liquid culture containing 50 μg ml−1 rifampicin at 28 °C for 8 to 12 hrs, until bacterial culture reached OD600 of 0.6 to 1.0. Thereafter the virulent Pst DC3000 was diluted to OD600 of 0.002, and the bacterial suspension containg 10 mM MgCl2 and 0.05% silwet L-77 were infected in the abaxial side of 28-day-old plant leaves. After syringe infection, plants were covered with plastic dome to maintain humidity for 2 days at 23 °C in the growth chamber.
At 0, 2 and 4 dpi, syringe infected leaves were harvested and placed in 70% ethanol solution for 1 minute with gently mixed occasionally, thereafter the leaves were removed and rinsed in sterile distilled water for 1 minute, the leaves are then dry on paper towel. At least 15 independent leaf discs of 1 cm2 within the infected area in each treatment of one independent experiment were excised, and the bacterial populations in the leave discs were determined in KB medium containing 1.5% (m/v) agar and 50 μg ml−1 rifampicin using 10 μl five 10-fold dilutions of homogenate of Pst DC3000-infected leaves as described in Shi et al.41,51,52. Six biological repeats were performed for the innate disease resistance assay.
Extraction, identification and quantification of primary metabolites in Arabidopsis leaves
Extraction, identification and quantification of primary metabolites of plant leaves were performed as previously described53,54. Briefly, 100 mg plant leaves was ground in liquid nitrogen and the dry powder was transferred to a new EP tube, and 1.4 ml pre-cooled 100% methanol was added and vortexed for 10 seconds, and thereafter 60 μl 0.2 mg mL−1 ribitol was added as an internal standard and vortexed for 10 another 10 seconds. After shaking at 70 °C for 10 min, the mixture was centrifuged at 12560 g for 10 min, and the supernatant was transferred to a new tube with 750 μl chloroform and 1.4 ml dd H2O. After vortexed for 30 seconds, the mixture was centrifuged at 12560 for 10 min, and 150 μl supernatant was transferred to a new EP tube with 40 μl methoxyamination reagent. The mixture was shaked at 37 °C for 2 hrs and 70 μl MSTFA reagent was added for shaking at 37 °C for another 0.5 hr.
For each sample in every biological repeat, 1 μl derivatizated extract was injected into a DB-5MS capillary (30 m × 0.25 mm × 0.25 μm, Agilent J&W GC column, California, USA). The GC-TOF-MS analysis was performed as previously described54, which was determined using electron impact ionization (70 eV) in full scan mode (m/z from 30 to 550). Different metabolites were identified by comparing retention time index specific masses from samples with reference spectra in mass spectral libraries (NIST 2005, Wiley 7.0), and quantified based on the pre-added internal standard (ribitol).
Hierarchical cluster analysis and construction of heatmap
The hierarchical cluster analysis of different primary metabolites in Arabidopsis leaves with various treatments was performed using CLUSTER program (http://bonsai.ims.u-tokyo.ac.jp/~mdehoon/software/cluster/), and then the heatmap was constructed using Java Treeview (http://jtreeview.sourceforge.net/).
Quantification of endogenous melatonin and NO levels in Arabidopsis leaves
Extraction and quantification of endogenous melatonin in Arabidopsis leaves were determined using melatonin enzyme-linked immunosorbent assay (ELISA) Kit (EK-DSM; Buhlmann Laboratories AG, Schonenbuch, Switzerland) as previously described6,24,25,26,27,41,42. Extraction and quantification of endogenous NO in Arabidopsis leaves were determined using the hemoglobin assay by examining the conversion of oxyhemoglobin to methemoglobin spectrophotometrically and the NO-sensitive fluorescence assay using 3-Amino,4-aminomethyl-2′,7′-difluorescein, diacetate (DAF-FM DA) as previously described41,51,55,56,57.
