Abstract
The prevalence of non-alcoholic fatty liver disease (NAFLD) has markedly increased, especially in patients exhibit one or more features of the metabolic syndrome. This study investigates the effect of rosuvastatin (RSV) and/or β-carotene (βC) in NAFLD-induced rats. Rats were classified into nine groups; normal (I), NAFLD-induced with high-fat diet (HFD; II), NAFLD switched to regular diet (RD; III), NAFLD-HFD or NAFLD-RD treated with RSV (IV, V), βC (VI, VII) or both RSV+βC (VIII, IX), respectively. After four weeks, rats were sacrificed to obtain serum samples and liver tissues. Liver histology, lipid profile, liver oxidative stress markers, and adipocytokines were measured. Liver sections of rats with NAFLD-HFD revealed steatosis, lose of hepatic architecture, inflammation and hepatocyte vacuolation with high percentage of cell fibrosis. Serum levels of ALT, AST, ALP, gamma glutamyl transferase (GGT) and lipid profile (triglycerides, cholesterol, LDL and VLDL) were significantly increased (P<0.05) compared with normal. Also, hepatic malondialdehyde level and serum leptin, tumor necrosis factor-alpha (TNF-α) and transforming growth factor-β1 (TGF-β1) were increased. Meanwhile, superoxide dismutase (SOD) activity, GSH content in liver, serum HDL and adiponectin were decreased (P<0.05) vs normal. These changes were observed to a lesser extent in NAFLD-RD group. Administration of RSV or/and βC almost improved all previously mentioned parameters. Moreover, hepatic steatosis was decreased and inflammation was markedly ameliorated with reduction of TNF-α and TGF-β. These results were more pronounced in the groups VIII and IX vs each drug alone. In conclusion RSV and βC could be beneficial for the treatment and prevention of NAFLD. Combined RSV with βC is more effective than RSV alone.
Keywords: NAFLD, Rosuvastatin, β-carotene, TNF-α, TGF-β1, Oxidative stress
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD), regarded as the hepatic manifestation of the metabolic syndrome, currently represents the most common cause of chronic liver disease (1). NAFLD ranges from simple hepatic fat accumulation (steatosis) to non-alcoholic steatohepatitis (NASH), where fat is accompanied by hepatocyte injury, and necroinflammation. This condition poses an increased risk of cirrhosis and hepatocellular carcinoma (1). Depending on the assessment tools, the prevalence of NAFLD in adults ranges between 20% and 30% (2), reaching up to 46% in some studies (3). Furthermore, NAFLD has become the most common cause of chronic liver disease in children and adolescents, with prevalence of 3–10%, and even up to 70% in obese children (2,4). The dramatic increase in prevalence of obesity, metabolic syndrome, and NAFLD has been linked to the excess caloric intake due to increased consumption of processed food and beverages, coupled with a more sedentary lifestyle (5). The condition is strongly associated with overweight and insulin resistance, thus the promoting of changes in lifestyle, e.g. focusing on regular exercise and a reduction in fat and carbohydrate intake, is a key factor in the treatment of patients with NAFLD (6).
Approximately 70% of patients with NASH also have concurrent dyslipidemia (7) making treatment with a lipid-lowering medication appear to be a reasonable approach. Rosuvastatin (RSV) is a lipid-lowering agent that competitively inhibits 3-hydroxy-3-methylglutaryl coenzyme A reductase. RSV exhibits the highest efficacy in the reduction of low-density lipoprotein (LDL), total cholesterol (TC), and triglycerides (TG) compared with other statins at comparable doses (8). It has been reported that RSV ameliorates hepatic insulin resistance in rodents and humans (9). In addition, RSV reduces the risk of cardiovascular disease, decrease vascular reactive oxygen species (ROS) generation independently of cholesterol reduction (10) and exerts several pleiotropic effects, but also achieve significant improvement in endothelial function effect (11).
In fatty liver disease, ROS induces lipid peroxidation acting on polyunsaturated fatty acids and producing highly reactive aldehydic derivatives (e.g. malondialdehyde, MDA) which have long-term adverse effects on liver cells (12). There is a characteristic cytokine pattern in liver steatosis which includes increasing tumor necrosis factor-alpha (TNF-α), transforming growth factor-β1 (TGF-β1) and interleukin 6, but decreasing adiponectin, ghrelin and leptin (13). β-carotene (βC), a lipid-soluble antioxidant, has an important function as a precursor of vitamin A, and it has a direct impact on cholesterol synthesis (14). Vitamin A participates in several functions that are primordial to the human system, acting in visual acuity, in cellular proliferation and differentiation, and immunological activity (15). Retinol and carotenoids, especially βC, have received a prominent position for their role against ROS, protecting the organism against oxidative stress (OS) and, consequently, preventing damages and tissue lesions related to several chronic diseases (16). Considering the role of OS in NAFLD pathogenesis and the potent action of βC as a precursor of vitamin A in the fight against ROS, it is probable that individuals with NAFLD will have lower levels of this vitamin, as OS much increases the intake of substances with antioxidant function (17). Moreover, βC serves as a prehormone that, through metabolism, is converted into retinoic acid, which functions as a ligand, regulating the expression of genes involved in metabolic processes. The efficacy and safety profile of pharmacotherapy in the treatment of NAFLD has yet been remained uncertain (18) and since there is no effective specific therapy for NAFLD, this study was conducted to evaluate whether the addition of βC to RSV could improve NAFLD characteristics in a rat model subjected to different life styles.
MATERIALS AND METHODS
Animals
Seventy-two male Sprague-Dawley rats, weighting 150–200 g, were provided by the Schistosome Biology Supply Center of Theodor Bilharz Research Institute, Giza, Egypt. The rats were housed in cages with free access to food (standard commercial pelleted diet, El-Kahira Company for oils and soap) and water for one week before initiation of the experiment in an air-conditioned animal house at 20–22 °C. The experimental study protocol was conducted at the animal unit according to the international ethical guidelines that was approved by Institutional Animal Ethics Committee of Theodor Bilharz Research Institute.