Statistical analysis
All the experiments were repeated at least three biological repeats with similar results, and plant leave samples in each biological repeat were extracted from at least 10 plants. All the data were expressed as means ± SDs of the biological repeats. The statistical analysis was performed using AVOVA and student t-test, and p < 0.05 was considered as significant difference that was marked with asterisk (*).
Additional Information
How to cite this article: Qian, Y. et al. Comparative metabolomic analysis highlights the involvement of sugars and glycerol in melatonin-mediated innate immunity against bacterial pathogen in Arabidopsis. Sci. Rep. 5, 15815; doi: 10.1038/srep15815 (2015).
Supplementary Material
Acknowledgments
AcknowledgementsWe thank Prof. Jianmin Zhou for sharing the bacterial strain Pst DC3000. This research was supported by the National Natural Science Foundation of China (No. 31570249) and the startup funding of Hainan University (No. kyqd1531) to Haitao Shi and by the Basic Research Fund of National Non-profit Research Institutions (No. CAFYBB2012043, RIF2014-03) to Yongqiang Qian.
Footnotes
The authors declare no competing financial interests.
Author Contributions S.H. conceived and directed this study, designed and performed the experiments, analyzed the data, wrote and revised the manuscript. Q.Y. performed the experiments, analyzed the data, wrote and revised the manuscript. T.D. and R.R. provided suggestions and revised the manuscript.
References
- Dubbels R. et al. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J. Pineal Res. 18, 28–31 (1995). [DOI] [PubMed] [Google Scholar]
- Hattori A. et al. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem. Mol. Biol. Int. 35, 627–634 (1995). [PubMed] [Google Scholar]
- Reiter R. J. et al. Melatonin in plants. Nutr. Rev. 59, 286–290 (2001). [DOI] [PubMed] [Google Scholar]
- Manchester L. C. et al. High levels of melatonin in the seeds of edible plants: possible function in germ tissue protection. Life Sci. 67, 3023–3029 (2000). [DOI] [PubMed] [Google Scholar]
- Burkhardt S. et al. Detection and quantitation of melatonin in Montmorency and Balaton cherries. J. Agric. Food Chem. 49, 4898–4902 (2001). [DOI] [PubMed] [Google Scholar]
- Pape C. & Lűning K. Quantification of melatonin in phototrophic organisms. J. Pineal Res. 41, 157–165 (2006). [DOI] [PubMed] [Google Scholar]
- Hernández-Ruiz J. & Arnao M. B. Distribution of melatonin in different zones of lupin and barley plants at different ages in the presence and absence of light. J. Agr. Food Chem. 56, 10567–10573 (2008). [DOI] [PubMed] [Google Scholar]
- Murch S. J. et al. Melatonin and serotonin in flowers and fruits of Datura metel L. J Pineal Res 2009 47, 277–283 (2009). [DOI] [PubMed] [Google Scholar]
- Arnao M. B. & Hernández-Ruiz J. Chemical stress by different agents affects the melatonin content of barley roots. J. Pineal Res. 46, 295–299 (2009). [DOI] [PubMed] [Google Scholar]
- Ramakrishna A. et al. Melatonin and serotonin profiles in beans of coffea species. J. Pineal Res. 52, 470–476 (2012). [DOI] [PubMed] [Google Scholar]
- Arnao M. B. & Hernández-Ruiz J. Growth conditions influence the melatonin content of tomato plants. Food Chem. 138, 1212–1214 (2013). [DOI] [PubMed] [Google Scholar]
- Reiter R. J. et al. Phytomelatonin: assisting plants to survive and thrive. Molecules 20, 7396–7437 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnao M. B. et al. Estimation of free radical-quenching activity of leaf pigment extracts. Phytochem. Analysis 12, 138–143 (2001). [DOI] [PubMed] [Google Scholar]
- Zhao Y. et al. Melatonin and its potential biological functions in the fruits of sweet cherry. J. Pineal Res. 55, 79–88 (2001). [DOI] [PubMed] [Google Scholar]
- Cano A., Hernández-Ruiz J. & Arnao M. B. Changes in hydrophilic antioxidant activity in Avena sativa and Triticum aestivum leaves of different age during de-etiolation and high-light treatment. J. Pineal Res. 119, 321–327 (2006). [DOI] [PubMed] [Google Scholar]
- Wang P. et al. Delayed senescence of apple leaves by exogenous melatonin treatment: toward regulating the ascorbate-glutathione cycle. J. Pineal Res. 53, 11–20 (2012). [DOI] [PubMed] [Google Scholar]
- Tan D. X. et al. Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J. Exp. Bot. 63, 577–597 (2012). [DOI] [PubMed] [Google Scholar]
- Wang P. et al. Delay in leaf senescence of Malus hupehensis by long-term melatonin application is associated with its regulation of metabolic status and protein degradation. J. Pineal Res. 55, 424–434 (2013). [DOI] [PubMed] [Google Scholar]
- Wang P. et al. Melatonin regulates proteomic changes during leaf senescence in Malus hupehensis. J. Pineal Res. 57, 291–307 (2014). [DOI] [PubMed] [Google Scholar]
- Zhang N. et al. The RNA-seq approach to discriminate gene expression profiles in response to melatonin on cucumber lateral root formation. J. Pineal Res. 56, 39–50 (2014). [DOI] [PubMed] [Google Scholar]
- Wang P. et al. Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J. Pineal Res. 54, 292–302 (2013). [DOI] [PubMed] [Google Scholar]
- Yin L. et al. Exogenous melatonin improves Malus resistance to Marssonina apple blotch. J. Pineal Res. 54, 426–434 (2013). [DOI] [PubMed] [Google Scholar]
- Weeda S. et al. Arabidopsis transcriptome analysis reveals key roles of melatonin in plant defense systems. PLoS ONE 9, e93462 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H. & Chan Z. The cysteine2/histidine2-type transcription factor ZINC FINGER OF ARABIDOPSIS THALIANA 6-activated C-REPEAT-BINDING FACTOR pathway is essential for melatonin-mediated freezing stress resistance in Arabidopsis. J. Pineal Res. 57, 185–191 (2014). [DOI] [PubMed] [Google Scholar]
- Shi H., Reiter R. J., Tan D. & Chan Z. INDOLE-3-ACETIC ACID INDUCIBLE 17 positively modulates natural leaf senescence through melatonin-mediated pathway in Arabidopsis. J. Pineal Res. 58, 26–33 (2015). [DOI] [PubMed] [Google Scholar]
- Shi H. et al. Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in bermudagrass [Cynodon dactylon (L). Pers.] by exogenous melatonin. J. Exp. Bot. 66, 681–694 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Tan D., Reiter R. J., Yang F. & Chan Z. Melatonin induces class A1 heat shock factors (HSFA1s) and their possible involvement of thermotolerance in Arabidopsis. J. Pineal Res. 58, 335–342 (2015). [DOI] [PubMed] [Google Scholar]
- Okazaki M. et al. Lowering intercellular melatonin by transgenic analysis of indoleamine 2,3-dioxygenase from rice in tomato plants. J. Pineal Res. 49, 239–247 (2010). [DOI] [PubMed] [Google Scholar]
- Byeon Y. et al. Microarray analysis of genes differentially expressed in melatonin-rich transgenic rice expressing a sheep serotonin N-acetyltransferase. J. Pineal Res. 55, 357–363 (2013). [DOI] [PubMed] [Google Scholar]
- Byeon Y., Park S., Lee H. Y., Kim Y. S. & Back K. Elevated production of melatonin in transgenic rice seeds expressing rice tryptophan decarboxylase. J. Pineal Res. 56, 275–282 (2014). [DOI] [PubMed] [Google Scholar]