Experimental design
A rat model of NAFLD was induced by a high-fat diet containing 25% fats, 1% cholesterol (Win lab Laboratory chemicals), and 0.25% bile salts (Alpha chemika, India) for 12 weeks (19). After 12 weeks, rats in NAFLD groups were administered orally with RSV (Astrazeneca-Egypt) at a dose of 10 mg/kg/day or/and βC (Arab Co. for Pharmaceuticals and Medicinal Plants; MEPACO-Egypt) at a dose of 70 mg/kg every alternative day, for 4 weeks (starting from the 13th week to the 16th week). Rats in the NAFLD-RD treated groups were switched and maintained on the standard chow diet (weeks 13-16). After establishing NAFLD (after 12 weeks) rats were randomly divided into nine groups (eight rats each); normal control (I), NAFLD-induced with high-fat diet (HFD; II), NAFLD switched to regular diet (RD; III), NAFLD-HFD or NAFLD-RD treated with RSV (IV, V), βC (VI, VII) or the combined RSV+βC (VIII, IX) respectively. Rats in the normal group were maintained on the standard chow diet for 16 weeks. At the end of the study, all rats were weighted and then sacrificed by rapid decapitation after 12 h of fasting to insure that the change in lipid profile and other serum biomarkers is due to NAFLD or drug treatment and not to instant food intake. Blood samples were collected for biochemical assays. The liver was immediately removed and weighted after rinsing with ice-cold saline, and sampled for assessment of liver reduced glutathione (GSH), superoxide dismutase (SOD), lipid peroxidation as malondialdehyde (MDA), and for histological study. Liver weight index (%) was calculated as liver weight/body weight × 100.
Biochemical analysis
Blood biochemical parameters including; serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma glutamyl transferase (GGT), triglycerides (TG), total cholesterol (TC), HDL, LDL and VLDL were assayed spectrophotometrically using the commercially available kits. Serum levels of leptin and TNF-α (RayBiotech Inc., Norcross, GA, USA), adiponectin (Assaypro, St. Louis, MO, USA), and TGF-β1 (eBioscience, Vienna, Austria) were measured using enzyme-linked immunosorbent assay (ELISA) kits.
Assay of oxidative stress and lipid peroxidation
Liver tissues were immediately cooled on ice and 0.5 g of liver was homogenized (Pro Scientific Inc., U.S.A. homogenizer) in 4 volumes (w/v) of ice-cold 0.1 M KH2 PO4 buffer containing 1 mM EDTA adjusted to pH 7.4 and centrifuged at 10,000 × g for one hour at 4 °C. The supernatant was collected and kept at –80 °C for subsequent analysis for determination of GSH (20), SOD activity (21). Degree of lipid peroxidation in liver tissue homogenates was determined in terms of thiobarbituric acid-reactive substances (TBARs) as expressed by MDA formation (22).
Histological studies
The sections of liver tissues were fixed in 10% buffered formaldehyde, and then embedded in paraffin wax. A 5 μm-thick section cut from a paraffin-embedded block and at a distance of 250 μm from the preceding section was stained with H&E and Masson's trichrome. Steatosis was assessed by a morphological semi-quantitative approach and graded as follows: mild=5–30%, moderate=30–60%, and severe >60% of hepatocytes affected (23). The specimens were also examined for histological features including; ballooning degeneration, hepatocytes with cytoplasmic vacuolation, acidophilic necrosis, sinusoidal fibrosis and polymorph nuclear infiltration.
Statistical analysis
All values are expressed as mean ± SEM. Calculations were carried out using SPSS, software package version 16.0 (Chicago, IL, USA). Groups of data were compared with the one-way analysis of variance (ANOVA) followed by post-hoc LSD test for multiple comparison among groups of treatments with the NAFLD and normal control groups. P values of ≤0.05 were considered statistically significant.
RESULTS
Effects on liver gross manifestations
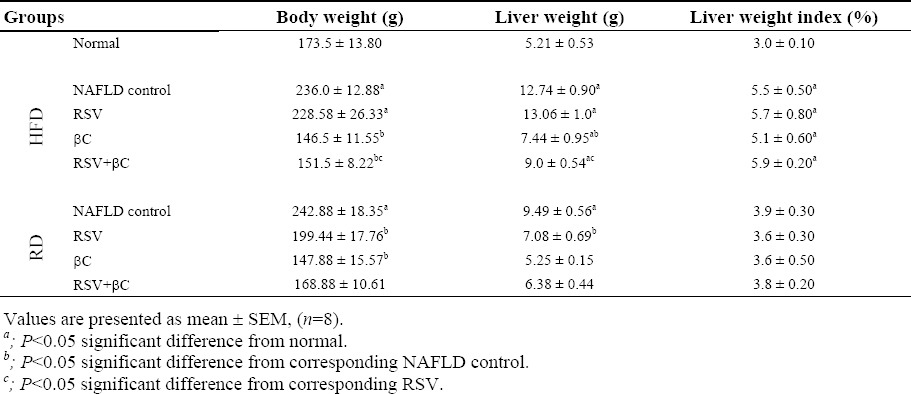
The body weight, liver weight and liver index in NAFLD-HFD or NAFLD-RD groups were significantly increased (P<0.05) compared with those of normal control group. Compared with NAFLD-HFD, there is no significant difference in body and liver weight, and liver index in the group treated with RSV-HFD (Table 1). Meanwhile, a tendency for normalization with significant decrease (P<0.05) in the previously mentioned parameters in the groups treated with βC or RSV either alone or in combination and the group treated with RSV-RD relative to their corresponding NAFLD control group.
Table 1.
Effect of rosuvastatin and β-carotene alone or in combination on liver gross manifestations in NAFLD-induced rats.
Effects on hepatic function, lipid profile and adipocytokine markers
The serum levels of ALT, AST, ALP, GGT, TG, TC, LDL and VLDL as well as the levels of the adipocytokines; leptin, TNF-α and TGF-β1 were significantly increased (P<0.05) in NAFLD-HFD group and to a lesser extent in NAFLD-RD group when compared with normal control group (Table 2; Figs. 1 and 2). At the same time, there was a significant decrease (P<0.05) in the levels of HDL and adiponectin. When compared with corresponding NAFLD control group, serum levels of ALT, AST, ALP, GGT, TG, TC, LDL, VLDL, leptin, TNF-α and TGF-β1 were VLDL, leptin, TNF-α and TGF-β1 were significantly decreased (P<0.05) in the groups treated with either RSV alone or in combination with βC. Meanwhile, an insignificant decreasing tendency in almost all these parameters was observed in the group treated with βC alone especially in lipid profile and adipocytokines. On the other hand, serum levels of HDL and adiponectin were significantly increased (P<0.05) in either RSV or βC-treated groups and their combination except βC-HFD (for HDL) or βC-RD (for adiponectin) compared with their corresponding NAFLD control groups (Table 2 Figs. 1 and 2).
Table 2.
Effect of rosuvastatin and β-carotene alone or in combination on lipid profile in NAFLD-induced rats.
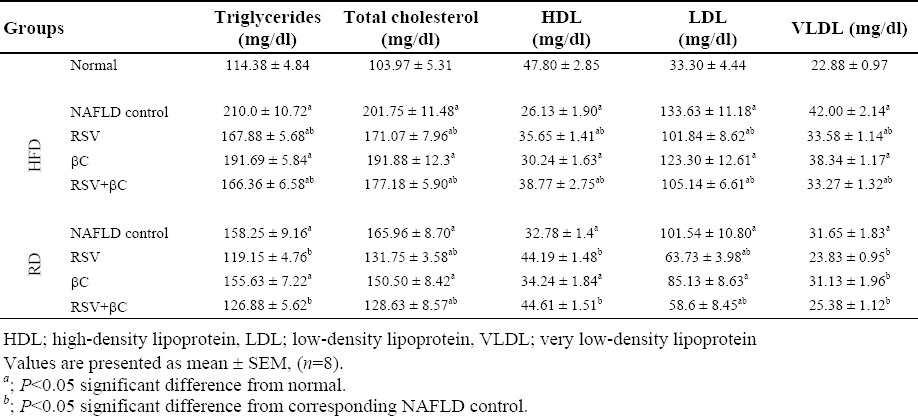
Fig. 1.
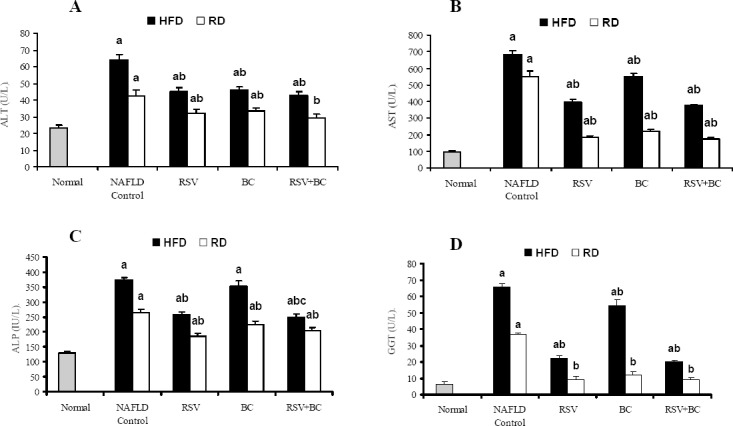
Effect of rosuvastatin and β-carotene alone or in combination on liver functions in NAFLD-induced rats. A; ALT, B; AST, C; ALP, D; GGT. Values are presented as mean ± SEM, (n=8). a; P<0.05 significant difference from normal. b; P<0.05 significant difference from corresponding NAFLD control.
Fig. 2.
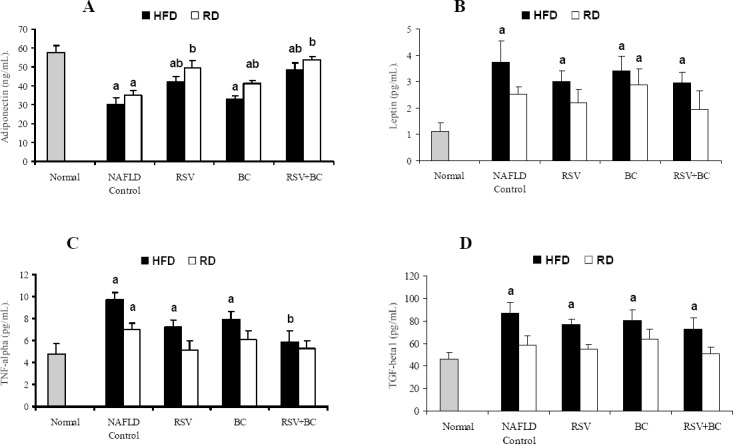
Effect of rosuvastatin and β-carotene alone or in combination on adipocytokine markers, A; adiponectin, B; leptin, C; TNF-α and D; TGF-β1 in NAFLD-induced rats. Values are presented as mean ± SEM, (n=8). a; P<0.05 significant difference from normal. b; P<0.05 significant difference from corresponding NAFLD control.
Effects on oxidative stress and lipid peroxidation
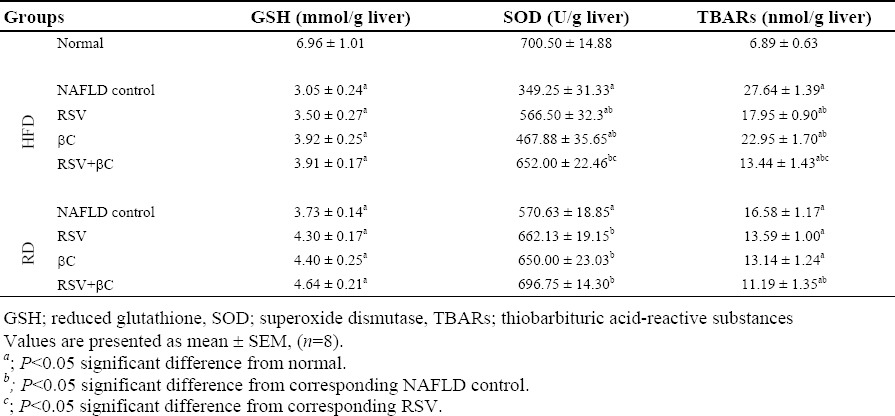
Compared with normal control group, the hepatic GSH content and SOD activities in NAFLD-HFD group were significantly decreased (P<0.05), meanwhile MDA level was significantly increased. These changes were to a less extent in NAFLD-RD group. Compared with the corresponding NAFLD control groups, oxidative stress and lipid peroxidation in the hepatic tissue were restored by decreasing SOD activities and MDA levels significantly (P<0.05) upon administration of either RSV or βC alone or both except the RSV-RD and βC-RD treated groups for MDA (Table 3).
Table 3.
Effect of rosuvastatin and β-carotene alone or in combination on oxidative stress parameters and lipid peroxidation in NAFLD-induced rats.
In the RSV+βC group, all the measured serumic and hepatic markers gave better results over those of single treated group whether maintained on HFD or switched to regular diet. Moreover, the best results were obtained in all groups switched to regular diet.