- Byeon Y. & Back K. An increase in melatonin in transgenic rice causes pleiotropic phenotypes, including enhanced seedling growth, delayed flowering, and low grain yield. J. Pineal Res. 56, 408–414 (2014). [DOI] [PubMed] [Google Scholar]
- Lee H. Y., Byeon Y. & Back K. Melatonin as a signal molecule triggering defense responses against pathogen attack in Arabidopsis and tobacco. J. Pineal Res. 57, 262–268 (2014). [DOI] [PubMed] [Google Scholar]
- Lee H. Y., Byeon Y., Tan D. X., Reiter R. J. & Back K. Arabidopsis serotonin N-acetyltransferase knockout mutant plants exhibit decreased melatonin and salicylic acid levels resulting in susceptibility to an avirulent pathogen. J. Pineal Res. 58, 291–299 (2015). [DOI] [PubMed] [Google Scholar]
- Wang L. et al. Changes in melatonin levels in transgenic ‘Micro-Tom’ tomato overexpressing ovine AANAT and ovine HIOMT genes. J. Pineal Res. 56, 134–142 (2014). [DOI] [PubMed] [Google Scholar]
- Kolář J. & Macháčkova I. Melatonin in higher plants: occurrence and possible functions. J. Pineal Res. 39, 333–341 (2005). [DOI] [PubMed] [Google Scholar]
- Arnao M. B. & Hernández-Ruiz J. The physiological function of melatonin in plants. Plant Signal. Behav. 1, 89–95 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes S. D. et al. Phytomelatonin: a review. J. Exp. Bot. 60, 67–69 (2009). [DOI] [PubMed] [Google Scholar]
- Arnao M. B. & Hernández-Ruiz J. Melatonin: plant growth regulator and/or biostimulator during stress? Trends Plant Sci. 19, 789–797 (2014). [DOI] [PubMed] [Google Scholar]
- Zhang N. et al. Roles of melatonin in abiotic stress resistance in plants. J. Exp. Bot. 66, 647–656 (2014). [DOI] [PubMed] [Google Scholar]
- Tan D. X. et al. Novel rhythms of N1-acetyl-N2-formyl-5-methoxykynuramine and its precursor melatonin in water hyacinth: importance in phytoremediation. FASEB J. 21, 2104–2108 (2007). [DOI] [PubMed] [Google Scholar]
- Shi H., Chen Y., Tan D. X., Reiter R. J., Chan Z. & He C. Melatonin induces nitric oxide and the potential mechanisms relate to innate immunity against bacterial pathogen infection in Arabidopsis. J. Pineal Res. 59, 102–108 (2015). [DOI] [PubMed] [Google Scholar]
- Shi H., Wang X., Tan D. X., Reiter R. J. & Chan Z. Comparative physiological and proteomic analyses reveal the actions of melatonin in the reduction of oxidative stress in bermudagrass (Cynodon dactylon (L). Pers.). J. Pineal Res. 59, 120–131 (2015). [DOI] [PubMed] [Google Scholar]
- Tan D. X. et al. Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocr J. 1, 57–60 (1993). [Google Scholar]
- Tan D. X. et al. Mechanistic and comparative studies of melatonin and classic antioxidants in terms of their interactions with the ABTS cation radical. J. Pineal Res. 34, 249–259 (2003). [DOI] [PubMed] [Google Scholar]
- Galano A., Tan D. X. & Reiter R. J. Melatonin as a natural ally against oxidative stress: a physicochemical examination. J. Pineal Res. 51, 1–16 (2011). [DOI] [PubMed] [Google Scholar]
- Venegas C. et al. Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. J. Pineal Res. 52, 217–227 (2012). [DOI] [PubMed] [Google Scholar]
- Calvo J. R., González-Yanes C. & Maldonado M. D. The role of melatonin in the cells of the innate immunity: a review. J. Pineal Res. 55, 103–120 (2013). [DOI] [PubMed] [Google Scholar]
- Reiter R. J., Tan D. X. & Galano A. Melatonin: exceeding expectations. Physiology (Bethesda) 29, 325–333 (2014). [DOI] [PubMed] [Google Scholar]