Histopathological evaluation
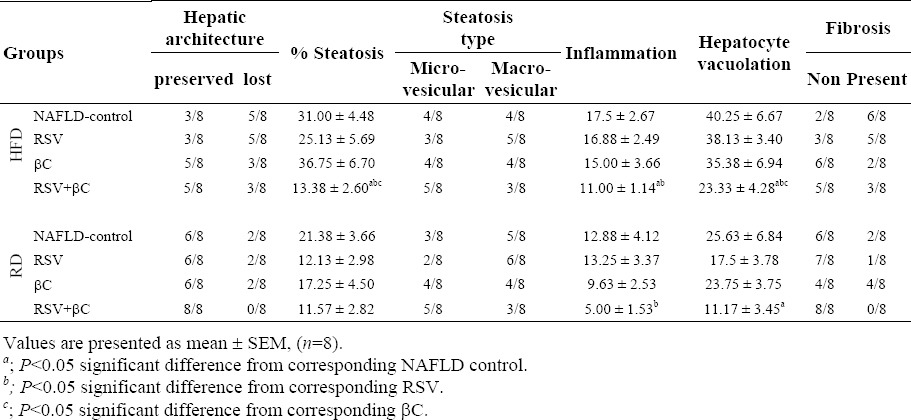
In normal control group, the liver lobules were distinct (preserved lobular architecture) and the liver cell cords arranged regularly (Fig. 3A), whereas, the liver in NAFLD-HFD group (Fig. 3B) showed typical steatosis (31.0 ± 4.48, micro and macrosteatotic changes), lobular infiltration (17.5 ± 2.67) by mononuclear cell and lymphocyte, hepatocytes with vacuolation (40.25 ± 6.67), fat droplets accumulation, spotty necrosis and focal necrosis. These findings suggest that the animal model of NAFLD was successfully established. However, the degree of hepatic injury including steatosis, hepatocytes with cytoplasmic vacuolation and lobular inflammation were to a less degree in NAFLD-RD group (21.38 ± 3.66, 12.88 ± 4.12 and 25.63 ± 6.84 respectively; Table 4; Fig. 3D). Treatment of NAFLD-induced rats maintained on either HFD or RD, markedly attenuated steatosis with either RSV or βC alone or their combination (25.13 ± 5.69 and 12.13 ± 2.98 RSV; 36.75 ± 6.70 and 17.25 ± 4.50 βC; 13.38 ± 2.60 and 11.57 ± 2.82 RSV+βC, respectively), inflammation (16.88 ± 2.49 and 13.25 ± 3.37 RSV; 15.00 ± 3.66 and 9.63 ± 2.53 βC; 11.0 ± 1.14 and 5.0 ± 1.53 RSV+βC, respectively) and hepatocytes with cytoplasmic vacuolation (38.13 ± 3.40 and 17.50 ± 3.78 RSV; 35.38 ± 6.94 and 23.75 ± 3.75 βC; 23.33 ± 4.28 and 11.17 ± 3.45 RSV+βC, respectively) 4 weeks post treatment (Table 4; Fig. 3(E–J)). In the RSV+βC combined group, steatosis and extension of lobular inflammation were relieved significantly over those of each treated group, and in all NAFLD-RD groups over those groups maintained on HFD (Table 4; Fig. 3 (I and J)).
Fig. 3.
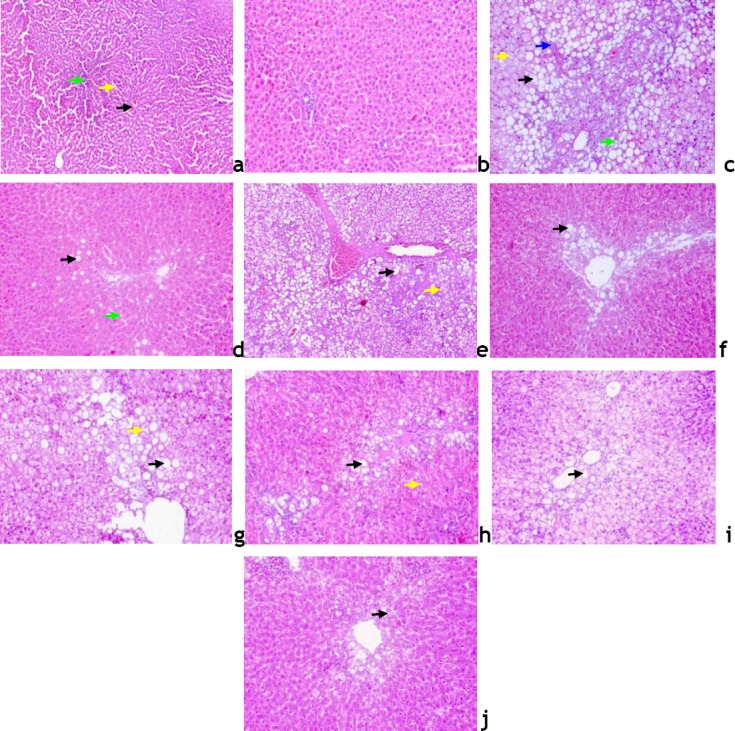
Liver sections from; normal untreated rats, a; ×100; composed of hexagonal or pentagonal lobules with central veins (black arrow) and portal tract (bright green arrow) embedded in connective tissue. Hepatocytes are arranged in trabecules running radiantly from the central vein and are separated by sinusoids (yellow arrow) containing Kuppfer cells, b; ×200; NAFLD-HFD rats, c; showing lost hepatic architecture, formation of fibrous septa (blue arrow) moderate macro 50% (black arrow) and micro (bright green arrow) steatotic changes, many hepatocytes with cytoplasmic vacuoles (yellow arrow), and to a less extent in NAFLD-RD rats, d; RSV-HFD-treated rats, e; showing loss of hepatic architecture 62.5%, macro (black arrow) and micro (yellow arrow) 37.5% steatotic changes, RSV-RD-treated rats, f; showing intact hepatic architecture 75%, large lipid droplets as macro (black arrow) 75% steatotic changes, βC-HFDtreated rats, g; showing partial loss of hepatic architecture 37.5%, macro (black arrow) and micro (yellow arrow) 50% steatotic changes, βC-RD-treated rats, h; showing intact hepatic architecture 75%, micro (yellow arrow) and macro (black arrow) 50% steatotic changes, RSV+βC-HFD-treated rats, i; showing preserved hepatic architecture 62.5%, macro (black arrow) 37.5% steatotic changes, and RSV+βC-RD-treated rats, j; showing normal preserved hepatic architecture 100%, liver appear within normal limit with micro (black arrow) lipid droplets (H&E, × 200).
Table 4.
Effect of rosuvastatin and β-carotene alone or in combination on histological parameters of liver in NAFLD-induced rats.
DISCUSSION
NAFLD represents a wide spectrum of disorders, the hallmark of which is hepatic steatosis. NAFLD was considered a benign condition, but is now increasingly recognized as a major cause of liver-related morbidity and mortality (24). Although the exact physiopathology of NAFLD is not fully understood (25), it was described as a “two hit model”. The first hit is supposed to be the increase of free fatty acids in hepatocytes, which results in a decrease of β-oxidation, which aggravates accumulation of fatty acids and insulin resistance. The second step includes all mechanisms contributing to the generation of proinflammatory cytokines, oxidative species and thereby enhances lipid peroxidation of the hepatocyte membrane (26) and development of inflammation and fibrosis (27).
In this study hypercholestermic model of NAFLD was established by feeding animals with HFD for 12 weeks (19) followed by RD for 4 weeks concomitant with drugs administration.
Increased body weight, liver weight and liver weight index, elevated serum liver enzymes, lipid profile levels, oxidative stress, enhanced lipid peroxidation and altered liver histopathology including steatosis, inflammation and fibrosis were observed in the NAFLD-HFD rats, and to a less extent in NAFLD-RD rats. These abnormalities were significantly improved 4 weeks after treatment with either RSV or βC or both.
Body weight significantly increased with the high-fat diet, this might be due to a metabolic imbalance of carbohydrate, protein, and fat. Treatment of NAFLD-HFD with RSV resulted in significant increase in body weight versus normal but not significant with NAFLD-HFD control; this was in agreement with Valero-Muñoz and coworkers (28) who reported that RSV did not modify body weight or the weight of the adipose packages in HFD rat. Treatment with βC alone or in combination with RSV resulted in significant decrease of body weight, liver weight and its index.
This was in accordance with Chung and colleagues (29) who observed that most of the carotenoids appeared to be inversely correlated to fat mass, suggesting that during obesity carotenoids are sequestered in adipose tissue, decreasing their plasma concentrations. Moreover, Van Helden and coworkers (30) showed that the anti-obesity effect of βC has been demonstrated to be linked to its pro-vitaminic A effect. However, hepatic index was higher in the group with steatosis as compared to the RSV or βC-treated and normal groups, which means that βC and, at least in part, RSV acts by decreasing fat accumulation in the liver and fat weight, and therefore decreases hepatic index.
In the present investigation, the observed elevation in all liver enzymes in NAFLD model is in agreement with Al-Dosari and colleagues (31) who reported that there were increased plasma activities of AST, ALT, ALP and GGT in HFD-fed rats. ALT and AST are biomarkers in the diagnosis of hepatic damage because they are released into the circulation after hepatocellular damage (32). Moreover, data also showed significant increase in serum TG, TC, LDL, VLDL, leptin as well as TNF-α and TGF-β1 levels accompanied with significant decrease in serum HDL and adiponectin in the NAFLD group as in previous reports (33,34,35). Rizos and coworkers (36) reported that in hyperlipidemic patient clinically significant elevations in AST/ALT values were defined as >3-fold the upper limit of normal. This was in agreement with the result of this study where AST/ALT was >3-fold the upper limit. Body fat distribution appears to be even more important than the total amount of adipose tissue, and visceral fat mass is strongly linked to insulin resistance and NAFLD (37). Visceral fat released FFAs are transported to the liver by the portal vein and may contribute to hepatic steatosis, production of triglyceride rich VLDL and elevated β-oxidation (38).
These abnormalities were significantly improved, and hepatic steatosis was significantly decreased in all RSV and βC-treated groups and to a less extent in the group treated with βC alone. The results of the present study showed that RSV treatment in NAFLD model resulted in normalization of GGT and a significant decrease in ALT, AST and ALP. This was in agreement with Antonopoulos and colleagues (39) and Ahmed and coworkers (40) who reported that the use of RSV has been improved liver abnormalities in NAFLD patients. Moreover, RSV treatment reduced the steatosis likely due to a decreased input of FFAs and an increased output of FFAs through increased β-oxidation. In addition, RSV treatment in NAFLD-RD model showed normalization in lipid profile; TG, TC, LDL, VLDL and HDL. This was in accordance with Awad and Kamel (41) who indicated that RSV ameliorated hepatic injury, inflammation and lipid perixodation, and in contrast with those of Ansari and coworkers (42) who reported that oral administration of RSV at 10 mg/day in rats fed with HFD resulted in significant elevation of HDL. Kostapanos and colleagues (43) showed that RSV treatment at 10 mg/day in patients was associated with a significant hypotriglyceridemic effect, a reduction in the cholesterol concentration of all LDL particles. Moreover, Malaguarnera and coworkers (44) reported that RSV improves lipid profiles, hepatic parameters, and the histology of HCV. Furthermore, RSV limits the effects of cytokines, improving insulin resistance and TNF-α produced by monocytes and macrophages, which impairs insulin signaling and plays a crucial role in NASH progression (45).
Data from the present investigation showed that RSV treatment increased adiponectin level significantly but not leptin compared with NAFLD control. On contrary to this, De las Heras and colleagues (46) and Valero-Muñoz and coworkers (28) reported that RSV decreased leptin levels and did not modify adiponectin levels in treated HFD rats. Hasegawa and colleagues (47) reported that TGF-β1 mediates the transformation of quiescent hepatic stellate cells into myofibroblasts-like cells with an increased production of extra cellular matrix proteins including type I collagen. Activated kupffer cells and hepatic stellate cells secrete TGF-β1 and its plasma concentration is elevated in NASH patients compared to patients with hepatic steatosis and healthy subjects, suggesting that this cytokine is involved in fibrogenesis in NASH. Yang and coworkers (48) reported that adiponectin also regulates hepatic expression of TGF-β1, a pro-fibrotic factor involved in hepatic stellate cell activation that plays an important role in neo-fibrogenesis of NAFLD. In this study, the improvement in TNF-α and TGF-β1 with RSV-HFD rats was not significant from its NAFLD-HFD control, while a tendency for normalization was achieved using RSV-RD with respect to normal. This is in agreement with De las Heras and colleagues (46) who observed that RSV treatment did not affect the elevated expression of TNF-α in adipose tissue of HFD rats with respect to controls. Also, Ma and coworkers (49) reported that RSV in a dose dependent manner reduces TGF-β1 expression and inhibits the development of myocardial fibrosis in diabetic rats.
In the current study, βC supplementation resulted in a significant decrease in liver enzymes except ALP from NAFLD control. This is in agreement with the study of Vardi and colleagues (50) who reported that βC given for 21 days before the methotrexate application provided significant protection from the hepatotoxicity of methotrexate. On the other hand, it showed no significant changes in lipids serum markers from NAFLD control. These results are in accordance with Nierenberg and coworkers (51) who observed that high intakes of βC had no effect on serum lipid concentrations in human beings. This could be due to βC not accumulates in rats tissues when fed at physiologic levels (52,53). Moreover, the coadministration of RSV+βC herein, resulted in further improvements in liver enzymes and lipid profile versus each drug alone. In this context, Rydén and colleagues (54) reported that there is a risk to misinterpret the carotenoids status in individuals with cholesterol-lowering therapy.
In this study, administration of βC alone did not produce significant reduction in adiponectin or leptin levels from NAFLD control. This is conversely to Canas and coworkers (55) who observed that there were inverse correlations between βC and abdominal fat mass at baseline. Meanwhile, co-administration of RSV with βC resulted in significant increase in adiponectin concomitant with reduction in leptin and TGF-β1 and normalization in TNF-α level versus NAFLD and normal control. In this context, Kameji and colleagues (56) reported that the anti-inflammatory effects of βC in adipocytes were suggested to arise through limitation of TNF-α-mediated down-regulation of genes linked to adipocyte biology.