- Zhao H., Xu L., Su T., Jiang Y., Hu L. & Ma F. Melatonin regulates carbohydrate metabolism and defenses against Pseudomonas syringae pv. tomato DC3000 infection in Arabidopsis thaliana. J. Pineal Res. 59, 109–119 (2015). [DOI] [PubMed] [Google Scholar]
- Gaffney T. et al. Requirement of salicylic acid for the induction of systemic acquired resistance. Science 261, 754–756 (1993). [DOI] [PubMed] [Google Scholar]
- Shi H. T., Li R. J., Cai W., Liu W., Wang C. L. & Lu Y. T. Increasing nitric oxide content in Arabidopsis thaliana by expressing rat neuronal nitric oxide synthase resulted in enhanced stress tolerance. Plant Cell Physiol. 53, 344–357 (2012). [DOI] [PubMed] [Google Scholar]
- Shi H. et al. The Cysteine2/Histidine2-type transcription factor ZINC FINGER OF ARABIDOPSIS THALIANA 6 modulates biotic and abiotic stress responses by activating salicylic acid-related genes and C-REPEAT-BINDING FACTOR genes in Arabidopsis. Plant Physiol. 165, 1367–1379 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisec J. et al. Gas chromatography mass spectrometry-based metabolite profiling in plants. Nature Protocols 1, 387–396 (2006). [DOI] [PubMed] [Google Scholar]
- Shi H., Ye T., Zhong B., Liu X., Jin R. & Chan Z. Comparative proteomic and metabolomic analyses reveal mechanisms of improved cold stress tolerance in bermudagrass (Cynodon dactylon (L). Pers.) by exogenous calcium. J. Inter. Plant Biol. 56, 1064–1079 (2014). [DOI] [PubMed] [Google Scholar]
- Shi H. et al. Manipulation of arginase expression modulates abiotic stress tolerance in Arabidopsis: effect on arginine metabolism and ROS accumulation. J. Exp. Bot. 64, 1367–1379 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Ye T. & Chan Z. Nitric oxide-activated hydrogen sulfide is essential for cadmium stress response in bermudagrass (Cynodon dactylon (L). Pers.). Plant Physiol. Biochem. 74, 99–107 (2014). [DOI] [PubMed] [Google Scholar]
- Shi H., Ye T., Zhu J. K. & Chan Z. Constitutive production of nitric oxide leads to enhanced drought stress resistance and extensive transcriptional reprogramming in Arabidopsis. J. Exp. Bot. 65, 4119–4131 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denancé N. et al. Disease resistance or growth: the role of plant hormones in balancing immune responses and fitness costs. Front. Plant Sci. 4, 155 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huot B. et al. Growth-defense tradeoffs in plants: a balancing act to optimize fitness. Mol. Plant 7, 1267–1287 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bent A. F. & Mackey D. Elicitors, effectors, and R genes: the new paradigm and a lifetime supply of questions. Annu. Rev. Phytopathol. 45, 399–436 (2007). [DOI] [PubMed] [Google Scholar]
- Chun H. J. et al. Constitutive expression of mammalian nitric oxide synthase in tobacco plants triggers disease resistance to pathogens. Mol Cells 34, 463–471 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delledonne M., Xia Y. & Dixon R. A. Lamb C: Nitric oxide functions as a signal in plant disease resistance. Nature 394, 585–588 (1998). [DOI] [PubMed] [Google Scholar]
- Delledonne M. et al. Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. P. Natl Acad. Sci. USA 98, 13454–13459 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidler D. et al. Innate immunity in Arabidopsis thaliana: lipopolysaccharides activate nitric oxide synthase (NOS) and induce defense genes. P. Natl Acad. Sci. USA 101, 15811–15816 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Ye T., Han N., Bian H., Liu X. & Chan Z. Hydrogen sulfide regulates abiotic stress tolerance and biotic stress resistance in Arabidopsis. J. Inter. Plant Biol. 57, 628–640 (2015). [DOI] [PubMed] [Google Scholar]