Oxidative stress is believed to play an important role in pathogenesis of NAFLD. It has been shown that chronic oxidative stress, generated through oxidation of cytotoxic free fatty acids, may lead to cytokine upregulation and depletion of hepatic antioxidant levels (57). In addition, enhanced lipid peroxidation leads to the generation of by-products, such as MDA, which have been shown to further stimulate cytokine production (58). In the current study, there was a significant decrease in GSH content and SOD activity in NAFLD rats and a significant increase in MDA levels. These findings are in agreement with those of Khan and coworkers (59) who reported that liver GSH and SOD activity was decreased in hypercholesterolemic rats, while MDA content was significantly higher in comparison with normal control group. As, hypercholesterolemia induces cellular oxidative stress, to protect cells or tissues antioxidant systems have to fight against this (60).
RSV treatment showed antioxidant properties as it normalized SOD activity and significantly decreased MDA levels in rats fed with HFD. These findings are in accordance with those of Ansari and colleagues (42) who reported that oral administration of RSV at 10 mg/day in rats fed with HFD resulted in significant elevation in antioxidant properties of cardiac SOD activity and GSH content accompanied with significant decrease in cardiac TBARs level.
Data in the present study showed that βC treatment significantly increased SOD activity whereas the increase in GSH was not significant from NAFLD control. These results are in accordance with Vardi and coworkers (50) who observed that the oxidative damage increasing with methotrexate in liver tissue was prevented by dosing βC for 21 days prior to methotrexate administration. Also, El-Demerdash and colleagues (61) reported that βC plays an important role in protecting cell membrane against oxidative damage because of its property of scavenging lipid and peroxyl radicals. Moreover, Shih and colleagues (62) observed that βC attenuated the diet-induced oxidative stress in rats fed with high fat, high-cholesterol diet, which was due in part to up regulated antioxidant defenses. At the same time, the significant decrease in MDA level from NAFLD control after βC administration herein is in agreement with Berryman and coworkers (63) who reported that the addition of βC returned MDA levels back to normal in the liver of diabetic rats.
CONCLUSION
The current study shows that weight loss through dietary control is one of the most effective therapeutic strategies and is the first step in NAFLD management (64). As hypertriglyceridemia and hypercholesterolemia is often associated with NAFLD, hence the rationale for using the effective lipid lowering agent RSV in its management. In addition, βC recognized as the most potent retinol precursor and provitamin A (65), which responsible for most of its antioxidative and pharmacological effects. Accordingly, the combined administration of RSV and βC in this study has an enhancement effect in modulating the biochemical and histological markers related to NAFLD disease compared to either drug alone. This may be due to their synergistic antioxidative, anti-inflammatory and lipid lowering effects. Moreover, combined treatment with RSV and βC has a modulating effect on the extent of fatty infiltration and on lipid peroxidation compared with each drug alone. Reduction of leptin, TGF-β1, TNF-α and oxidative stress remains the best-targeted treatment to date for NAFLD.
ACKNOWLEDGMENTS
This work was supported by the internal research project 95/A for basic and applied research, a grant from Theodor Bilharz Research Institute.
REFERENCES
- 1.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi KN, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, American College of Gastroenterology and the American Gastroenterological Association. Am J Gastroenterol. 2012;142:1592–1609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010;28:155–161. doi: 10.1159/000282080. [DOI] [PubMed] [Google Scholar]
- 3.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and non-alcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 4.Loomba R, Sirlin CB, Schwimmer JB, Lavine JE. Advances in pediatric non-alcoholic fatty liver disease. Hepatology. 2009;50:1282–1293. doi: 10.1002/hep.23119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anania FA. Non-alcoholic fatty liver disease and fructose: bad for us, better for mice. J Hepatol. 2011;55:218–220. doi: 10.1016/j.jhep.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjornsson E, Angulo P. Non-alcoholic fatty liver disease. Scand J Gastroenterol. 2007;42:1023–1030. doi: 10.1080/00365520701514529. [DOI] [PubMed] [Google Scholar]
- 7.Hyogo H, Tazuma S, Arihiro K, Iwamoto K, Nabeshima Y, Inoue M, et al. Efficacy of atorvastatin for the treatment of nonalcoholic steatohepatitis with dyslipidemia. Metabolism. 2008;57:1711–1718. doi: 10.1016/j.metabol.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 8.Vijan S, Hayward RA. Pharmacologic lipid-lowering therapy in type 2 diabetes mellitus: background paper for the American College of Physicians. Ann Intern Med. 2004;140:650–658. doi: 10.7326/0003-4819-140-8-200404200-00013. [DOI] [PubMed] [Google Scholar]
- 9.Fraulob JC, Souza-Mello V, Aguila MB, Mandarim-de-Lacerda CA. Beneficial effects of rosuvastatin on insulin resistance, adiposity, inflammatory markers and non-alcoholic fatty liver disease in mice fed on a high-fat diet. Clin Sci (Lond) 2012;123:259–270. doi: 10.1042/CS20110373. [DOI] [PubMed] [Google Scholar]
- 10.Puccetti L, Pasqui AL, Scarpini F, Cappellone R, Ghezzi A, Ceccatelli L, et al. Statins discontinuation in compliant chronic users induces atherothrombotic profile despite baseline clinical setting and treatments. Int J Cardiol. 2011;3:328–329. doi: 10.1016/j.ijcard.2011.09.042. [DOI] [PubMed] [Google Scholar]
- 11.Parson HK, Bundy MA, Dublin CB, Boyd AL, Paulson JF, Vinik AI. Pleiotropic effects of rosuvastatin on microvascular function in type 2 diabetes. Diabetes Metab Syndr Obes. 2010;3:19–26. [PMC free article] [PubMed] [Google Scholar]
- 12.Day CP. From fat to inflammation. Gastroenterology. 2006;130:207–210. doi: 10.1053/j.gastro.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 13.Kolak M, Westerbacka J, Velagapudi VR, Wågsäter D, Yetukuri L, Makkonen J, et al. Adipose tissue inflammation and increased ceramide content characterize subjects with high liver fat content independent of obesity. Diabetes. 2007;56:1960–1968. doi: 10.2337/db07-0111. [DOI] [PubMed] [Google Scholar]
- 14.Burri BJ. Lycopene and human health. In: Meskin MS, Bidlack WR, Davies AJ, Omaye ST, editors. Phytonutrients in health and disease. Boca Raton: CRC Press; 2002. pp. 157–172. [Google Scholar]
- 15.Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington: National Academy Press; 2001. Institute of Medicine (IOM). Vitamin A; pp. 82–161. [PubMed] [Google Scholar]
- 16.Delport R, Ubbink JB, Human JA, Becker PJ, Myburgh DP, Vermaak WJ. Antioxidant vitamins and coronary artery disease risk in South African males. Clin Chim Acta. 1998;278:55–60. doi: 10.1016/s0009-8981(98)00131-4. [DOI] [PubMed] [Google Scholar]
- 17.Sarni RO, Ramalho RA. Serum retinol and total carotene concentrations in obese children. Med Sci Monit. 2005;11:510–514. [PubMed] [Google Scholar]
- 18.Duvnjak M, Tomasic V, Gomercic M, Smircic-Duvnjak L, Barsic N, Lerotic I. Therapy of nonalcoholic fatty liver disease: current sta-tus. J Physiol Pharmacol. 2009;60:57–66. [PubMed] [Google Scholar]
- 19.Zulet MA, Barber A, Garcin H, Higueret P, Martinez JA. Alterations in carbohydrate and lipid metabolism induced by a diet rich in coconut oil and cholesterol in a rat model. J Am Coll Nutr. 1999;1:36–42. doi: 10.1080/07315724.1999.10718825. [DOI] [PubMed] [Google Scholar]
- 20.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 21.Winterbourn CC, Hawkins RE, Brian M, Carrell RW. The estimation of red cell superoxide dismutase activity. J Lab Clin Med. 1975;85:337–342. [PubMed] [Google Scholar]
- 22.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 23.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Nonalcoholic steatohepatitis clinical research network design and validation of a histological scoring system for non-alcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 24.Xiao J, Fai SK, Liong EC, Tipoe GL. Recent advances in the herbal treatment of non-alcoholic fatty liver disease. J Tradit Complement Med. 2013;3:88–94. doi: 10.4103/2225-4110.110411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaser S, Ebenbichler CF, Tilg H. Pharmacological and non-pharmacological treatment of non-alcoholic fatty liver disease. Int J Clin Pract. 2010;64:968–983. doi: 10.1111/j.1742-1241.2009.02327.x. [DOI] [PubMed] [Google Scholar]
- 26.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 27.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 28.Valero-Muñoz M, Martín-Fernández B, Ballesteros S, Cachofeiro V, Lahera V, de Las Heras N. Rosuvastatin improves insulin sensitivity in overweight rats induced by high fat diet. Role of SIRT1 in adipose tissue. Clin Investig Arterioscler. 2014;26:161–167. doi: 10.1016/j.arteri.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Chung HK, Kang B, Lee JH, Shim JY, Park S, Lee SH, et al. Increased arterial stiffness is associated with reduced plasma levels of beta-carotene in treated hypertensive patients with type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis. 2009;6:e9–e11. doi: 10.1016/j.numecd.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 30.van Helden YG, Godschalk RW, Swarts HJ, Hollman PC, van Schooten FJ, Keijer J. Beta-carotene affects gene expression in lungs of male and female Bcmo1 (-/-) mice in opposite directions. Cell Mol Life Sci. 2011;68:489–504. doi: 10.1007/s00018-010-0461-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.AL-Dosari M, ALqasoumi S, Ahmad M, AL-yahya M, Ansari MN, Rafatullah S. Effect of Beta vulgaris L. on cholesterol rich diet-induced hypercholesterolemia in rats. Farmacia. 2011;59:669–678. [Google Scholar]
- 32.Naik SR, Panda VS. Antioxidant and hepatoprotective effects of Ginkgo biloba phytosomes in carbon tetrachloride-induced liver injury in rodents. Liver Int. 2007;27:393–399. doi: 10.1111/j.1478-3231.2007.01463.x. [DOI] [PubMed] [Google Scholar]
- 33.El-Attar M, El-Melegy N. Serum levels of leptin and adiponectin in patients with nonalcoholic fatty liver disease: potential biomarkers. JASMR. 2010;5:101–108. [Google Scholar]
- 34.Olorunnisola OS, Bradley G, Afolayan AJ. Protective effect of Tulbaghia violacea Harv. on aortic pathology, tissue antioxidant enzymes and liver damage in diet-induced atherosclerotic rats. Int J Mol Sci. 2012;13:12747–12760. doi: 10.3390/ijms131012747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neto-Ferreira R, Rocha VN, Souza-Mello V, Mandarim-de-Lacerda CA, de-Carvalho JJ. Pleiotropic effects of rosuvastatin on the glucose metabolism and the subcutaneous and visceral adipose tissue behavior in C57Bl/6 mice. Diabetol Metab Syndr. 2013;5:32. doi: 10.1186/1758-5996-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rizos CV, Milionis HJ, Kostapanos MS, Florentin M, Kostara CE, Elisaf MS, et al. Effects of rosuvastatin combined with olmesartan, irbesartan, or telmisartan on indices of glucose metabolism in Greek adults with impaired fasting glucose, hypertension, and mixed hyperlipidemia: a 24-week, randomized, open-label, prospective study. Clin Ther. 2010;32:492–505. doi: 10.1016/j.clinthera.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 37.Calamita G, Portincasa P. Present and future therapeutic strategies in non-alcoholic fatty liver disease. Expert Opin Ther Targets. 2007;11:1231–1249. doi: 10.1517/14728222.11.9.1231. [DOI] [PubMed] [Google Scholar]
- 38.Jensen MD. Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab. 2008;93:S57–S63. doi: 10.1210/jc.2008-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Antonopoulos S, Mikros S, Mylonopoulou M, Kokkoris S, Giannoulis G. Rosuvastatin as a novel treatment of non-alcoholic fatty liver disease in hyperlipidemic patients. Atherosclerosis. 2006;184:233–234. doi: 10.1016/j.atherosclerosis.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 40.Ahmed MH, Barakat S, Almobarak AO. Non-alcoholic fatty liver disease and cardiovascular disease: has the time come for cardiologists to be hepatologists? J Obes 2012. 2012 doi: 10.1155/2012/483135. ID 483135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Awad AS, Kamel R. Effect of Rosuvastatin on cholestasis-induced hepatic injury in rat livers. J Biochem mol Toxicol. 2010;24:89–94. doi: 10.1002/jbt.20315. [DOI] [PubMed] [Google Scholar]