- Natarajan S. et al. Transgenic soybeans and soybean protein analysis: an overview. J. Agric. Food Chem. 61, 11736–11743 (2013). [DOI] [PubMed] [Google Scholar]
- Wang E. H. et al. Effects of 90-day feeding of transgenic Bt rice TT51 on the reproductive system in male rats. Food Chem. Toxicol. 62, 390–396 (2013). [DOI] [PubMed] [Google Scholar]
- Chandler S. F. et al. Expression of flavonoid 3′, 5′-hydroxylase and acetolactate synthase genes in transgenic carnation: assessing the safety of a nonfood plant. J. Agric. Food Chem. 61, 11711–11720 (2013). [DOI] [PubMed] [Google Scholar]
- Fu Z. Q. et al. NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature 486, 228–232 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S. & Dong X. Perception of the plant immune signal salicylic acid. Curr. Opin. Plant Biol. 20, 64–68 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada Y. et al. Plant immunity requires conformational charges of NPR1 via S-nitrosylation and thioredoxins. Science 321, 952–956 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindermayr C. et al. Redox regulation of the NPR1-TGA1 system of Arabidopsis thaliana by nitric oxide. Plant Cell 22, 2894–2907 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun A., Nie S. & Xing D. Nitric oxide-mediated maintenance of redox homeostasis contributes to NPR1-dependentplant innate immunity triggered by lipopolysaccharides. Plant Physiol. 160, 1081–1096 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun A. & Li Z. Regulatory role of nitric oxide in lipopolysaccharides-triggered plant innate immunity. Plant Signal. Behav. 8, e22554 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. et al. Free radicals mediate systemic acquired resistance. Cell Rep. 7, 348–355 (2014). [DOI] [PubMed] [Google Scholar]
- Wendehenne D. et al. Free radical-mediated systemic immunity in plants. Curr. Opin. Plant Biol. 20, 127–134 (2014). [DOI] [PubMed] [Google Scholar]
- Agurla S., Gayatri G. & Raghavendra A. S. Nitric oxide as a secondary messenger during stomatal closure as a part of plant immunity response against pathogens. Nitric Oxide 43, 89–96 (2014). [DOI] [PubMed] [Google Scholar]
- Trapet P. et al. NO signaling in plant immunity: A tale of messengers. Phytochemistry 112, 72–79 (2015). [DOI] [PubMed] [Google Scholar]
- Yun B. W. et al. S-nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature 478, 264–268 (2011). [DOI] [PubMed] [Google Scholar]
- Yu M. et al. A sleigh ride through the SNO: regulation of plant immune function by protein S-nitrosylation. Curr. Opin. Plant Biol. 15, 1–7 (2012). [DOI] [PubMed] [Google Scholar]
- Thibaud M. C. et al. Sucrose increases pathogenesis-related PR-2 gene expression in Arabidopsis thaliana through an SA-dependent but NPR1-independent signaling pathway. Plant Physiol. Biochem. 42, 81–88 (2004). [DOI] [PubMed] [Google Scholar]
- Tsutsui T., Nakano A. & Ueda T. The plant-specific RAB5 GTPase ARA6 is required for starch and sugar homeostasis in Arabidopsis thaliana. Plant Cell Physiol. 56, 1073–1083 (2015). [DOI] [PubMed] [Google Scholar]
- Mandal M. K. et al. Oleic acid-dependent modulation of NITRIC OXIDE ASSOCIATED1 protein levels regulates nitric oxide-mediated defense signaling in Arabidopsis. Plant Cell 24, 1654–1674 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.