- 42.Ansari JA, Bhandari U, Pillai KK, Haque SE. Effect of rosuvastatin on obesity-induced cardiac oxidative stress in Wistar rats--a preliminary study. Indian J Exp Biol. 2012;50:216–222. [PubMed] [Google Scholar]
- 43.Kostapanos MS, Milioni HJ, Filippatos TD, Nakou ES, Bairaktari ET, Tselepis AD, et al. A 12-weeks, prospective, open-label analysis of the effect of rosuvastatin on triglyceride-rich lipoprotein metabolism in patients with primary dyslipidemia. Clin Ther. 2007;7:1403–1414. doi: 10.1016/j.clinthera.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 44.Malaguarnera M, Vacante M, Russo C, Gargante MP, Giordano M, Bertino G, et al. Rosuvastatin reduces nonalcoholic fatty liver disease in patients with chronic hepatitis C treated with α-interferon and ribavirin. Hepat Mon. 2011;2:92–98. [PMC free article] [PubMed] [Google Scholar]
- 45.Kamada Y, Takehara T, Hayashi N. Adipocytokines and liver disease. J Gastroenterol. 2008;43:811–822. doi: 10.1007/s00535-008-2213-6. [DOI] [PubMed] [Google Scholar]
- 46.De las Heras N, Valero-Munoz M, Ballesteros S, Gomez-Hernandez A, Martýn-Fernandez B, Blanco-Rivero J, et al. Factors involved in rosuvastatin induction of insulin sensitization in rats fed a high fat diet. Nutr Metab Cardiovasc Dis. 2013;23:1107–1114. doi: 10.1016/j.numecd.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 47.Hasegawa T, Yoneda M, Nakamura K. Plasma transforming growth factor-beta1 level and efficacy of alpha-tocopherol in patients with non-alcoholic steatohepatitis: a pilot study. Aliment Pharmacol Ther. 2001;15:1667–1672. doi: 10.1046/j.1365-2036.2001.01083.x. [DOI] [PubMed] [Google Scholar]
- 48.Yang Z, Wang X, We J, Ye Z, Li Q, He M, et al. Prevalence of non-alcoholic fatty liver disease and its relation to hypoadiponectinaemia in the middle-aged andelderly Chinese population. Arch Med Sci. 2011;7:665–672. doi: 10.5114/aoms.2011.24137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma YX, Li WH, Xie Q. Rosuvastatin inhibits TGF-beta1 expression and alleviates myocardial fibrosis in diabetic rats. Pharmazie. 2013;5:355–358. [PubMed] [Google Scholar]
- 50.Vardi N, Parlakpinar H, Cetin A, Erdogan A, Cetin Ozturk I. Protective effect of beta-carotene on methotrexate-induced oxidative liver damage. Toxicol Pathol. 2010;38:592–597. doi: 10.1177/0192623310367806. [DOI] [PubMed] [Google Scholar]
- 51.Nierenberg DW, Bayrd GT, Stukel TA. Lack of effect of chronic administration of oral b-carotene on serum cholesterol and triglyceride concentrations. Am J Clin Nutr. 1991;53:652–654. doi: 10.1093/ajcn/53.3.652. [DOI] [PubMed] [Google Scholar]
- 52.Erdman JWJr, Breirer TL, Guggr ET. Absorption and transport of carotenoids. Ann N Y Acad Sci. 1993;691:76–85. doi: 10.1111/j.1749-6632.1993.tb26159.x. [DOI] [PubMed] [Google Scholar]
- 53.Erhardt A, Stahl W, Sies H, lirussi F, Donner A, Häussinger D. Plasma levels of vitamin E and carotenoids are decreased in patient with non alcoholic steatohepatitis (NASH) Eur J Med Res. 2011;16:76–78. doi: 10.1186/2047-783X-16-2-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rydén M, Leanderson P, Kastbom KO, Jonasson L. Effects of simvastatin on carotenoid status in plasma. Nutr Metab Cardiovasc Dis. 2012;1:66–71. doi: 10.1016/j.numecd.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 55.Canas JA, Damaso L, Altomare A, Killen K, Hossain J, Balagopal P. Insulin resistance and adiposity in relation to serum â-carotene levels. J Pediatr. 2012;161:58–64. doi: 10.1016/j.jpeds.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 56.Kameji H, Mochizuki K, Miyoshi N, Goda T. Beta-carotene accumulation in 3T3-L1 adipocytes inhibits the elevation of reactive oxygen species and the suppression of genes related to insulin sensitivity induced by tumor necrosis factor-alpha. Nutrition. 2010;26:1151–1156. doi: 10.1016/j.nut.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 57.Garcia-Ruiz C, Colell A, Morales A, Kaplowitz N, Fernandez Checa JC. Role of oxidative stress generated form the mitochondrial electron transport chain and mitochondrial glutathione status in loss of mitochondrial function and activation of transcription factor nuclear factor-kappa B: studies with isolated mitochondria and rat hepatocytes. Mol Pharmacol. 1995;48:825–834. [PubMed] [Google Scholar]
- 58.Thong-Ngam D, Samuhasaneeto S, Kulaputana O, Klaikeaw N. N-acetylcysteine attenuates oxidative stress and liver pathology in rats with non-alcoholic steatohepatitis. World J Gastroenterol. 2007;13:5127–5132. doi: 10.3748/wjg.v13.i38.5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khan MA, Rahman MM, Tania M, Uddin MN, Ahmed S. Pleurotus sajor-caju and Pleurotus florida mushrooms improve some extent of the antioxidant systems in the liver of hypercholesterolemic rats. Open Nutraceuticals J. 2011;4:20–24. [Google Scholar]
- 60.Chenni A, Yahia DA, Boukortt FO, Prost J, Lacaille-Dubois MA, Bouchenak M. Effect of aqueous extract of Ajuga iva supplementation on plasma lipid profile and tissue antioxidant status in rats fed a high-cholesterol diet. J Ethnopharmacol. 2007;109:207–213. doi: 10.1016/j.jep.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 61.El-Demerdash FM, Yousef MI, Kedwany FS, Baghdadi HH. Cadmium-induced changes in lipid peroxidation, blood hematology, biochemical parameters and semen quality of male rats: protective role of vitamin E and β-carotene. Food. doi: 10.1016/j.fct.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 62.Shih T, Vourvahis M, Singh M, Papay J. Pharmacogenetics: from bench science to the bedside. Ther Innov Regul Sci. 2008;42:503–513. [Google Scholar]
- 63.Berryman AM, Maritim AC, Sanders RA, Watkins JB. Influence of treatment of diabetic rats with combinations of pycnogenol, β-carotene, and alfa-lipoic acid on parameters of oxidative stress. J Biochem Mol Toxicol. 2004;18:345–352. doi: 10.1002/jbt.20046. [DOI] [PubMed] [Google Scholar]
- 64.Huang MA, Greenson JK, Chao C, Anderson L, Peterman D, Jacobson J, et al. One-year intense nutritional counseling results in histological improvement in patients with non-alcoholic steatohepatitis: a pilot study. Am J Gastroenterol. 2005;100:1072–1081. doi: 10.1111/j.1572-0241.2005.41334.x. [DOI] [PubMed] [Google Scholar]
- 65.Mecocci P, Polidori MC, Troiano L, Cherubini A, Cecchetti R, Pini G, et al. Plasma antioxidants and longevity: a study on healthy centenarians. Free Radic Biol Med. 2000;28:1243–1248. doi: 10.1016/s0891-5849(00)00246-x. [DOI] [PubMed] [Google Scholar]


